Abstract
Recently, the Urmia salamander, Neurergus crocatus Cope, 1862 was classified as a vulnerable species. The age structure of a breeding population portion of N. crocatus from Turkey was studied by using skeletochronology performed on the phalanges. According to the analysis of the age structure based on counting lines of arrested growth (LAGs), ages ranged from 5 to 14 years (mean = 9.4 ± 2.3 years) for males and from 8 to 17 years (mean = 11.6 ± 2.2 years) for females in N. crocatus. The mean snout–vent length was 69.2 ± 3.6 mm in males and 76.2 ± 3.8 in females. The sexual dimorphism index was 0.10. The difference between the sexes in age and size was statistically significant.
Introduction
Newts of the genus Neurergus (Salamandridae) are confined to Turkey and the Middle East. Four species have been described (Leviton et al. Citation1992; Schmidtler Citation1994; Sparreboom et al. Citation2000): Neurergus crocatus Cope, 1862 from northwest Iran, northern Iraq and southeast Turkey; Neurergus strauchii (Steindachner, 1887) from the western side of Van Lake to Malatya in Eastern Turkey; Neurergus microspilotus (Nesterov, 1916) from the border area between Iraq and Iran; and Neurergus kaiseri Schmidt, 1952 from the surroundings of Shah-Bazan of Luristan Province, Iran. All species of Neurergus can be easily distinguished by their morphological characters and on ecological grounds such as living and breeding habitats (Schmidtler & Schmidtler Citation1970, Citation1975; Schmidtler Citation1994).
The genus Neurergus is represented by two species in Turkey, N. crocatus Cope, 1862 and N. strauchii (Steindachner, 1887) (Baran & Öz Citation1986). The Turkish locality for N. crocatus is only the vicinity of Beytüssebap (Vilayet Şirnak), southeast Anatolia. The Urmia salamander, N. crocatus, like its congeneric species, is quite closely related to the Calotriton species (Steinfartz et al. Citation2002). Thus, it is an inhabitant of flowing water. This species is a montane form and lives in cool and well-oxygenated streams (Özeti & Yilmaz Citation1994; Baran & Atatür Citation1998). There is hardly any information on its life cycle, but eggs and larvae of different lengths were observed at the end of May and June. Breeding season is dependent upon altitude (Özeti & Yilmaz Citation1994).
The distribution of this species in Turkey is expected to undergo significant development due to different human activities (e.g. the construction of several dams is planned within the species range) over the next 10 years, and presumably the species will be impacted by these changes. There is a continuing decline in the extent and quality of its habitat in Turkey, Iran and Iraq. N. crocatus is categorized as a vulnerable species [VU B2ab (iii)] in the IUCN Red List of Threatened Animals (Papenfuss et al. Citation2004). Despite the significance of its conservation, nothing is known about the life history (e.g. individual growth, longevity, and other demographic parameters) of this species. Although N. crocatus is relatively common in Iran, there is no information regarding its population status in Turkey (Papenfuss et al. Citation2008). The aim of this study is to present original data on body size and age structure (minimum and maximum age for reproduction activity) of N. crocatus by using skeletochronology the most reliable method of age estimation in amphibians (Castanet & Smirina Citation1990; Smirina Citation1994).
Materials and methods
The specimens of Neurergus crocatus were collected from Basaran Village, Beytüssebap, Şırnak, Turkey (37°29' N, 43°07' E) at an altitude of 1135 m above sea level. Newts (34 males and 19 females) were caught by dip netting and by hand from the water during the breeding season (April and May 2004). This species breeds in mountain streams (egg laying) during the spring. Adults are known to leave the streams in favor of surrounding areas after breeding, but the terrestrial habitat remains unknown. It is presumed that the adults occur under rocks and other cover during the winter (Papenfuss et al. Citation2004).
The sex of each individual was determined by examining the cloacae: the male cloaca is turgid and shaped like male cloacae of Triturus, Ichthyosaura and Ommatotriton during the breeding season, whereas the female cloaca is not significantly different during breeding and non-breeding seasons.
Snout–vent lengths (SVL) of the newts were measured from the tip of the snout to the posterior margin of the vent by using a dial caliper with an accuracy of 0.02 mm (Marunouchi et al. Citation2000). Sexual size dimorphism (SSD) was calculated only on SVL. Sexual size dimorphism was estimated with the Lovich and Gibbons (Citation1992) sexual dimorphism index (SDI):
Individual age was estimated by skeletochronology (see Castanet & Smirina Citation1990; Olgun et al. Citation2005). The longest finger of the forelimb was excised and preserved in 70% ethanol. The preserved fingers were dissected; the larger bone of the phalange was washed in running water for 24 h, decalcified in 5% nitric acid for 2 h, and then washed again in running water for about 12 h. The cross-sections (15–18 μm) from the diaphyseal part of the phalange were obtained using a freezing microtome and then stained with Ehrlich's hematoxylin. These sections were placed in glycerin for observation with a light microscope. Lines of arrested growth (LAGs) were clearly visible in the phalange sections. The number of LAGs was counted for each individual and each slide was photographed for future reference since the preparation can fade. The slides were independently read and interpreted by two authors (N. Üzüm and N. Özdemir), who have similar experience. Since no additional line such as an aestivation line (Castanet & Smirina Citation1990) was observed, it was assumed that one LAG was formed in each year after hibernation. Consequently, individual age was assumed to be equivalent to the number of LAGs. For identification of how many LAGs were destroyed by endosteal resorption, sections with small and non-destroyed marrow cavities were selected and the perimeter of their inner LAGs were compared with the sections having endosteal resorption.
Both SVL and age in both sexes have a normal distribution (Kolmogorov–Smirnov test, P > 0.05), and show homogeneity of variance (Levene test, P > 0.05) and consequently, no data transformations have been applied prior to significance testing. Student's t-test was used to compare variables between sexes and correlation analysis was used to infer the pattern of relationships between SVL and age. All analyses were conducted by using the SPSS 11.0 statistical package.
Results
Cross-sections of phalanges of Neurergus crocatus showed not only periosteal but also endosteal bone separated by a resorption line. Both layers had varying numbers of rather concentric hematoxylinophilic lines. These lines were interpreted as LAGs (Castanet & Smirina Citation1990; Lima et al. Citation2000; Jakob et al. Citation2002). Although juvenile specimens were not caught, almost half of the cross-sections from adults (43.1%) exhibited a first thin line very close to the edge of the marrow cavity (A). This line was interpreted as a metamorphosis line (Castanet & Smirina Citation1990). LAGs were evident in all individuals, but varied in their degree of distinctness and regularity. LAGs were generally closer to each other toward to the periphery of the bone (A). This situation generally causes some difficulties, especially in old individuals, while counting LAGs (Castanet & Smirina Citation1990). Nonetheless, exact counts of the LAGs in the sample were obtained by comparing the independent results from two of the authors (N. Üzüm and N. Özdemir).
Figure 1. Cross-sections through phalanges of adult Neurergus crocatus. A, Male 71.52 mm SVL. Ten LAGs were observed in the periosteal bone. Metamorphosis line (m.l.) and the first LAG were distinct. This individual was 10 years old. B, Female 72.02 mm SVL. Ten LAGs were observed in the periosteal bone. The first LAG was completely destroyed by endosteal resorption. This individual was 11 years old. Since all specimens were collected in the breeding season, the outer margin of the bone did not regard as a LAG. e.b., endosteal bone; e.r., endosteal resorption; m.c., marrow cavity; m.l., metamorphosis line; p, periphery.
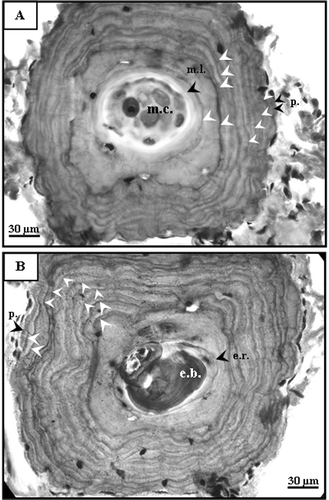
Endosteal resorption which creates an erosion of the periosteal bone on the edge of the marrow cavity, was observed in 40 specimens (78.4%); affecting only the first LAG (B).
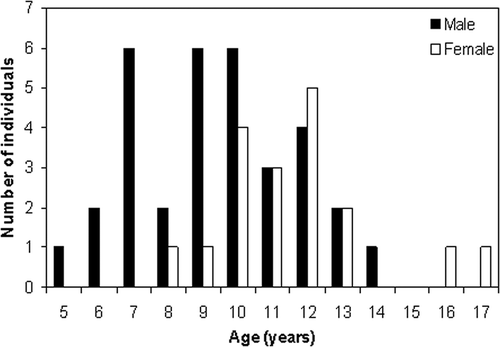
Fifty-three bones (representing 34 males and 19 females) were scored and 96.2% were suitable for an estimation of the individual's age. The minimum age was determined as 5 and 8 years for adult males and females, respectively, while maximum ages were determined as 14 and 17 years, respectively (). The mean age of newts was 9.4 ± 2.3 years (age ± SD; n = 33, range = 5–14 years) in males, and 11.6 ± 2.2 years (n = 18, range = 8–17 years) in females. There was a significant difference between males and females in terms of age (Student's t-test; t = –3.349, df = 49, P < 0.001), and females were older than males on the average. The age at which a significant decrease in growth occurs, based on the thickness of the growth rings was taken as the age of sexual maturity (Ryser Citation1988). The data suggest that sexual maturity in this population was reached by 2–3 years.
Mean SVL was 69.2 ± 3.6 mm (mean ± SD; range = 60.3–76.6, n = 34) in males and 76.2 ± 3.8 mm (range = 67.9–81.6, n = 19) in females. The sexual dimorphism index, on the other hand, was calculated as 0.10. The mean SVL of females was larger than the mean SVL of males. The mean SVL values were significantly different between sexes (Student's t-test; t = –6.567, df = 51, P < 0.001).
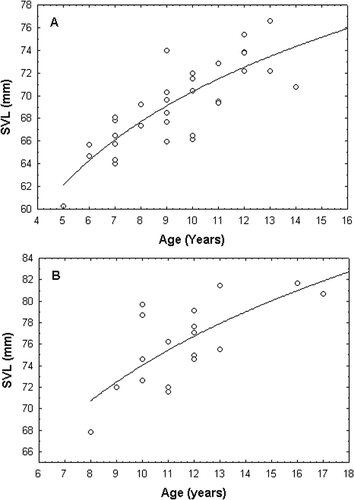
The age and body size were significantly correlated in males as well as in females (Pearson's correlation, males: r = 0.799, P < 0.01; females: r = 0.689, P < 0.01). The best regression model was selected as an S-curve model according to R 2 values; and the regression equations were ln(SVL) = 4.4014–(1.4574/age) for males and ln(SVL) = 4.5340–(2.3004/age) for females (). The regression model was statistically significant for both males (F = 64.854, P < 0.01, df = 31) and females (F = 17.1059, P < 0.01, df = 16). The model shows that the initial stage of growth is approximately exponential; then, as saturation begins the growth slows and it stops at maturity.
Discussion
The LAGs were clearly visible and allowed for an estimation of individual's age in Neurergus crocatus. The presence of LAGs in ectotherms living in relatively constant natural environments (e.g. Pancharatna et al. Citation2000) or under controlled conditions (Castanet Citation1985) causes an argument for an internal rhythm that is synchronized and amplified by the seasonal cycle (Alcobendas & Castanet Citation2000). As in other temperate species (e.g. Triturus marmoratus, Jakob et al. Citation2002; Triturus karelinii, Olgun et al. Citation2005; Calotriton asper, Miaud & Guillaume Citation2005), it was assumed that only one LAG was laid down during each hibernating period and they could easily be identified in the specimens. Bone remodeling, especially endosteal resorption, is one of the serious difficulties in skeletochronological interpretation, because it can cause the complete loss of one or more periosteal LAGs and therefore, result in underestimation of age (Castanet et al. Citation1993). Endosteal resorption was observed in 40 specimens (78.4%) in the sample for this study. By comparing the diameter of eroded marrow cavities with the diameter of non-eroded marrow cavities, it was determined that endosteal resorption destroys only (completely or partially) the first LAG. Several authors suggested that resorption may be linked to environmental conditions (Smirina Citation1972) with, for example, less resorption for populations living in high altitudes than for lowland populations (Esteban et al. Citation1996, Citation1999) or the opposite (Caetano & Castanet Citation1993). The presence of a line of metamorphosis seems to be an individual phenomenon documented for species in which larval development is completed in a single year (Hemelaar Citation1985).
Age distribution of N. crocatus ranged from 5 to 14 years for males and from 8 to 17 years for females. These results are similar to the results obtained for high-elevation populations of the sister species of Neurergus: Miaud et al. (Citation2000) found the youngest males and females were 11 and 9 years while longevity reached 20 and 19 years for males and females, respectively, of a population of Ichthyosaura alpestris living at 2200 m above sea level. Similarly, Kutrup et al. (Citation2005) reported that age distribution of a high-elevation population of Ommatotriton ophryticus ranged from 4 to 16 years; the minimum age in adults was 6 years for males and 8 years for females in a stream population of Calotriton asper (1400 m a.s.l.), whereas the maximum age was 22 and 29 years for males and females, respectively (Miaud & Guillaume Citation2005). Age differences between the sexes was found to be statistically significant (Student's t‐test, P < 0.01); indicating that adult females were older than the males on average. Similar results were also found for Chioglossa lusitanica (Lima et al. Citation2000) and in a stream population of Calotriton asper (Miaud & Guillaume Citation2005). On the other hand, the age distribution between the sexes did not differ significantly in Triturus karelinii (Olgun et al. Citation2005), Triturus marmoratus (Diaz-Paniagua et al. Citation1996), and Ichthyosaura alpestris (Miaud et al. Citation2000).
This study conducted on N. crocatus shows significant variations in mean SVL between sexes. Sexual size dimorphism was observed in many amphibians; females are often larger than males in many salamanders and newts (e.g. Diaz-Paniagua et al. Citation1996; Diaz-Paniagua & Mateo Citation1999; Miaud et al. Citation2000; Olgun et al. Citation2001, 2005). Similar results were also found for frogs (e.g. Miaud et al. Citation1999; Yılmaz et al. Citation2005; Guarino et al. Citation2008). Males often mature earlier than females at a smaller size in salamanders (Tilley Citation1973; Caetano et al. Citation1985; Miaud et al. Citation1993; Rebelo & Caetano Citation1995). This much variation between sexes could be explained by the fact that females allocate much more stored energy to gonad and embryo development than do males (Miaud et al. Citation2001).
Since the oldest specimen was 17 years old in the newts, we can evaluate that the Urmia salamander, N. crocatus, is a long-lived amphibian species. Therefore, it was assumed that the locality where N. crocatus was living offers a good habitat for these animals, and they can live until older ages. Moreover, low micronucleus number, as an indicator of healthy populations, was also recorded for this population by Başımoğlu-Koca et al. (Citation2006). These findings indicate that the population that lives in Başaran village is a healthy population and the area where these animals live serves as a suitable habitat for this population. Nonetheless, age composition of a population in amphibians varies depending upon the other environmental conditions and habitat characteristics (e.g. climatic conditions, trophic resources, interspecific competition and predator–prey interactions). Consequently, more interpopulational research is needed to clarify the life-history characteristics of this species and to determine the proximate effects of various environments on newt life-history traits.
Acknowledgment
We are most grateful to M. Stackhowitsch from Vienna University for English corrections on an earlier manuscript version.
References
- Alcobendas , M and Castanet , J . 2000 . Bone growth plasticity among populations of Salamandra salamandra: Interactions between internal and external factors . Herpetologica , 56 : 14 – 26 .
- Baran , İ and Atatür , MK . 1998 . Turkish herpetofauna (amphibians and reptiles) , Ankara : Republic of Turkish Ministry of Environment .
- Baran , İ and Öz , M . 1986 . On the occurrence of Neurergus crocatus and N. strauchii in Southeast Anatolia . Zoology in the Middle East , 1 : 96 – 104 .
- Başımoğlu-Koca , Y , Koca , S , Olgun , K , Beytaş , P and Üzüm , N . 2006 . Blood cell morphology, erythrocyte size and micronucleus counts of Neurergus crocatus (Cope, 1862) (Urodela: Salamandridae) in Turkey . Russian Journal of Herpetology , 13 : 83 – 88 .
- Caetano , MH and Castanet , J . 1993 . Variability and microevolutionary patterns in Triturus marmoratus from Portugal: Age, size longevity and individual growth . Amphibia–Reptilia , 14 : 117 – 129 .
- Caetano , MH , Castanet , J and Francillon , H . 1985 . Détermination de l’âge de Triturus marmoratus (Latreille, 1800) du Parc National de Peneda Gerês (Portugal) par squelettochronologie . Amphibia–Reptilia , 6 : 117 – 132 .
- Castanet , J . 1985 . La squelettochronologie chez les reptiles. I. Résultats expérimentaux sur la signification des marques de croissance squelettiques utilisé escomme critére d’âge chez les lézards et les tortues . Annales des Sciences Naturelles-Zoologie et Biologie Animale , 7 : 23 – 40 .
- Castanet , J , Francillon-Vieillot , H , Meunier , FJ and de Ricqlés , A . 1993 . Bone and individual aging , Edited by: Hall , BK . 245 – 283 . Boca Raton, FL : CRC Press .
- Castanet , J and Smirina , EM . 1990 . Introduction to the skeletochronological method in amphibians and reptiles . Annales des Sciences Naturelles-Zoologie et Biologie Animale , 11 : 191 – 196 .
- Diaz-Paniagua , C and Mateo , JA . 1999 . Geographic variation in body size and life-history traits in Bosca's Newt (Triturus boscai) . Herpetological Journal , 9 : 21 – 27 .
- Diaz-Paniagua , C , Mateo , JA and Andreu , AC . 1996 . Age and size structure of populations of small marbled newts (Triturus marmoratus pygmaeus) from Donana National Park (SW Spain) . A case of dwarfism among dwarfs. Journal of Zoology , 239 : 83 – 92 .
- Esteban , M , Garcia-Paris , M , Buckley , D and Castanet , J . 1999 . Bone growth and age in Rana saharica, a water frog living in a desert environment . Annales Zoologici Fennici , 36 : 53 – 62 .
- Esteban , M , Garcia-Paris , M and Castanet , J . 1996 . Use of bone histology in estimating the age of frogs (Rana perezi) from a warm temperate climate area . Canadian Journal of Zoology , 74 : 1914 – 1921 .
- Guarino , FM , Di Gia , I and Sindaco , R . 2008 . Age structure in a declining population of Rana temporaria from Northern Italy . Acta Zoologica Academiae Scientiarum Hungaricae , 54 : 99 – 112 .
- Hemelaar , AS . 1985 . An improved method to estimate the number of year rings resorbed in phalanges of Bufo bufo (L) and its application to populations from different latitudes and altitudes . Amphibia–Reptilia , 6 : 323 – 341 .
- Jakob , C , Seitz , A , Crivelli , AJ and Miaud , C . 2002 . Growth cycle of the marbled newt (Triturus marmoratus) in the Mediterranean region assessed by skeletochronology . Amphibia–Reptilia , 23 : 407 – 418 .
- Kutrup , B , Bülbül , U and Yılmaz , N . 2005 . Age structure in two populations of Triturus vittatus ophryticus at different altitudes . Amphibia–Reptilia , 26 : 49 – 54 .
- Leviton , AE , Anderson , SC , Adler , K and Minton , A . 1992 . Handbook to Middle East amphibians and reptiles , Oxford, OH : Society for the Study of Amphibians and Reptiles .
- Lima , V , Arntzen , JW and Ferrand , NM . 2000 . Age structure and growth pattern in two populations of the golden-striped salamander Chioglossa lusitanica (Caudata, Salamandridae) . Amphibia–Reptilia , 22 : 55 – 68 .
- Lovich , JE and Gibbons , JW . 1992 . A review of techniques for quantifying sexual size dimorphism . Growth Development & Aging , 56 : 269 – 281 .
- Marunouchi , J , Ueda , H and Ochi , O . 2000 . Variation in age and size among breeding populations at different altitutes in the Japanese newts, Cynops pyrrhogaster . Amphibia–Reptilia , 21 : 381 – 396 .
- Miaud , C , Andreone , F , Riberon , A , De Michelis , S , Clima , V , Castanet , J , Francillon-Vieillot , H and Guyétant , R . 2001 . Differences in age, size at maturity and gestation duration among two neighboring populations of the alpine salamander Salamandra lanza . Journal of Zoology , 254 : 251 – 260 .
- Miaud , C and Guillaume , O . 2005 . Variation in age, body size and growth among surface and cave-dwelling populations of the Pyrenean newt, Euproctus asper (Amphibia; Urodela) . Herpetologica , 61 : 241 – 249 .
- Miaud , C , Guyétant , R and Elmberg , J . 1999 . Variations in life-history traits in the common frog Rana temporaria (Amphibia: Anura): A literature review and new data from the French Alps . Journal of Zoology , 249 : 61 – 73 .
- Miaud , C , Guyétant , R and Faber , H . 2000 . Age, size and growth of the Alpine newt, Triturus alpestris (Urodela, Salamandridae), at high altitude and a review of life history trait variation throughout its range . Herpetologica , 56 : 135 – 144 .
- Miaud , C , Joly , P and Castanet , J . 1993 . Variation of age structures in a subdivided population of Triturus cristatus . Canadian Journal of Zoology , 71 : 1874 – 1879 .
- Olgun , K , Miaud , C and Gautier , P . 2001 . Age, size and growth of the terrestrial Salamander Mertensiella luschani in an arid environment . Canadian Journal of Zoology , 79 : 1559 – 1567 .
- Olgun , K , Üzüm , N , Avcı , A and Miaud , C . 2005 . Age, size and growth of the Southern Crested Newt Triturus karelinii (Strauch, 1870) in a population from Bozdağ (Western Turkey) . Amphibia–Reptilia , 26 : 223 – 230 .
- Özeti , N and İ , Yılmaz . 1994 . Türkiye amfibileri (The amphibians of Turkey) , İzmir : Ege Üniversitesi Fen Fakültesi Kitaplar Serisi .
- Pancharatna , K , Chandran , S and Kumbar , S . 2000 . Phalangeal growth marks related to testis development in the frog Rana cyanophlyctis . Amphibia–Reptilia , 21 : 371 – 379 .
- Papenfuss T, Sparreboom M, Uğurtaş İH, Rastegar-Pouyani N, Kuzmin S, Anderson S, Eken G, Kılıç T, Gem E, Kaya U. 2008. Neurergus crocatus. In: IUCN 2008. IUCN Red List of Threatened Species. Version 2009.2. http://www.iucn.org (http://www.iucn.org)
- Rebelo , R and Caetano , MH . 1995 . Use of skeletochronological method for ecodemographical studies on Salamandra salamandra gallaica from Portugal . Scientia Herpetologica , 1995 : 135 – 140 .
- Ryser , J . 1988 . Determination of growth and maturation in the common frog, Rana temporaria, by skeletochronology . Journal of Zoology (Lond.) , 216 : 673 – 685 .
- Schmidtler , JF . 1994 . Eine Übersicht neuerer Untersuchungen und Beobachtungen an der vorderasiatischen Molchgattung Neurergus . Abhandlungen und Berichte für Naturkunde Magdeburg , 17 : 193 – 198 .
- Schmidtler , JJ and Schmidtler , JF . 1970 . Morphologie, Biologie und Verwandtschafts- beziehungen von Neurergus strauchii aus der Turkei . Senckenbergiana Biologica , 51 : 41 – 53 .
- Schmidtler , JJ and Schmidtler , JF . 1975 . Untersuchunge an westpersischen Bergbachmolchen der Gattung Neurergus . Salamandra , 11 : 84 – 98 .
- Smirina , EM . 1972 . Annual layers in bones of Rana temporaria . Zoolicheskii Zhurnal , 51 : 1529 – 1534 .
- Smirina , EM . 1994 . Age determination and longevity in amphibians . Gerontology , 40 : 133 – 146 .
- Sparreboom , M , Steinfartz , S and Schultschik , G . 2000 . Courtship behavior of Neurergus . Amphibia–Reptilia , 21 : 1 – 11 .
- Steinfartz , S , Wook-Hwang , U , Tautz , D , Öz , M and Veith , M . 2002 . Molecular phylogeny of the salamandrid genus Neurergus: Evidence for an intrageneric switch of reproductive biology . Amphibia–Reptilia , 23 : 419 – 431 .
- Tilley , SG . 1973 . Life histories and natural selection in populations of the salamander Desmognathus ochropaeus . Ecology , 54 : 3 – 17 .
- Yılmaz , N , Kutrup , B , Ü , Çobanoglu and Özoran , Y . 2005 . Age determination and some growth parameters of Rana ridibunda population in Turkey . Acta Zoologica Academiae Scientiarum Hungaricae , 51 : 67 – 74 .