Abstract
Timing and habitat preferences for settlement of juvenile fishes were investigated at the Marine Protected Area (MPA) of Torre Guaceto (SE Adriatic Sea) from April 2005 to March 2006. Data were obtained by visual census (on a fortnightly basis) in 10 habitat types identified within the depth range 0–6 m. A total of 22 taxa of juvenile fish was recorded: juveniles of 14 taxa were analysed for settlement timing and habitat preferences, whereas the other 8 taxa were only occasionally observed. Diplodus vulgaris and Sarpa salpa showed two settlement peaks, while the other 12 species displayed a single annual peak. Most species (10 out of 14) settled between late spring and early autumn. Juvenile labrids and Oblada melanura were mostly associated with exposed shallow rocks, while Diplodus sargus, D. vulgaris, D. puntazzo and S. salpa chiefly settled in shallow sheltered coves. Juveniles of Chromis chromis were found in sublittoral rocks and Posidonia oceanica beds. Spondilyosoma cantharus, Diplodus annularis and Dicentrarchus labrax mostly associated to P. oceanica and small-sized seagrasses for settlement, while Mullus surmuletus chiefly used sublittoral sands. This study provides evidence of clear coherence during settlement of many fishes in terms of habitat for settlement, while some discrepancy was found in terms of timing across the year. In addition, this study provided suggestive evidence of the potential of the MPA, relative to the habitats included within its borders, in hosting juvenile fish stages and thus contributing to sustain local diversity of the coastal fish fauna. Similar data would deserve to be properly considered to design MPAs and refine conservation targets.
Introduction
Juvenile stages represent crucial phases in the life history of many littoral fishes (Brothers & McFarland Citation1981). The success of settlement and recruitment, in fact, is crucial for the replenishment of local populations (Meekan et al. Citation1983; Jones Citation1990; Sheaves et al. Citation2006), especially those that are impacted by fishing and/or concerned by conservation measures (Beck et al. Citation2001; Jones et al. Citation2009 and references therein).
In this general context, it is worth carefully defining the two subsequent phases of the early life history of coastal fish, i.e. ‘settlement’ and ‘recruitment’ (Biagi et al. Citation1998; Macpherson Citation1998). ‘Settlement’ refers to the very early phase when post-larvae move from the pelagic environment towards the coast and metamorphose into the benthic juvenile. ‘Recruitment’, instead, identifies the phase following the settlement, when juveniles join the adult population, leaving the nursery areas or expanding home range beyond the nursery itself (Biagi et al. Citation1998). Settlement, depending on the fish species considered, may or may not occur in the same habitat(s) where recruitment occurs. Many fishes, in fact, show dramatic ontogenetic changes in the habitat use, with different habitat types or depth ranges being occupied by specimens of different sizes/ages (Harmelin-Vivien et al. Citation1995; Newman & Dunk Citation2002).
As far as the Mediterranean Sea is concerned, the presence of juvenile fishes in coastal habitats was firstly noticed by Aristotle in the Historia Animalium. Aristotle, therefore, first introduced, to some extent, the concept of ‘nursery habitat’ (Balme Citation2002). Relatively recent studies dating back to the first decades of the past century (Lo Bianco Citation1908; Ranzi Citation1930) reported qualitative but important information on juvenile stages of coastal fishes, specifically about where (e.g. in terms of habitat type and depth) and when (i.e. period of the year) juveniles were found. Recent studies reported on quantitative data about the presence and abundance of juveniles of many Mediterranean fishes (Garcia-Rubies & Macpherson Citation1995; Harmelin-Vivien et al. Citation1995; Biagi et al. Citation1998; Macpherson Citation1998; Vigliola et al. Citation1998) emphasising that: (1) many species are typically associated with specific habitats and depth ranges in relation to individual size (and age) (Harmelin-Vivien et al. Citation1995); (2) several fishes show typical and sometimes dramatic peaks in settlement at specific periods of the year (Vigliola et al. Citation1998); (3) some congeneric species avoid competition by using different habitat types as nurseries or by settling in the same habitat but in different periods of the year (e.g. sea breams of the genus Diplodus; Macpherson Citation1998; Vigliola et al. Citation1998); and (4) some species show remarkable ontogenetic shifts in the use of different habitats (e.g. sea breams of the genus Diplodus), while other species settle and recruit in the same habitat where adults typically live (e.g. most labrid fishes) (Garcia-Rubies & Macpherson Citation1995). The available information, however, is fairly restricted in space (it mostly concerns the NW Mediterranean Sea) and time (most of the data available concern a time window of about 2–3 years, corresponding to specific EU projects carried out in the 1990s). Distribution patterns of marine species, however, are well known to change in space (e.g. across latitudinal gradients) and time (e.g. from year to year), also considering the implications of current climate change in the Mediterranean Sea (Astraldi et al. Citation1995; Guidetti et al. Citation2002a; Bianchi & Morri Citation2003; Guidetti & Dulcic Citation2007; Nykjaer Citation2009). There is thus the need to widen the spatio-temporal scales for the assessment of distribution patterns of juvenile stages of many coastal fishes to see whether or not the information coming from the NW Mediterranean Sea is coherent at a larger scale. There could be the risk, in fact, that conclusions based on data restricted to a geographical sector become a paradigm extended to larger scales, which may have negative consequences on the effectiveness of conservation/management measures established elsewhere.
The ‘nursery role’ is one of the most important biological criteria to prioritise coastal sectors/habitats (Heck et al. Citation2003), e.g. to be included into Marine Protected Areas (hereafter MPAs). The information on juvenile presence, abundance and diversity of fish is suggestive of how an area, with its own mosaic of habitats, has the potential to sustain the local diversity and abundance of fishes, with important implications in terms of conservation targets, but also of fishing revenues (Guidetti & Claudet Citation2010). From this perspective, an MPA that is potentially able to self-sustain local populations of fish can be more effective in terms of recovery within its boundaries (Halpern & Warner Citation2002; Guidetti & Sala Citation2007) and enhancement of fishing outside (e.g. Guidetti Citation2007; Harmelin-Vivien et al. Citation2008).
The present study, therefore, is aimed at reporting data on timing and habitat preferences for settlement of juvenile fishes at the MPA of Torre Guaceto located in the southern Adriatic Sea.
Material and methods
Study area
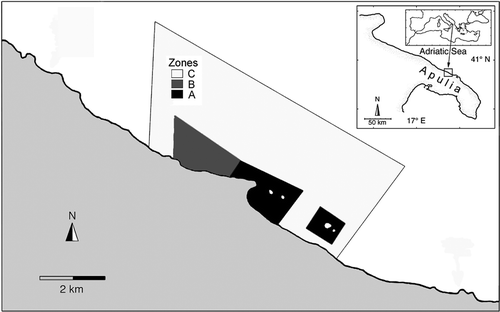
This study was carried out at the MPA of Torre Guaceto (40°42′N; 17°48′ about 10 km of coastline, north to the city of Brindisi; ) in the south-western Adriatic Sea.
It extends for about 8 km along the coast and off shore for about 2.5 km (corresponding opproximately to the bathymetry of 50 m). The whole MPA covers about 2227 ha and is subdivided into subzones that include both no-take and buffer zones (Guidetti & Claudet Citation2010).
The area is characterised by a rocky coast alternating with small to fairly wide beaches in front of which shallow substrates are often sandy with rare and small beds of small-sized seagrasses (e.g. Cymodocea nodosa) or Posidonia oceanica beds. Where the coastline is rocky, a calcarenitic rocky plateau with a gentle to medium slope declines from the water surface to a depth of about 10–12 m over coarse sand and P. oceanica beds. From about the surface to 8 m depth, the rocky plateaux are continuous and fairly flat, while around 10 m the rocky habitat shows high structural complexity. In the MPA these rocky bottoms are often covered by photophilous algae (Guidetti Citation2006). Posidonia oceanica beds (sometimes in the form of dead mat) alternate with sandy bottoms from 10–12 m to about 22–25 m depth. Deeper, the substrate is constituted by coralligenous formations alternating with sandy–muddy patches down to ∼50 m (Fraschetti et al. Citation2005).
For the specific goal of our study, we considered only the depth range of 0–6 m. This depth range is the most suitable for hosting early stages of fishes on the basis of the available literature (Garcia-Rubies & Macpherson Citation1995; Harmelin-Vivien et al. Citation1995; Guidetti et al. Citation2002b). We also conducted preliminary surveys to refine characterisation of habitats included into the depth range investigated. This step was necessary as some environmental characteristics that may affect juvenile fish distribution (e.g. freshwater inputs) are not included into biocoenotic maps. We thus selected 10 habitats as potentially suitable in hosting settlers of coastal fishes. They included six shallow (0–3 m depth) and four deeper (3–6 m depth) habitats listed as follows: (1) sandy bays (0–1.5 m depth) with freshwater inputs (habitat code: SBFI); (2) sandy bays (0–1.5 m) without freshwater inputs (SB); (3) shallow rocks with macroalgae (0–1.5 m) (SR); (4) exposed indented shallow rocks (0–1.5 m) (EISR); (5) exposed linear shallow rocks (0–1.5 m) (ELSR); (6) shallow substrata in sheltered coves (0–1.5 m) (SSC); (7) small-sized seagrasses (e.g. Cymodocea nodosa) (3–6 m) (SSS); (8) Posidonia oceanica beds (3–6 m) (POC); (9) sublittoral rocks with macroalgae and zoobenthos (3–6 m) (SRMZ); and (10) sublittoral sand (3–6 m) (SAND).
Sampling procedure and presentation of data
Data were collected at each habitat type on a fortnightly basis, from April 2005 to March 2006 (for a total of 24 sampling sessions across the study year). We used snorkelling in shallow habitats and SCUBA in deeper habitats. Density of juvenile fish, from settlers (identified as early stages, including semitransparent post-larvae of ∼1.5–2.0 cm) to young fish (corresponding to 1/10 of total length of each species; Froese & Pauly Citation2009), was evaluated by means of visual census along strip transects of 25 × 2 m (Harmelin-Vivien et al. Citation1985). Each transect was run in about 10–15 min, using a reel of pre-established length (i.e. 25 m). Five replicate censuses were performed for each habitat type at each time of sampling, for a total of 1200 replicated transects during the study year.
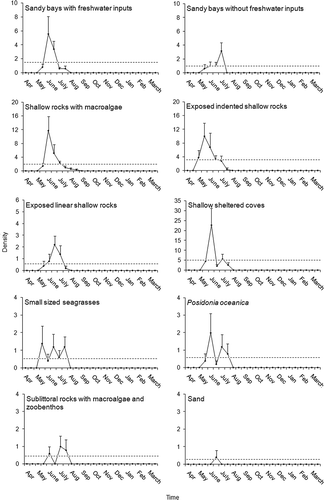
Settlement patterns usually show peaks. At the beginning of the settlement process there is, in general, a progressive increase in the arrival of juveniles and mortality is low until a peak is achieved. Then, juvenile mortality increases, the number of arriving settlers decreases and the relative abundance of settlers consequently decreases. For the sake of simplicity, we identified ‘settlement peaks’ according to the following operational definition: a settlement peak includes those values of density of settlers (i.e. average density calculated on each series of five replicate censuses per each habitat and sampling date) that exceed the 66% of the overall mean density calculated across the year (excluding zero values; see as an example for Diplodus sargus).
Figure 2. Temporal trends in juvenile Diplodus sargus density (no. individuals/50 m2) with settlement peaks (i.e. months above the dotted line; see Methods for details) in each of the 10 habitat types at the Torre Guaceto MPA.
Temporal trends and peaks, and habitat preferences were investigated on 14 juvenile fish taxa (see Results), due to the occasional presence of juveniles belonging to 8 out of the 22 taxa recorded.
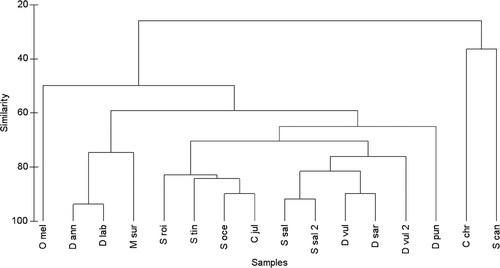
Hierarchical clustering was performed to evaluate similarities in habitat preferences of juvenile fishes at the Torre Guaceto MPA. Juvenile fish abundance at the 10 habitat types investigated were fourth-root transformed, and the triangular similarity matrix was obtained using Bray–Curtis similarity index. Dendrograms were constructed using group-average link for delineating groups of species with possible distinct choices in terms of habitat for juvenile stages. A Principal Component Analysis (PCA) was then performed on standardised data of juvenile abundance to assess the degree to which juveniles of the different species associated with the 10 habitat types investigated (Harmelin-Vivien et al. Citation1995). Cluster and PCA analyses were run using Primer 6 software package (Clarke & Warwick Citation2001).
Results
During the whole study year, juvenile fish belonging to 22 fish taxa were recorded. Due to the paucity of observations on juveniles of 8 taxa, however, a more detailed analysis was performed only on 14 taxa ().
Table I. Complete list of juvenile fish taxa recorded at the MPA of Torre Guaceto during the study year (April 2005–March 2006). *, occasional observation
Peaks of juvenile settlement were usually clear and easy to identify for all 14 species. As an example, in the peak in the arrival of juvenile Diplodus sargus at each of the 10 habitat types is reported. Temporal windows for the presence of juveniles were mostly coherent among the different habitats, and lasted approximately 1 or, at maximum, 2 months. In terms of timing, peaks of juvenile settlement of two species, i.e. Diplodus vulgaris and Sarpa salpa, showed two well distinct periods, while the remaining 12 taxa displayed a single annual settlement event (). Half of fish taxa (i.e. 7 out of 14) were found to settle in summer to early autumn (Coris julis, Chromis chromis, Dicentrarchus labrax, Diplodus annularis, Symphodus ocellatus, Symphodus roissali, Symphodus tinca). Juvenile settlement of D. vulgaris (first peak), Mullus surmuletus and S. salpa (first peak) took place in spring, while juveniles of D. sargus, Oblada melanura and Spondyliosoma cantharus were mostly found in late spring–early summer. Juvenile settlement of Diplodus puntazzo occurred in autumn, whereas the second peak of juvenile settlement of D. vulgaris and S. salpa was observed in winter ().
Table II. Timing of peaks in the presence of juvenile fishes in the 10 habitats investigated at the Torre Guaceto MPA. Habitat codes: SBFI, sandy bays with freshwater inputs; SB, sandy bays without freshwater inputs; SR, shallow rocks with macroalgae; EISR, exposed indented shallow rocks; ELSR, exposed linear shallow rocks; SSC, shallow sheltered coves; SSS, small-sized seagrasses; POC, Posidonia oceanica; SRMZ, sublittoral rocks with macroalgae and zoobenthos; SAND, sublittoral sand. Grey: unique or first peak; dark: second peak
In terms of number of habitat types used by juvenile fishes, only D. sargus and M. surmuletus were found (although in different proportions) in all 10 habitat types included in this study. On the contrary, juveniles of C. chromis, S. cantharus and O. melanura were recorded only in a few habitats (i.e. 2, 2 and 3 habitat types, respectively; ).
The hierarchical analysis showed that, in terms of similarity in habitat use by the 14 fish species investigated, juveniles of C. chromis and S. cantharus, and to a lesser extent O. melanura, clearly differed among them and when compared to the remaining 11 species (). Juvenile labrid fishes tended to cluster apart from the remaining species that mostly included sparid fishes. Among sparids, D. annularis clustered with D. labrax and, to a lesser extent, with M. surmuletus, and well separated from S. salpa and the remaining three Diplodus fishes (i.e. D. sargus, D. vulgaris and D. puntazzo). These latter fishes tended to cluster together, especially D. sargus and D. vulgaris, indicating similar habitat requirements for settlement by these three congeneric species, although D. puntazzo separated a little bit from the other two Diplodus fishes, being able to use a wider array of habitat types for settlement ().
Figure 3. Hierarchical clustering of juvenile fishes based on proportional abundances (standardised data) assessed in the 10 habitat types at the Torre Guaceto MPA.
PCA analysis (with PC1 and PC2 explaining 51.4% and 18.0%, respectively, of the overall variation) provided evidence of clear differences in the habitat use by juvenile fishes ().
Figure 4. Principal component analysis ordination model (PCA) of the 14 fish species investigated in this study with superimposed the vectors of the 10 habitat types at the Torre Guaceto MPA. Habitat codes: SBFI, sandy bays with freshwater inputs; SB, sandy bays without freshwater inputs; SR, shallow rocks with macroalgae; EISR, exposed indented shallow rocks; ELSR, exposed linear shallow rocks; SSC, shallow sheltered coves; SSS, small-sized seagrasses; POC, Posidonia oceanica; SRMZ, sublittoral rocks with macroalgae and zoobenthos; SAND, sublittoral sand.
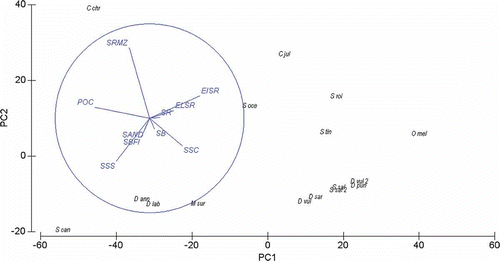
Juvenile labrid fishes and O. melanura mostly associated with exposed indented and linear shallow rocks (EISR and ELSR), and to a lesser extent with shallow sheltered coves (SSC). Diplodus sargus, D. vulgaris, D. puntazzo and S. salpa mostly settled in shallow sheltered coves (SSC) and, secondly, in other rocky habitats (i.e. SR, EISR or ELSR). Juveniles of C. chromis were mostly associated to sublittoral rocks with macroalgae and zoobenthos (SRMZ) and to a lesser extent to P. oceanica beds (POC). Juveniles of S. cantharus were found to be associated in similar proportions to P. oceanica and small-sized seagrasses (SSS). Juveniles of D. annularis and D. labrax were found to settle in a wide array of habitat types, but proportionally they were mostly found in seagrass habitats (both POC and SSS in similar proportions) and, secondly, in sandy bays with freshwater inputs (SBFI). Settlers of M. surmuletus were found in all 10 habitat types, but proportionally sublittoral sands (SAND) contributed most in hosting juvenile stages of this species, followed by sandy bays with freshwater inputs (SBFI), small-sized seagrasses (SSS) and shallow sheltered coves (SSC).
Discussion
In terms of timing, settlement peaks of juvenile fishes in south-eastern Apulia appeared to be mostly (although not completely) coherent with those observed elsewhere. In the W Mediterranean (similarly to our results in SE Italy, Adriatic Sea), juveniles of many species were found to settle in late spring and summer (e.g. for many labrids and C. chromis; Lo Bianco Citation1908; Garcia-Rubies & Macpherson Citation1995; Tunesi et al. Citation1997; Biagi et al. Citation1998). In SE Apulia the two observed arrivals of S. salpa took place around January and April. In Spain, other authors (Garcia-Rubies & Macpherson Citation1995) mostly recorded juveniles of S. salpa in two different temporal windows, i.e. May and October, while Fernandez-Jover et al. (Citation2009) reported two peaks, specifically in May and July. Near Marseille (France) juveniles of S. salpa were recorded from February to April, even though no distinctions between two different peaks were reported (Harmelin-Vivien et al. Citation1995). In the Tuscany Archipelago (Tyrrhenian Sea, Italy) settlers of S. salpa were found in February and December (Biagi et al. Citation1998), while in the Liguria Sea they were reported in November–December and March (Tunesi et al. Citation1997). In Apulia we found O. melanura in the June–early July period, and M. surmuletus in May. Juveniles of O. melanura and M. surmuletus were recorded in June and the June–July period, respectively, in the Tyrrhenian Sea (Biagi et al. Citation1998), while in Spanish waters and the Ligurian Sea they are reported in July–August (Garcia-Rubies & Macpherson Citation1995; Tunesi et al. Citation1997; Deudero Citation2002; Fernandez-Jover et al. Citation2009). Dicentrarchus labrax is reported to settle during spring in the Ligurian and Tyrrhenian Seas (Tunesi et al. Citation1997; Biagi et al. Citation1998), while juveniles of this fish were found some months later (early summer) in SE Apulia. Considering the juvenile size of D. labrax we have censused, however, we are likely to have recorded post-recruits and not just settlers, which could explain our later observation compared to the literature information. Our data, instead, fit well with data from the Gulf of Naples, where juveniles D. labrax about 8 cm long were found in July (Lo Bianco Citation1908). Juveniles of Diplodus annularis and S. cantharus were observed during the entire summer season, similar to other areas in the Mediterranean Sea (see Harmelin-Vivien et al. Citation1995, and references therein; Guidetti & Bussotti Citation1997; Tunesi et al. Citation1997; Biagi et al. Citation1998). More information is available for the three species belonging to the genus Diplodus. Juveniles of D. vulgaris were reported between November and February–March in the NW Mediterranean, with the general indication that there were two pulses (Vigliola et al. Citation1998; Fernandez-Jover et al. Citation2009; see also Lo Bianco Citation1908). Near Marseille, juveniles of this species were found from early March to June (Harmelin-Vivien et al. Citation1995). In the Tyrrhenian Sea settlement took place in December–January, but juveniles were found in nursery areas until June (Biagi et al. Citation1998), while in the Ligurian Sea settlers were found from December to March (Tunesi et al. Citation1997). We found two well distinct events of settlement in December and April in SE Apulia. Juveniles of D. sargus were found to mostly settle in shallow waters in May–June in Apulia, similar to other locations in Italy, Spain and France (Lo Bianco Citation1908; Biagi et al. Citation1998; Vigliola et al. Citation1998; Fernandez-Jover et al. Citation2009). Diplodus puntazzo was found in October–November in this study, as well as in many other locations in the NW Mediterranean (Lo Bianco Citation1908; Garcia-Rubies & Macpherson Citation1995; Biagi et al. Citation1998; Vigliola et al. Citation1998; Fernandez-Jover et al. Citation2009), while other authors near Marseille recorded juveniles of this species from March to early June (Harmelin-Vivien et al. Citation1995).
Data and patterns reported above need some consideration to be properly interpreted. Firstly, most of the data available refer to the NW Mediterranean, which makes it difficult to generalise the observed patterns to the whole Mediterranean. Secondly, as data were mostly collected within single or very few years, it is difficult to distil between spatial differences and temporal (among years) differences. Although specific data are not available, there is more than an impression that settlement events each year in each area of the Mediterranean may take place sooner or later depending on specific environmental conditions. Thirdly, there is some (maybe apparent) temporal discrepancy in the presence of juvenile stages reported in the literature. Although temporal patterns of settlement seems to change at large spatial scale (i.e. among areas within the Mediterranean basin), without precise data about juvenile size it is difficult to understand whether or not such discrepancies actually represent spatial differences or are simply attributable to the fact that when authors refer to ‘juveniles’, some refer to settlers and others to recruits or bigger juvenile-subadult fishes.
With regard to the habitat(s) used by fish for settlement, it is noteworthy to stress that many species showed clear preferences, with some species being found exclusively in some habitats, and other species that showed higher densities in specific habitats although their presence was recorded in other additional/alternative habitats. In terms of habitat preferences, investigated using the juvenile density as a proxy, Spondilyosoma cantharus and Diplodus annularis were mostly associated to marine phanerogams (especially small-sized seagrasses) for settlement. Similar results for these two species were reported from other Western Mediterranean locations (Francour & Le Direach Citation1994; Harmelin-Vivien et al. Citation1995; Biagi et al. Citation1997; Guidetti & Bussotti Citation1997; Tunesi et al. Citation1997; Guidetti Citation2000; Guidetti & Bussotti Citation2000). This shows their strict association with marine phanerogams, even though it is long been known that they can use additional/alternative vegetated habitats (e.g. rocks covered by arborescent macroalgae) for settlement (Lo Bianco Citation1908; Guidetti & Bussotti Citation1997). Mullus surmuletus chiefly used sublittoral sandy habitats in SE Italy. Other authors found similar patterns, but also reported that juveniles of this species can be found associated with marine phanerogams (Garcia-Rubies & Macpherson Citation1995; Guidetti & Bussotti Citation2000) or can be collected in open waters far from the coast (Deudero Citation2002). Juveniles of Chromis chromis were always found to be chiefly associated with sublittoral rocks composed by blocks and boulders. Even though the species may use additional/alternative habitats for settlement, like the eroded mat of the seagrass P. oceanica, the habitat preference for rocky boulders rich in crevices and shelters seems to be quite a general pattern (Lo Bianco Citation1908; Garcia-Rubies & Macpherson Citation1995; Guidetti Citation2000). Juveniles of Oblada melanura and some labrid fishes were found to be mostly associated with exposed shallow rocks, especially where rocks were covered by arborescent macroalgae (e.g. for juveniles belonging to Symphodus species and Coris julis) both in SE Apulia and in other areas of the Mediterranean Sea (Lo Bianco Citation1908; Garcia-Rubies & Macpherson Citation1995; Harmelin-Vivien et al. Citation1995). The available data about juvenile settlement of Dicentrarchus labrax are pretty scant. In the NW Mediterranean this fish was found to settle and form large groups in relatively open waters (Tunesi et al. Citation1997; Biagi et al. Citation1998), while in the Gulf of Naples juveniles of this species were usually found in harbour areas (Lo Bianco Citation1908). We found juveniles of D. labrax in shallow sheltered habitats, small-sized seagrasses and sandy substrates. Sarpa salpa, Diplodus sargus, D. puntazzo and D. vulgaris chiefly settled in shallow sheltered coves. In more detail, S. salpa was also found (although with lower densities compared to sheltered coves) in other shallow rocky and vegetated habitats. These latter habitats have been indicated elsewhere as hosting larger densities of juvenile S. salpa (Lo Bianco Citation1908; Harmelin-Vivien et al. Citation1995), even though this species seems to be able to settle in a wide range of habitat types (Garcia-Rubies & Macpherson Citation1995). The three above-mentioned Diplodus fishes (i.e. Diplodus sargus, D. puntazzo and D. vulgaris), on the contrary, showed quite similar habitat preferences for settlement. Shallow and gently sloped sheltered habitats composed by sand, gravels, pebbles or rocks seemed to be the favourite habitat types in the Adriatic, Tyrrhenian and NW Mediterranean Seas (Lo Bianco Citation1908; Ranzi Citation1930; Garcia-Rubies & Macpherson Citation1995; Harmelin-Vivien et al. Citation1995; Tunesi et al. Citation1997; Biagi et al. Citation1998; this study). It is also interesting to note that these congeneric species settle in the same habitat types but in different temporal windows across the year (Lo Bianco Citation1908; Biagi et al. Citation1997; Tunesi et al. Citation1997; Biagi et al. Citation1998; Macpherson Citation1998; Vigliola et al. Citation1998; this study). The patterns of habitat use reported in this study, on the whole, were found to be fairly similar to those reported by other authors in the available literature, which suggests that fish species use similar habitat types for settlement in different geographic areas.
This paper seems to suggest that seagrass habitats do not have a primary role as nursery areas. Quite a few species, in fact, seem to strictly depend on seagrass habitats for settlement in the Mediterranean region, which is also in agreement with available data (e.g. Franco et al. Citation2006). Nevertheless, this is not in agreement with the evidence available about the important nursery role of seagrasses for multispecies fish assemblages observed in other geographical regions outside the Mediterranean (Heck et al. Citation2003). This conclusion should be taken with major caution, however. There is the risk of dismissing the importance of seagrasses as nurseries and, therefore, attributing low priority to seagrass habitats when conservation measures are set up (e.g. during the establishment of MPAs). In this study, in fact, the conclusion that seagrasses are not that important as nurseries is drawn assessing the effectiveness as nursery only based on juvenile density, i.e. the abundance of juveniles per surface area. However, this calculation does not properly take into account the overall and actual contribution of a habitat in providing new individuals to the local population, which depends also on the overall extent of each habitat. In other words, even though the density of juveniles of a species is low in a specific habitat, the overall number of juveniles recruiting to the adult population can be high if this habitat occupies a large surface. On the contrary, a habitat may contribute little if the juvenile density is high but habitat extension is limited. Such different perspectives to assess nursery effectiveness have also been debated (Beck et al. Citation2001; Dahlgren et al. Citation2006; Sheaves et al. Citation2006), along with the choral call for quantitative approaches to assess nursery roles of coastal habitats.
Whatever the approach used to assess the nursery role of coastal habitats, the need for protecting/managing mosaics of habitats rather than single or few habitats in order to achieve the goal of protecting multispecies fish assemblages is more and more evident. This seems to be quite obvious in order to maintain local biodiversity (from species to ecosystems; Roberts et al. Citation2003), along with the need not to neglect particular habitats that may contribute to local diversity by hosting exclusive species (e.g. marine caves; Bussotti & Guidetti Citation2009).
In conclusion, the role of coastal habitats as nurseries for juvenile fish is extremely important in management considerations for identifying critical habitats for conservation and restoration and for maintenance and/or recovery of local fish populations (Lipcius et al. Citation2008). Management of nursery habitats, from these perspectives, is critical for biodiversity conservation and fishery management (Beck et al. Citation2001; Sala et al. Citation2002). The nursery role of coastal habitats, however, has been often addressed to single species and poorly investigated from a quantitative viewpoint (Beck et al. Citation2001; Dahlgren et al. Citation2006), especially in the Mediterranean region. This stresses the urgency of providing quantitative data on the nursery potential of coastal areas to improve management of human activities (e.g. fisheries) and prioritising inclusion of specific habitats into MPAs.
Acknowledgements
Research was funded by the Regione Puglia (‘Ecofishmod’ POR project).
References
- Astraldi , M , Bianchi , CN , Gasparini , GP and Morri , C. 1995 . Climatic fluctuations, current variability and marine species distribution: A case study in the Ligurian Sea (north-west Mediterranean) . Oceanologica Acta , 18 : 139 – 149 .
- Balme , DM. 2002 . “ Historia Animalium. Aristotle, Vol. I, Books I–X: Text ” . In Cambridge Classical Texts and Commentaries, No. 38 , Cambridge : Cambridge University Press .
- Beck , MW , Heck Jr , KL , Able , KW , Childers , DL , Eggleston , DB , Gillanders , BM , Halpern , B , Hays , CG , Hoshino , K , Minello , TJ , Orth , RJ Sheridan , PF . 2001 . The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates . BioScience , 51 : 633 – 641 .
- Biagi , F , Gambaccini , S and Zazzetta , M. 1997 . Insediamento e microhabitat di specie ittiche nella fascia costiera toscana . Biologia Marina Mediterranea , 4 : 195 – 203 .
- Biagi , F , Gambaccini , S and Zazzetta , M. 1998 . Settlement and recruitment in fishes: The role of coastal areas . Italian Journal of Zoology , 65 : 269 – 274 .
- Bianchi , CN and Morri , C. 2003 . Climate change and biological response in Mediterranean Sea ecosystems – A need for broad-scale and long-term research . Ocean Challenge , 13 : 32 – 36 .
- Brothers , EB and McFarland , WN. 1981 . Correlations between otolith microstructure, growth, and life history transitions in newly recruited French grunts (Haemulon flavolineatum) (Desmaret) Haemulidae . Rapports et Procès-Verbaux des Reunions Commission Internationale pour l'Exploration de la Mer Mediterranée Monaco , 178 : 369 – 374 .
- Bussotti , S and Guidetti , P. 2009 . Do Mediterranean fish assemblages associated with marine caves and rocky cliffs differ? . Estuarine, Coastal and Shelf Science , 81 : 65 – 73 .
- Clarke , KR and Warwick , RM. 2001 . Change in marine communities: An approach to statistical analysis and interpretation , 2nd , Plymouth, , UK : PRIMER-E .
- Dahlgren , CP , Kellison , GT , Adams , AJ , Gillanders , BM , Kendall , MS , Layman , CA , Ley , JA , Nagelkerken , I and Serafy , JE. 2006 . Marine nurseries and effective juvenile habitats: Concepts and applications . Marine Ecology Progress Series , 312 : 291 – 295 .
- Deudero , S. 2002 . Unexpected large numbers of Mullus surmuletus juveniles in open waters of the Mediterranean sampled with light attraction devices . Journal of Fish Biology , 61 : 1639 – 1642 .
- Fernandez-Jover , D , Sanchez-Jerez , P , Bayle-Sempere , JT , Arechavala-Lopez , P , Martinez-Rubio , L , Lopez Jimenez , JA and Martinez Lopez , FJ . 2009 . Coastal fish farms are settlement sites for juvenile fish . Marine Environmental Research , 68 : 89 – 96 .
- Franco , A , Franzoi , P , Malavasi , S , Riccato , F , Torricelli , P and Mainardi , D. 2006 . Use of shallow habitats by fish assemblages in a Mediterranean coastal lagoon . Estuarine, Coastal and Shelf Science , 66 : 67 – 83 .
- Francour , P and LeDireach , L. 1994 . Recrutement de l'ichtyofauna dans l'herbier superficiel à Posidonia oceanica de la Réserve Naturelle de Scandola (Corse, Méditerranée nord-occidentale): Données préliminaires . Travaux Scientifiques du Parc Naturel Régional & des Réserves Naturelles de Corse , 46 : 71 – 91 .
- Fraschetti , S , Terlizzi , A , Bussotti , S , Guarnieri , G , D'Ambrosio , P and Boero , F. 2005 . Conservation of Mediterranean seascapes: Analyses of existing protection schemes . Marine Environmental Research , 59 : 309 – 332 .
- Froese R, Pauly D. 2009. FishBase. 09/2009. http://www.fishbase.org (http://www.fishbase.org)
- Garcia-Rubies , A and Macpherson , E. 1995 . Substrate use and temporal pattern of recruitment in juvenile fishes of the Mediterranean littoral . Marine Biology , 124 : 35 – 42 .
- Guidetti , P. 2000 . Differences among fish assemblages associated with nearshore Posidonia oceanica seagrass beds, rocky-algal reefs and unvegetated sand habitats in the Adriatic Sea . Estuarine Coastal Shelf Science , 50 : 515 – 529 .
- Guidetti , P. 2006 . Marine reserves reestablish lost predatory interactions and cause community changes in rocky reefs . Ecological Applications , 16 : 963 – 976 .
- Guidetti , P. 2007 . Potential of marine reserves to cause community-wide changes beyond their boundaries . Conservation Biology , 21 : 540 – 545 .
- Guidetti , P , Bianchi , CN , La Mesa , G , Modena , M , Morri , C , Sara , G and Vacchi , M. 2002a . Abundance and size structure of Thalassoma pavo (Pisces: Labridae) in the western Mediterranean Sea: Variability at different spatial scales . Journal of the Marine Biological Association of the United Kingdom , 82 : 495 – 500 .
- Guidetti , P and Bussotti , S. 1997 . Recruitment of Diplodus annularis and Spondyliosoma cantharus (Sparidae) in shallow seagrass beds along the Italian coasts (Mediterranean Sea) . Marine Life , 7 : 47 – 52 .
- Guidetti , P and Bussotti , S. 2000 . Fish fauna of a mixed meadow composed by the seagrasses Cymodocea nodosa and Zostera noltii in the western Mediterranean . Oceanologica Acta , 23 : 759 – 770 .
- Guidetti , P and Claudet , J. 2010 . Co-management practices enhance fisheries in marine protected areas . Conservation Biology , 24 : 312 – 318 .
- Guidetti , P and Dulcic , J. 2007 . Relationships among predatory fish, sea urchins and barrens in Mediterranean rocky reefs across a latitudinal gradient . Marine Environmental Research , 63 : 168 – 184 .
- Guidetti , P and Sala , E. 2007 . Community-wide effects of marine reserves in the Mediterranean Sea . Marine Ecology Progress Series , 335 : 43 – 56 .
- Guidetti , P , Terlizzi , A , Fraschetti , S and Boero , F. 2002b . Spatio-temporal variability in fish assemblages associated with coralligenous formations in south-eastern Apulia (SE Italy) . Italian Journal of Zoology , 69 : 325 – 331 .
- Halpern , BS and Warner , RR. 2002 . Marine reserves have rapid and lasting effects . Ecology Letters , 5 : 361 – 366 .
- Harmelin-Vivien , M , Harmelin , JG , Chauvet , C , Duval , C , Galzin , R , Lejeune , P , Barnabe , G , Blanc , F , Chevalier , R Duclerc , J . 1985 . Evaluation des peuplements et populations de poissons. Méthodes et problèmes . Revue Ecologie (Terre Vie) , 40 : 467 – 539 .
- Harmelin-Vivien , M , Harmelin , JG and Leboulleux , V. 1995 . Microhabitat requirements for settlement of juvenile sparid fishes on Mediterranean rocky shores . Hydrobiologia 300– , 301 : 309 – 320 .
- Harmelin-Vivien , M , Le Diréach , L , Bayle-Sempere , J , Charbonnel , E , García-Charton , JA , Ody , D , Pérez-Ruzafa , A , Reñones , O , Sánchez-Jerez , P and Valle , C. 2008 . Gradients of abundance and biomass across reserve boundaries in six Mediterranean marine protected areas: Evidence of fish spillover? . Biological Conservation , 141 : 1829 – 1839 .
- Heck , KL , Hays , G and Orth , RJ. 2003 . Critical evaluation of the nursery role hypothesis for seagrass meadows . Marine Ecology Progress Series , 25 : 123 – 136 .
- Jones , GP. 1990 . The importance of recruitment in the dynamics of a coral reef fish population . Ecology , 71 : 1691 – 1698 .
- Jones , GP , Russ , GR , Sale , PF and Steneck , RS. 2009 . Theme section on ‘Larval connectivity, resilience and the future of coral reefs’ . Coral Reefs , 28 : 303 – 305 .
- Lipcius , RN , Eggleston , DB , Schreiber , SJ , Seitz , RD , Shen , J , Sisson , M , Stockhausen , WT and Wang , HV. 2008 . Importance of metapopulation connectivity to restocking and restoration of marine species . Reviews in Fisheries Science , 16 : 101 – 110 .
- Lo Bianco , S. 1908 . Notizie biologiche riguardanti specialmente il periodo di maturità sessuale degli animali del golfo di Napoli . Mittheilungen aus der Zoologischen Station zu Neapel , 19 : 35 – 761 .
- Macpherson , E. 1998 . Ontogenetic shifts in habitat use and aggregation in juvenile sparid fishes . Journal of Experimental Marine Biology and Ecology , 220 : 127 – 150 .
- Meekan , MG , Milicich , MJ and Doherty , PJ. 1983 . Larval production drives temporal patterns of larval supply and recruitment of a coral reef damselfish . Marine Ecology Progress Series , 93 : 217 – 225 .
- Newman , SJ and Dunk , IJ. 2002 . Growth, age validation, mortality, and other population characteristics of the red emperor snapper, Lutianus sebae (Cuvier, 1828), off the Kimberley coast of north-western Australia . Estuarine Coastal Shelf Science , 55 : 67 – 80 .
- Nykjaer , L. 2009 . Mediterranean Sea surface warming 1985–2006 . Climate Research , 39 : 11 – 17 .
- Ranzi , S. 1930 . Stadi giovanili di Sparidi del Golfo di Napoli . Publicazione Stazione Zoologica di Napoli , 18 : 282 – 312 .
- Roberts , CM , Andelman , S , Branch , G , Bustamante , RH , Castilla , JC , Dugan , J , Halpern , BS , Lafferty , KD , Leslie , H Lubchenco , J . 2003 . Ecological criteria for evaluating candidate sites for marine reserves . Ecological Applications , 13 : 199 – 214 .
- Sala , E , Oropeza , O , Paredas , G , Parra , I , Barrera , JC and Dayton , PK. 2002 . A general model for designing networks of marine reserve . Science , 298 : 1991 – 1993 .
- Sheaves , M , Baker , R and Johnston , R. 2006 . Marine nurseries and effective juvenile habitats: An alternative view . Marine Ecology Progress Series , 318 : 303 – 306 .
- Tunesi , L , Mariani , L and Mori , M. 1997 . Insedimento di stadi giovanili di specie ittiche nelle acque costiere del Golfo del Tigullio (Mar Ligure) . Biologia Marina Mediterranea , 4 : 282 – 290 .
- Vigliola , L , Harmelin-Vivien , ML , Biagi , F , Galzin , R , Garcia-Rubies , A , Harmelin , JG , Jouvenel , JY , Le Direach-Boursier , L , Macpherson , E and Tunesi , L. 1998 . Spatial and temporal patterns of settlement among sparid fishes of the genus Diplodus in the northwestern Mediterranean . Marine Ecology Progress Series , 168 : 45 – 56 .