Abstract
The feeding habits of Padogobius bonelli (Bonaparte, 1846) were studied in two sites in the Orba stream (NW Italy), characterised by natural or altered flow conditions. The species fed mainly on aquatic insects, positively selecting Chironomidae, Simuliidae, Hydroptilidae and negatively Baetidae and other taxa. We hypothesised that size, mobility and handling time were on the basis of the detected feeding preferences, more than prey abundance in the substratum. When studying the variation of the diet with size, we detected that trophic spectrum of the species increases with fish dimensions. Comparing the populations of the two sites, we detected some interesting differences: fish from the natural flow site were generally larger and presented a broader trophic spectrum than fish from the altered flow site. Our study supports the hypothesis that fluctuating water levels may have evident impacts at different biotic scales, from biodiversity reductions to diet alterations.
Introduction
As stated by Monakov (Citation2003), there is no discipline in hydrobiology that does not require elements coming from the study of the feeding and nutrition of aquatic animals. In fact, understanding feeding relationships and characterising trophic positions is fundamental to better understand basic and applied elements of stream ecology. In the last few years, there has been a growing interest in the trophic ecology of aquatic organisms of Italian freshwater ecosystems, such as insects (e.g. Bo et al. Citation2007; Fenoglio et al. Citation2009), macrocrustaceans (e.g. Scalici & Gibertini Citation2007) and fishes (e.g. Balestrieri et al. Citation2006; Cammarata et al. Citation2008; Fochetti et al. Citation2008).
The family Gobiidae (Osteichthyes: Perciformes) is represented in the Italian inland waters by five species that show different geographical distribution and ecological habits. Padogobius bonelli (Bonaparte, 1846), earlier known under the name Padogobius martensi (Günther, 1861), is distributed in the northern Adriatic basin, from Vomano (Italy) to Krka drainages (Croatia) and in the subalpine lakes in Po drainages (Kottelat & Freyhof Citation2007). This species has been introduced in most of western and central Italy (Kottelat & Freyhof Citation2007). This fish exclusively inhabits freshwater, in a wide variety of stream, river and lake habitats with coarse substrata (Kottelat & Freyhof Citation2007). This little Gobiidae species reaches a mean length of 6–7 cm (exceptionally 9–10 cm) and has benthic habits during its juvenile and adult life (Zerunian Citation2002). The breeding season usually extends from the beginning of May to early July (Gandolfi et al. Citation1991). This species shows high territorial habits in both sexes: individuals defend small areas in the riverbed, with an evident preference for large cobbles and boulders located in fast flowing waters (Lugli et al. Citation1992). It is well known that erosive environments are the most colonised areas in the riverbed (Downes et al. Citation1998; Fenoglio & Bo Citation2009): in these microhabitats, P. bonelli takes advantage of high amounts of invertebrate preys and high levels of dissolved oxygen (Gandolfi & Tongiorgi Citation1974). Usually the dimensions of the territory are proportional to the size of the specimens and many studies underline the strong aggressive behaviour of this species (Bisazza et al. Citation1989). Like other Gobiidae, this species also shows acoustic communication mechanisms, which are particularly important in territorial and mating behaviours (Torricelli et al. Citation1987, Citation1990). The studies on feeding habits of Gobiidae started with estuarine and marine species and only in recent years have freshwater species been investigated (Miller Citation2003). Generally, Gobiidae are considered to be benthic feeders, with a diet dominated by crustaceans and molluscs (Charlebois et al. Citation1997). It is well known that some Gobiidae species are scarcely selective feeders, ingesting amounts of sediments and associated organisms (Carle & Hastings Citation1982), while many others show evident trophic preferences. For example, Adámek et al. (Citation2007), comparing the diet of four species from South Slovakia, reported that there are some differences among them, and also that Amphipoda, Diptera Chironomidae, Trichoptera Hydropsyche sp. and two Ephemeroptera species nymphs (Ephoron virgo, Potamanthus luteus) always represented the most important food items. Amphipoda constituted the most important food item in another Gobiidae, Neogobius gymnotrachelus, that also showed a marked preference for Chironomidae and, to a lesser extent, for Diptera Ceratopogonidae, Anellida Oligochaeta, adult Diptera and Copepoda (Grabowska & Grabowski Citation2005).
Padogobius bonelli is generally considered a benthic predator, feeding on stream invertebrates and fish eggs, but little information is available regarding its diet. The aim of our study was to analyse the diet of P. bonelli in an Apenninic lotic system, investigating the existence of feeding preference patterns and variations in relation to the size. We also hypothesised that hydrological conditions may have some influence on the fish diet. In fact, interestingly, the studied population inhabits a river reach affected by a small hydroelectrical plant. It is well known that reduced or altered flows, and in general fluctuating water levels, can have strong effects on lotic biota (Allan & Castillo Citation2007), such as habitat reduction (Dewson et al. Citation2007), functional ecosystem alterations (Young et al. Citation2008) and decrease of biological richness and diversity (Fenoglio et al. Citation2007), but few data are available about their effects on trophic ecology of freshwater fish.
Material and methods
The study area was a reach of the Orba stream (NW Italy), near Molare (Alessandria district). Samples were realised in two sites:
| • | Site 1: Marciazza (44°35’13.36” N; 8°36’41.79” E 264 m a.s.l.); | ||||
| • | Site 2: Cerreto (44°35’56.60” N; 8°36’10.81 E 216 m a.s.l.). | ||||
Only 3020 m separate the two stations, but they show very different hydrologic conditions. Site 1 has a natural flow, depending on natural precipitations, while Site 2 experiences high and unpredictable water level variations because it receives tailwater from a small hydroelectric plant: in this section, flow can augment or diminish in an unpredictable way, with changes that are in the range of 1–2 m3/s in a few minutes. Streambed width varies rapidly (with a 30% increase in a few minutes) and water current shows unpredictable variations, ranging from 0.2 to 0.9 m/s. Some physicochemical parameters were measured in both sites at the end of each sampling period; two ecotoxicological tests were also performed (). The fish community is characterised by the presence of few species, such as Barbus plebejus (Bonaparte, 1839), Squalius cephalus (Linnaeus, 1758), Alburnus alburnus alborella (De Filippi, 1844), and in depositional areas Cobitis taenia Linnaeus 1758.
Table I. Main chemical and ecotoxicological parameters of the two studied sites
Gobies were caught by using a Scubla IG200/2 electro-fishing device. In total, 120 specimens of Padogobius bonelli were collected: in two dates (5 October 2007 and 3 July 2008) 30 gobies were captured in each site (30 specimens/station/two dates). Each goby was measured (total length) with an accuracy of 1.0 mm. Digestive tracts were removed, stored in 90% ethanol and brought to the laboratory. Gut contents were analysed with a Nikon SMZ 1500 light microscope (60–100×) coupled with a JVC TK-C701EG videocamera. Identification of prey was based on sclerotised body parts, particularly head capsules, mouthparts and leg fragments. Organisms in guts were classified generally to genus or family level. Stewart and Stark (Citation2002) stated that the count of sclerotised fragments (i.e. head capsules or legs) can give a reasonably accurate count of prey consumed. Gut contents were also compared with the natural composition and abundance of macroinvertebrate communities. In fact, using a Surber net (20×20 cm; mesh 255 µm), 145 samples were collected in the same period in the two sites to assess the presence and abundance of the taxa of the natural benthic invertebrate population (n = 67 Surber samples in Site 1, 33 in October and 34 in July; and n = 78 in Site 2, 38 in October and 40 in July). Samples were preserved in 90% ethanol. In the laboratory, all organisms were counted and identified to genus or species level, except for Oligochaeta and early instars of some Trichoptera and Diptera, which were identified to family or sub-family level.
To investigate the existence of feeding preferences, we compared gut contents with natural composition and abundance of macroinvertebrate community in the riverbed using the trophic electivity index of Ivlev (Citation1961):
where ri = relative abundance of a particular taxon in the diet and pi = relative abundance of the same taxon in the benthic community. The formula considers the number of taxa (i) found in the diet. The index ranges from −1 to 1. A value of −1 means total avoidance, 1 indicates preference and 0 indicates indifference.
For statistical analysis, STATISTICA software (StatSoft Citation2005) was employed. Normality of the variables was assessed by means of a Kolmogorov–Smirnov test and, because variables studied were not normally distributed, non-parametric statistics were used in all cases. Thus, for assessing if there was a correlation between fish size and the number of prey items, between fish size and the number of taxa ingested, and between fish size and number of individuals of each taxonomic group of prey, a Gamma correlation test was used, which is the best choice when data present a high degree of range overlapping (Guisande González et al. Citation2006). To evaluate whether significant differences existed between sites in fish total body length, number of prey eaten, and number of taxa eaten, a Mann–Whitney test was employed.
Results
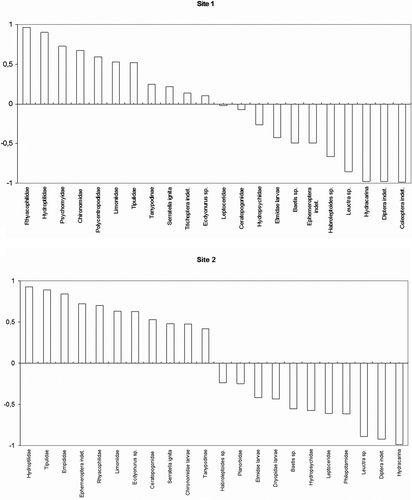
A list of total macroinvertebrate taxa collected (% of individuals) at each site is reported in . All analysed individuals, except one from Site 2, presented some kind of gut content. Padogobius bonelli in the studied sites fed mainly on macroinvertebrates, particularly aquatic insects (). Coarse and fine particulate organic matter, algae, vegetal matter, and sand were found only punctually in the guts, and they were probably ingested incidentally or came from the prey guts. The only non-macroinvertebrate prey widely consumed were Crustacea Daphnia spp., especially by the Site 2 population. The most important macroinvertebrate prey in the guts were larvae of Diptera Chironomidae: they constituted 46.0% of the total ingested items in the population from Site 1 and 30.4% in the Site 2 population, and they were present in almost all guts. Other important prey were, in order of abundance, Trichoptera Hydroptilidae, Trichoptera Hydropsychidae, and Ephemeroptera Baetidae (particularly Baetis sp.) in Site 1 and Trichoptera Hydroptilidae in Site 2. Unusual components of the gut contents were terrestrial insects and two fish scales, the latter probably ingested incidentally when feeding on other trophic resources. When comparing the macroinvertebrate community composition of the riverbed with the ingested prey by means of the Ivlev's index (), we observed in the Site 1 population a clear preference for Trichoptera Rhyacophilidae, Hydroptilidae and Psychomidae and Diptera Chironomidae, while some other taxa, such as Hydracarina, Baetis sp., Habroleptoides sp. and some Coleoptera and Diptera, also abundant in the riverbed, were consumed in smaller amounts. In the Site 2 population, Trichoptera Hydroptilidae, and Diptera Tipulidae and Empididae were preferred, while Hydracarina, indeterminate Diptera and Plecoptera Leuctridae (particularly Leuctra sp.) were less consumed, although some of them were common in the substratum.
Table II. Percentage of individuals of each taxa collected in the sample sites
Table III. Number of items, percent relative abundance in the gut, and mean, minimum and maximum values for macroinvertebrates found in the gut of the two Padogobius bonelli populations
Figure 1. Ivlev's electivity index for macroinvertebrate taxa in the Padogobius bonelli diet in the two stations of Orba creek.
Regarding the influence of the size in the diet of this species, we noticed that some prey tend to be more common in the gut of bigger specimens. This was the case in Site 1 of Ephemeroptera Baetis sp. and Trichoptera Hydroptilidae (Gamma correlation = 0.47 and 0.44, respectively, p < 0.05). In Site 2 there was a positive correlation between size and the number of Ephemeroptera Baetis sp., Trichoptera Philopotamidae, Hydropsichidae and Hydroptilidae, and Diptera Limoniidae and Tanypodinae (Gamma correlation = 0.34, 0.39, 0.33, 0.49, 0.33, 0.31, respectively, p < 0.05).
In Site 1, there was a positive correlation between the size of the individuals and the number of taxa eaten (Gamma correlation = 0.37, p < 0.05), but not with the number of prey (Gamma correlation = 0.15, p > 0.05). In Site 2, there was a very slight negative correlation between size and number of prey eaten (Gamma correlation = –0.19, p < 0.05), but not with the number of taxa eaten (Gamma correlation = 0.19, p > 0.05).
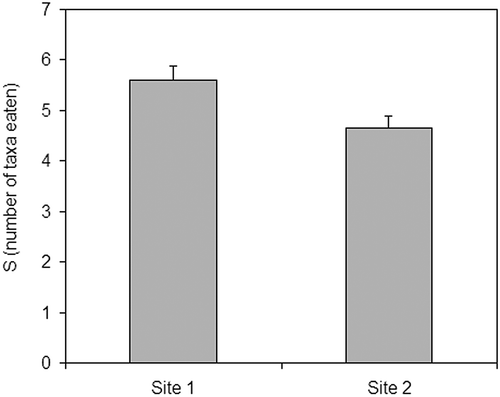
Comparing the two populations, there were differences in body size between sites (Mann–Whitney U = 1357.5, p < 0.05). Mean total body length in Site 1 was 49.15 mm ± 0.65 SD, while in Site 2 it was 46.15 mm ± 0.59 SD. Thus, fishes from Site 2 were smaller than those from Site 1, where the flow is natural, not unpredictably variable. Comparing diets, there were no differences in number of prey eaten by individuals from each population (Mann–Whitney U = 1438.0, p > 0.05). Nevertheless, there were differences in the number of taxa eaten by individuals from each population (Mann–Whitney U = 1371.5, p < 0.05), being higher in Site 1 ().
Discussion
In field studies, gut content analyses are the most diffused method to investigate prey choice and diet in lotic organisms (Allan & Castillo Citation2007). The analysis of the gut contents of Padogobius bonelli shows that, in the studied area, this species feeds mainly on aquatic insects, while Mollusca, Crustacea and other items are only present in their guts occasionally, contrary to findings in some other species of freshwater Gobiidae (e.g. Charlebois et al. Citation1997). Analysing diet preferences, we can assume that P. bonelli feed mainly on insects selected on the basis of some characteristics: preferred items are generally medium or large-sized, scarcely mobile, closely associated with the riverbed, generally soft-bodied and without hard exoskeleton, spines or some other morphological defences, such as Trichoptera Rhyacophilidae, Hydroptilidae and Polycentropodidae, and Diptera Chironomidae, Limoniidae and Tipulidae. This result agrees with the ‘Optimal foraging theory’ (Krebs Citation1978), that states that predators include in the diet the most profitable preys on the basis of different elements, such as energy contents (i.e. size), encounter rate, prey density, handling time and others. For this reason, other invertebrates, also abundant and widespread, were not positively selected: this is the case of the very mobile Ephemeroptera Baetidae and Leptophlebiidae and also of the small-sized Hydracarina. The small percentages of coarse and fine particulate organic matter in the guts, together with a lower percentage of sand and gravel, are probably a consequence of the feeding method of this species that collects its preys directly from the riverbed, as some other Gobiidae species (Carle & Hastings Citation1982; Charlebois et al. Citation1997).
The strong preference for benthic preys probably diminishes interspecific competition with the Brown Trout (Salmo trutta trutta L., 1758), a Salmonidae that usually lives in the same lotic environments, but that captures preys in the whole water column, ingesting high amounts of drifting and terrestrial insects (Montori et al. Citation2006; Fochetti et al. Citation2008).
In this study, a general increase of trophic spectrum was detected in larger fishes: bigger goby specimens ingested larger number of taxa. Probably, with the increase in length, there is an associated increase in the ability to handle and devour different taxa.
Comparing the two nearby located populations of P. bonelli, we detected some interesting differences: fishes from Site 1 were generally larger and ingested more taxa than fishes from Site 2. No significant differences were detected in the chemical characteristics and in the macrobenthic communities between the two sites, so we could hypothesise that differences in flow could be on the basis of the observed differences. In fact, fluctuating water levels may inhibit movements, habitat exploration and prey encounters in the downstream site: rapid changes in current velocity and riverbed area may have a strong impact on diet composition and subsequently on development and final size of gobies. Improvements of the autoecological knowledge, for example by means of diet analysis, could be very important for the protection of this species that recently seems to be vulnerable (Miller Citation2003).
Acknowledgements
We thank E. Coduti, M. Grenna, and F. Gallo for their help with field work. The final version of the manuscript benefited greatly from the insight provided by G.B. Delmastro and three anonymous reviewers. This work was supported by Provincia di Alessandria ATF Grants.
References
- Adámek , Z , Andrei , J and Gallardo , JM. 2007 . Food habits of four bottom-dwelling Gobiid species at the confluence of the Danube and Hron rivers (South Slovakia) . International Review of Hydrobiology , 92 : 554 – 563 .
- Allan , JD and Castillo , MM. 2007 . Stream ecology. Structure and function of running waters , 2nd , Dordrecht : Springer Editions .
- Balestrieri , A , Prigioni , C , Remonti , L , Sgrosso , S and Giuseppe , P. 2006 . Feeding ecology of Leuciscus cephalus and Rutilus rubilio in southern Italy . Italian Journal of Zoology , 73 : 129 – 135 .
- Bisazza , A , Marconato , A and Marin , G. 1989 . Male competition and female choice in Padogobius martensi (Pisces, Gobiidae) . Animal Behaviour , 38 : 406 – 413 .
- Bo , T , Fenoglio , S and Malacarne , G. 2007 . Diet of Dinocras cephalotes and Perla marginata (Plecoptera: Perlidae) in an Apennine stream (northwestern Italy) . Canadian Entomologist , 139 : 358 – 364 .
- Cammarata , M , Bo , T , Candiotto , A , Pessino , M , Fenoglio , S and Malacarne , G. 2008 . Analisi preliminare della dieta del barbo europeo (Barbus barbus) nel Fiume Bormida . Memorie della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano , 36 : 48
- Carle , KJ and Hastings , PA. 1982 . Selection of meiofaunal prey by the Darter Goby, Gobionellus boleosoma (Gobiidae) . Estuaries , 5 : 316 – 318 .
- Charlebois , PM , Marsden , JE , Goettel , RG , Wolfe , RK , Jude , DJ and Rudnicka , S. 1997 . The round goby, Neogobius melanostomus (Pallas), a review of European and North American literature , Zion, IL : Illinois Natural History Survey Press .
- Dewson , ZS , James , ABW and Death , RG. 2007 . Invertebrate responses to short-term water abstraction in small New Zealand streams . Freshwater Biology , 52 : 357 – 369 .
- Downes , BJ , Lake , PS , Schreiber , ESG and Glaister , A. 1998 . Habitat structure and regulation of local species diversity in a stony, upland stream . Ecological Monograph , 68 : 237 – 257 .
- Fenoglio , S and Bo , T. 2009 . Lineamenti di Ecologia Fluviale , Novara : Città Studi-DeAgostini scuola .
- Fenoglio , S , Bo , T , Cucco , M and Malacarne , G. 2007 . Response of benthic invertebrate assemblages to varying drought conditions in the Po river (NW Italy) . Italian Journal of Zoology , 74 : 191 – 201 .
- Fenoglio , S , Bo , T , López-Rodríguez , MJ , Tierno de Figueroa , JM and Malacarne , G. 2009 . Preimaginal feeding habits of Isoperla carbonaria Aubert, 1953 (Plecoptera: Perlodidae) . Aquatic Insects , 31 : 401 – 407 .
- Fochetti , R , Argano , R and Tierno de Figueroa , JM. 2008 . Feeding ecology of various age-classes of brown trout in River Nera, Central Italy . Belgian Journal of Zoology , 138 : 128 – 131 .
- Gandolfi , G and Tongiorgi , P. 1974 . Taxonomic position, distribution and biology of the gobies present in the Italian fresh-waters, Padogobius martensi (Günther) and Gobius niigricans Canestrini (Osteichthyes, Gobiidae) . Annali del Museo Civico di Storia Naturale di Genova , 80 : 92 – 118 .
- Gandolfi , G , Zerunian , S , Torricelli , P and Marconato , A. 1991 . I pesci delle acque interne italiane , Roma : Istituto Poligrafico e Zecca dello Stato .
- Grabowska , J and Grabowski , M. 2005 . Diel-feeding activity in early summer of racer goby Neogobius gymnotrachelus (Gobiidae): A new invader in the Baltic basin . Journal of Applied Ichthyology , 21 : 282 – 286 .
- Guisande González , C , Barreiro Felpeto , A , Maneiro Estraviz , I , Riveiro Alarcón , I , Vergara Castaño , AR and Vaamonde Liste , A . 2006 . Tratamiento de datos , Madrid : Ediciones Díaz de Santos .
- Ivlev , VS. 1961 . Experimental ecology of the feeding of fishes , New Haven, CT : Yale University Press .
- Kottelat , M and Freyhof , J. 2007 . Handbook of European freshwater fishes , Cornol : Publications Kottelat .
- Krebs , JR. 1978 . “ Behavioural ecology: An evolutionary approach ” . In Optimal foraging: Decision rules for predators , 23 – 63 . Oxford : Blackwell .
- Lugli , M , Bobbio , L , Torricelli , P and Gandolfi , G. 1992 . Breeding ecology and male spawning success in two hill-stream populations of the freshwater goby, Padogobius martensi . Environmental Biology of Fishes , 35 : 37 – 48 .
- Miller , PJ. 2003 . “ The freshwater fishes of Europe ” . In Vol.8/I. Mugilidae, Atherinidae, Atherinopsidae, Bleniidae, Odontobutidae, Gobiidae 1 , Wiebelsheim : Aula Verlag GmBH .
- Monakov , AK. 2003 . Feeding of freshwater invertebrates , Ghent : Kenobi Productions .
- Montori , A , Tierno de Figueroa , JM and Santos , X. 2006 . The diet of the brown trout Salmo trutta (L.) during the reproductive period: Size-related and sexual effects . International Review of Hydrobiology , 91 : 438 – 450 .
- Scalici , M and Gibertini , G. 2007 . Feeding habits of the crayfish Austropotamobius pallipes (Decapoda, Astacidae) in a brook in Latium (central Italy) . Italian Journal of Zoology , 74 : 157 – 168 .
- StatSoft Inc. 2005. STATISTICA (data analysis software system), version 7.1 www.statsoft.com (http://www.statsoft.com)
- Stewart , KW and Stark , BP. 2002 . Nymphs of North American stonefly genera (Plecoptera , Columbus : The Caddis Press .
- Torricelli , P , Lugli , M and Pavan , G. 1990 . Analysis of sounds produced by male Padogobius martensi (Pisces, Gobiidae) and factors affecting their structural properties . Bioacoustics , 2 : 261 – 275 .
- Torricelli , P , Pavan , G and Lugli , M. 1987 . Analisi di suoni aggressivi e di corteggiamento nei maschi del ghiozzo di fiume Padogobius martensi . Bollettino Accademia Gioenia di Scienze Naturali , 20 : 263 – 265 .
- Young , RG , Matthaei , CD and Townsend , CR. 2008 . Organic matter breakdown and ecosystem metabolism: functional indicators for assessing river ecosystem health . Journal of the North American Benthological Society , 27 : 605 – 625 .
- Zerunian , S. 2002 . Condannati all'estinzione? Biodiversità, biologia, minacce e strategie di conservazione dei pesci d'acqua dolce indigeni in Italia , Bologna : Agricole . Il Sole 24 Ore