Abstract
The similarity of the developmental anatomy of chicken with that of mammals has rendered it an appropriate animal model for understanding the anatomical and pathological aspects of human biological systems. The present study describes the age-dependent morphological differentiation stages of developing retina of chick embryos. The retinae of chicken embryos of ages 10 and 15 days incubation, of newly hatched chicks and also that of adult chicken were studied histologically. The retinae were processed both for routine microscopy and as araldite-embedded high-resolution sections. At all ages, different retinal layers were studied noting the changing shapes of nuclei and measuring the individual layer thickness. As the animal advances in age, the elongated to oval nuclear shape takes on a more round contour, the pigment content gradually increases, the initial cuboidal cells of the photoreceptor layer change over to tall columnar cells, their outer segments pushing their way into the epithelium layer, producing vacuolar appearances among the pigment granules, the inner and outer plexiform layers increase in thickness, while the nuclear layers, becoming more compact, decrease in thickness. The ganglion cell layer, initially multilayered, gradually becomes single-cell-layered with advancing age. This descriptive laboratory research presents a detailed retinal differentiation pattern, which contributes to the anatomical knowledge of retinal embryology as well as providing a comparative background for pathological deviations.
Introduction
The use of animal models over the years has remained immensely important for the advancement of knowledge of the aetiology and pathogenesis of human diseases. The chick (Gallus gallus) embryo, as a biological system, has certain methodological advantages. It develops and hatches in 20–21 days and has been used extensively in embryology studies. As the chick is an amniote vertebrate, its early embryogenesis involves developmental processes that are equivalent to those occurring in mammals, but it takes place at a higher rate. This warm-blooded higher vertebrate undergoes true growth during its morphogenesis in which the embryo and its developing organs increase dramatically in size, similar to the situation in the human embryo.
Moreover, as an oviparous organism, any response of the chick embryo to an external agent would be attributable to a direct interaction of the stimulus with the embryonic development process and not an influence of the field on the maternal organism. Therefore, studies of the chick are highly relevant for understanding human health effects.
Like other chicken structures, retinal morphology is commonly referred to in previous literature. The chicken retina forms from an optic cup, which evaginates from the neuroectodermal diencephalic vesicle. The optic vesicle remains attached to the developing brain; the connection between optic and diencephalic vesicles becomes the optic nerve. The optic vesicle itself collapses into a cup. The front surface of this vesicle (the hollow of the cup) becomes the neural retina, while the back surface becomes the retina's pigmented epithelium. In the early embryo, however, the future retina appears as a very simple neuroepithelium, in which all cells are morphologically homogeneous and mitotically active. The development of each retinal cell involves a series of divisions, including a critical ‘terminal’ mitosis, after which the cell migrates to one of the retinal layers, differentiates into a cell type consistent with its intraretinal position, and forms synaptic contacts with appropriate pre- and post-synaptic partners. In addition, many of the cells undergo programmed death during retinal development (Adler Citation1992).
The photosensitive retina of the adult chicken is composed of 10 layers, as in mammals but, unlike that in mammals, it is avascular, preventing shadows and light scattering. This improvement is possible because of a uniquely avian structure – the pecten. This is a thin, highly vascular, pleated membrane that protrudes into the cavity of the vitreous humour from the ventral surface of the optic disc. Its base is secured intermittently to the linear, optic disc. The apical surface is attached to a thickened mass of pectineal tissue called the bridge. The pecten is characterized by an extensive network of capillaries lined by thick endothelial cells with plump nuclei and nutrients and oxygen diffuse from it, through the vitreous body, to the retinal cells – the rods and/or cones. Polymorphic pigment cells fill the spaces between the capillaries and larger vessels. The pecten is draped by a covering membrane, which is thought to be continuous with the inner limiting membrane of the retina (Dellman Citation1993).
The cells of the pigment epithelium are tall and narrow. The nucleus occupies the smaller, basal region of each cell, which contains few or no pigment granules. The apical portion is filled with rod-shaped pigment granules that are oriented parallel to the long axis of the cell. The apical cytoplasm often appears to be separated into tufts or strands of pigment granules. The retinal pigment epithelium (RPE) lies at the interface between the neural retina and the choroid. The microvilli of its apical surface interdigitate with the outer segments of photoreceptors, whereas its highly infolded basal membrane interacts with Bruch's membrane.
The fovea, which is formed by the lateral displacement of the inner neurons and fibers, is not present in the chick's retina (Weller et al. Citation2009).
However, despite having detailed information of chicken retina, progressive age-related histological stages in retinal morphogenesis are not discussed in the existing literature.
Keeping in view the close resemblance of the developed chicken retina to that of humans, this project was designed to study and define the progressive developmental stages of chick embryo retinal differentiation.
Materials and methods
This descriptive laboratory study was carried out at the Department of Anatomy, Regional Centre College of Physicians and Surgeons Pakistan, Islamabad. The development of the chicken retina was traced from the embryonic period till hatching at different stages and comparison was made with the adult chicken retina. Fertilized chicken eggs of the ‘Desi’ breed were obtained from Poultry Research Institute, Rawalpindi. The retinal histomorphological study started at the tenth post-incubation day and compared at the fifteenth post-incubation embryonic day, at the time of hatching, and at adult age. Most cells of the retinal layers are said to be differentiated at day 10 (Gorovits et al. Citation1994) and so all the layers could be independently studied.
The eggs were incubated at 38 ± 0.5°C and relative humidity was kept between 60 and 70% (Mortell et al. Citation2003). At the onset of the experiments, the eggs were opened up, the embryos dissected out and fixed in 10% neutral buffered formalin for 48 h. The fixed embryos were decapitated and the heads were bisected in the sagittal plane. The right eyes were dissected out. The opened cup was again bisected at the coronal plane, 90° to the optic nerve. A square piece with nerve in the centre was taken for processing for paraffin-embedded preparations, and a 2-mm square portion was further taken from the right lateral margin of the above section for araldite-embedded preparations. The area of retina selected for study was chosen after our previous study (Zareen et al. Citation2009).
For the newly hatched chick and the adult chicken, the head was bisected in the sagittal plane and the eyeballs dissected out, fixed and sectioned as in the embryos.
The paraffin-embedded sections were cut at 5 μm and stained with Haematoxylin and Eosin (H&E), while the araldite-embedded sections cut with glass knives at 1 μm were stained with Toludine Blue stain. The morphology of different retinal layers was studied, more in detail in the high-resolution araldite sections, and the thickness of the layers was measured in H&E-stained paraffin sections under routine microscopy. The progressive changes of the retinal layers, noted and compared at different developmental ages, i.e. 10 and 15 post-incubation days, at the day of hatching and in the adult chicken, helped establish the histological stages of retinal differentiation. Mean measurement values of 29 animals are reported for 10 and 15 post-incubation day embryos, while for five animals they are reported for the newly-hatched chicks and as well as for adult chickens. The measurement points of the retina and its different layers were standardized as follows.
Thickness of retina: measured from the upper limit of the pigment epithelium to the lower limit of the internal limiting membrane.
The pigment epithelium: the outermost, brown-coloured granular layer.
Rods and cones: an empty-looking area lightly stained between the pigment epithelium and the external limiting membrane was measured as the rods and the cones, ignoring the processes projecting into the pigment epithelium for the precision of the measurement.
The external limiting membrane: a fine demarcating, eosinophilic, interrupted line between the rods and cones and their nucleated segment.
The outer nuclear layer: the densely packed nuclear layer between the external limiting membrane and the outer plexiform layer.
The outer plexiform layer: the fibrous layer deep to the outer nuclear layer was measured as the external plexiform layer.
The inner nuclear layer: the nuclear layer between the fibres of the outer and inner plexiform layers.
The inner plexiform layer: a fibrous layer between the inner nuclear and the ganglion cell layer.
The ganglion cell layer: a band of cells below the inner plexiform layer.
The nerve fiber layer: a fibrous layer beneath the ganglion cell layer, formed by the axons of the ganglion cells.
The internal limiting membrane: the inner-most membranous limit of the retina.
Results
Ten-day embryos: paraffin-embedded sections
The retinae showed different layers (). The outermost pigment epithelium was composed of small cuboidal cells filled with pigment granules. A few sections showed 4–5 cells undergoing mitosis in the outer nuclear layer, as identified by the Feulgen stain for DNA and also confirmed in the high-resolution sections (). The layer of rods and cones was seen as a small, pale-looking area between the external limiting membrane and the pigment epithelium. This layer was not a constant feature in all sections. In the sections where the external limiting membrane was not a prominent feature, the rods and cones layer could not be distinguished as a separate layer (). A fine demarcating, eosinophilic, interrupted line between the outer and inner segment of the photoreceptor cells denoted the external limiting membrane. This too was not a constant feature of all sections. The outer nuclear layer (ONL) was seen as a densely packed nuclear layer between the external limiting membrane and the outer plexiform layer. The identification of this layer depended on the presence of the outer plexiform layer (OPL).
Figure 1. Retinal section of 10-day chick embryos. A, H&E-stained section showing different layers of retina; B, section showing ill defined layering of retina (H&E stain); C, Toludine blue-stained high-resolution section. The arrow is pointing to a nucleus undergoing mitosis. The arrowhead is pointing external limiting membrane, while rod-shaped pigment granules are seen in the pigment epithelium; D, Toludine blue-stained high-resolution section showing inner nuclear layer mostly having elongated nuclei. The bar shown at the bottom of the figure represents approximately in A, 50 μm. B, 65 μm. C, 15 μm. D, 10 μm.
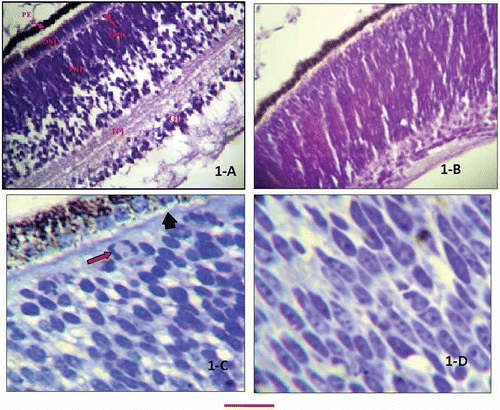
In sections in which the OPL was not present, the ONL could not be distinguished from the underlying inner nuclear layer (INL). The nuclei of ONL were mostly oval as confirmed through Cresyl violet stain, with a few sections showing elongated nuclei. The fibrous layer deep to the outer nuclear layer formed the outer plexiform layer (OPL) that, in the form of a thin fibrous band, separated the ONL from the INL. A wide inner nuclear layer was present between the OPL and the inner plexiform layer (IPL). The nuclei of this layer were elongated in the outer segment, taking a well-differentiated oval or even more mature round forms in the inner half of the layer (). This layer was separated from the ganglion cell layer by a fibrous band of the IPL. A few scattered cells could be seen in the IPL of some sections. These were perhaps the maturing cells, migrating towards the ganglion cell layer (, not labelled). The ganglion cell layer showed pear-shaped cells with a large round nucleus. The axons of the ganglion cell layer were forming the nerve fibre layer, continuing as the optic nerve. The inner limit of the retina was a thin membrane, the internal limiting membrane.
and , respectively, summarize the thicknesses of different layers of retinae, and the changing nuclear shapes at different ages.
Table I. Comparison of chicken retinal layers thickness at different developmental stages
Table II. Comparison of nuclear shapes of inner and outer nuclear cell layers at different developmental stages of chicken retinae
Ten-day embryos: araldite-embedded sections
The araldite-embedded sections of 10-day embryos cut at the thickness of 1 μm and stained with Toludine Blue stain showed discrete pigment granules in the pigment epithelium (). The nuclei could be seen towards the apices of epithelial cells and the basal portion was filled with rod-shaped pigment granules. A few mitotic figures were also observed in some of these sections, beneath the pigment epithelium. The next layer of rods and cones was separated from that of the ONL by the thin, interrupted, external limiting membrane (). In the ONL the cell cytoplasm or boundary could not be identified. The ONL nuclei were mostly oval in shape; however, some sections also showed elongated nuclei (). The mean length of the nuclei along the long axis was 6.230 μm. The OPL was seen as a thin fibrous band separating the inner and outer nuclear layers (not seen in all sections). The INL nuclei, as observed in the routine sections, were elongated in the outer segment, changing to oval in the inner segment. The mean length of the nuclei along the long axis was 6.315 μm. The next layer of IPL showed layers of fibres, which were separating the INL from the ganglion layer. The ganglion cells were large and conical (pear-shaped) with basal nuclei.
Fifteen-day embryos: paraffin-embedded sections
The sections were at a more advanced stage of development than that of 10-day embryos. All the 10 layers of retina were clearly identified in this age group (). The pigment epithelium comprised high cuboidal cells. However, no mitotic figures were seen in this subgroup, underneath the epithelium. The rods and cones could be seen as the pale-looking lightly stained area projecting towards the pigment epithelium. The portion of the rods/cones that was abutted into the pigment epithelium was ignored from the measurement of this layer to avoid inaccuracy. The ONL was a compact band of cells. The cell boundaries were not identified, but the nuclei were predominantly oval in shape.
Figure 2. Retinal differentiation stages. A, retinal section of 15-day chick embryo (H&E stain), showing all layers, starting from top, pigment epithelium, rods and cones (abutting tightly into the epithelium, compromising the measureable height/thickness of this layer), vague impression of external limiting membrane, ONL, a thin OPL, well-developed INL, IPL, ganglion cell layer and internal limiting membrane; B, retinal section of a newly hatched chick (H&E stain) showing morphological differences from day 15 retina, with obviously elongated photoreceptor cells protruding in the pigment epithelium producing vacuolated appearances. The INL is compressed between the tall photoreceptor cells and markedly developed IPL; C, magnified view of INL of 15-day embryo (H&E stain), showing interconnections between the inner nuclear and ganglion cell layer; D, retinal section of 15-day chick embryo (Cresyl violet stain). The pale-looking external limiting layer and external plexiform layers (not very clear in A) are highlighted. A few ganglion cells are seen migrating down the inner plexiform layer. The bar shown at the bottom of the figure represents approximately in A, B, D, 40 μm. C, 15 μm.
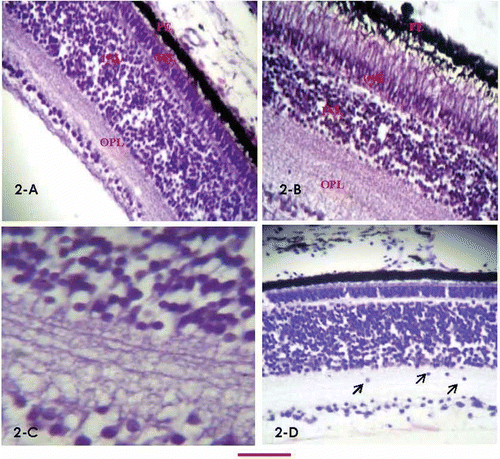
The OPL was a thin fibrous structure formed by the axonal connections of the outer and inner nuclear layers.
In the INL, cells were aligned in different patterns. Some were parallel and close to the OPL and others to the IPL. The nuclear shape in this layer, as confirmed through Cresyl violet stain, ranged from round to oval in some sections to predominantly round in other sections.
The IPL was demonstrated as a thick, multilayered fibrous band (). In some sections, cells presumably migrating downward through this layer towards their final destination in the ganglion layer could also be seen (). Similar cells were seen in 10-day embryos, but less frequently than at day 15. Underneath the IPL, cells were scattered to form the ganglion cell layer. In some sections the ganglion cells were aligned immediately under and parallel to the IPL, forming a single cell layer. However, some other sections showed two zones, one consisting of a single cell layer aligned underneath the IPL, and the other of cells underneath the above zone. The ganglion cells could be seen as large pear-shaped cells whose axons continued as the nerve fibre layer.
The inner limit of the retina was the thin internal limiting membrane.
Araldite-embedded sections
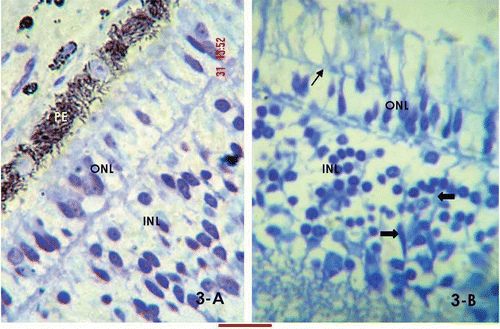
The araldite-embedded sections, cut at 1 μm, showed the same details as in 10-day embryos, except that the epithelium showed discrete rod-shaped pigment granules, and the nuclei of the ONL were more differentiated, attaining a round–oval shape (). The external limiting membrane stretched across the outer and inner segments of the photoreceptor cells (). The mean length of the nuclei along the long axis was 5.474 μm. The sections also showed bipolar cell nuclei in the inner nuclear layer (). The axonal connections between the inner and outer nuclear layer formed the OPL (). Similar connections forming the IPL could be observed between the INL and the ganglion cell layer. No mitotic figures were noticed in the embryos of this age group.
Figure 3. High-resolution, Toludine Blue-stained day 15 retinal sections. A, the section shows rod-shaped pigment granules and mostly oval nuclear morphology of the outer nuclear layer; B, the arrow marks the external limiting membrane stretching through the outer and inner segments of the photoreceptor cells. A few bipolar nuclei are identified in the inner nuclear layer (arrowheads). The bar shown at the bottom of the figure represents approximately in A, 10 μm. B, 15 μm.
Newly-hatched chick
All 10 layers of the retina were clearly identified in the transverse section (). The pigment epithelium comprised tall columnar cells, mostly demonstrating a moderate level of pigmentation with identifiable separate granules of pigment. The rods and cones could be seen as the pale-looking, lightly stained areas projecting towards and into the pigment epithelium, having a measurable thickness of 22 μm (measured as pale-looking area between the pigment epithelium and the external limiting membrane, ignoring the epithelial projection). The outer limiting membrane (OLM) stretched between the rods and cones and the ONL. The cell boundaries were not identified; the nuclei were predominantly round in shape. The OPL was a thin fibrous structure formed by the axonal connections of the outer and inner nuclear layers.
Next was the compact INL with predominantly round nuclear morphology. The IPL was demonstrated as thick multilayered fibrous band. Underneath the IPL the ganglion cells were mostly aligned parallel to it, with a tendency to form a single-layered ganglion cell layer.
The ganglion cells axons were observed as continuing as the nerve fibre layer. The inner limit of the retina was the thin internal limiting membrane.
The adult chicken
The adult chicken retina showed the same layers as the retinae of other age groups, but with structural differentiation ()
Figure 4. Retinal sections at different developmental ages of chicken. A, 10-day embryo retinal section showing undifferentiated layers. Appearance of a thin, inner plexiform layer is just demarcating the nuclear and ganglion cell layers; B, identifiable external limiting layer is stretching underneath the epithelium dividing the upper and lower segments of the photoreceptor cells. The OPL is present between the outer and inner nuclear layers. The thickness of the INL can be appreciated to be more as compared to the more advanced ages (C and D); C, at the time of hatching the sections revealed marked elongation of the phoreceptor cells, mature morphology of the ONL, identifiable OPL, a compact INL and broad band of the OPL; D, retinal sections of an adult chicken showing prominent vacuolation of the epithelium, thin outer nuclear layer, a more compromised ONL as compared to B and C, a very broad INL, and a single layer of ganglion cells (compare with A and B). The bar shown at the bottom of the figure represents approximately A, B, 60 μm. C, D, 50 μm.
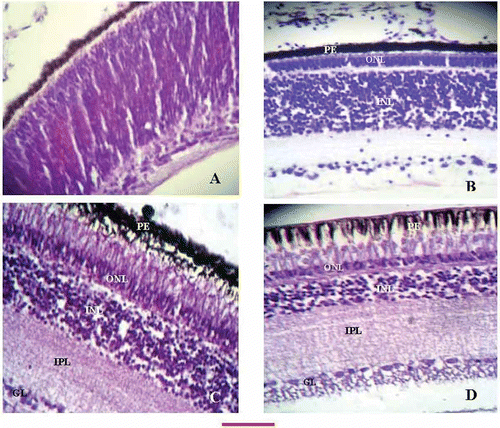
The pigment epithelium comprised tall columnar cells with wide empty gaps due to rods and cones projecting in creating a vacuolated appearance. The rods and cones could be seen as elongated, pale-looking lightly stained areas having a measurable thickness of 31.1 μm. The outer limiting membrane (OLM) was seen as an interrupted membrane stretching between the rods and cones layer and the ONL. The ONL was a compact band of cells with round nuclear morphology.
The OPL was a thin, fibrous, layered structure. Next were the compact INL with predominantly round nuclear morphology, and a wide multilayered fibrous band of IPL. Underneath the IPL, the single-layered ganglion cells layer ran parallel to it.
The ganglion cells axons extended down and continuing as the nerve fibre layer. The inner limit of the retina was the thin internal limiting membrane.
Discussion
The retinal developmental diversity has recently been mentioned by Andreazzoli (Citation2009) in a study that deals with the molecular regulation of retinal cell fate. Some studies reported on the role of different growth factors in differentiation of neuronal and pigment retina (e.g. Pittack et al. Citation1997). Genetic control regarding cytodifferentiation and morphogenesis of avian retinal pigmented epithelium has also been focused upon by Ishii et al. (Citation2009). However, the sequential age-related histological stages in the retinal layers need more elaborate description. The present study defines such developmental stages in chicken. From the comparative description of the features of adult chicken, newly hatched chicks, and the embryos of 15 and 10 days, a few inferences can be drawn regarding the development of the retina. The pigment epithelium consisting of small cuboidal cells at day 10, progressively changes from cuboidal (at day 15) to columnar (at hatching) to tall columnar cells at the adult age. The rods and cones also elongate towards the epithelium, progressively increasing in length (Table I) and projecting deeper into it, creating vacuoles by displacing the pigment granules at the adult age. The thickness of the outer nuclear layer decreases with increasing age, indicating a more compact and mature form (Table II). The nuclear morphology of this layer was elongated to oval at day 10, changing to predominantly oval at day 15, and attaining a definite round form by the time of hatching and adult age. The oval nuclear morphology in early stages of embryonic retina was also reported earlier in a tissue culture study of developing retina (Barr-Nea & Barishak Citation1970). The OPL, although maintaining the appearance of a thin band, showed some noticeable increase in thickness, attaining a layered appearance at the adult age. The INL showed a progressive decrease in thickness attaining the most compact form at the adult age. The nuclear morphology of this layer was elongated to round at day 10, changing to oval–round at day 15, and attaining a predominantly round form by the time of hatching and definitely so by the adult age. The IPL increased in thickness with age, acquiring a multilayered appearance at the time of hatching and adulthood. The ganglion layer also shrank in size reducing to a single-cell thick layer at the adult age ().
The characteristics of cellular proliferation in developing human retina has been mentioned at different developmental ages (Smirnov & Puchkov Citation2004). A study conducted on Mongolian gerbils showed that formation of all retinal layers occurs postnatally (Bytyqi & Layer Citation2005). The chicken retina in contrast, shows distinct lamina formation by as early as day 10 of embryonic life. The initial neuroblastic layers mature with advancing embryonic age and continue postnatally.
Conclusion
A columnar pigment epithelium, round nuclear morphology of the inner and outer nuclear layers, layering of the plexiform layers – especially IPL, thickness of inner plexiform layer equal to or more than that of inner nuclear layer, and presence of a single-cell-thick layer of ganglion cells are maturity indicators of the developing chick retina.
The results of this study will be helpful in understanding the differentiation pattern of chicken retina and formulating hypotheses for future interventional studies.
References
- Adler , R . 1992 . “ Cellular and molecular mechanisms regulating retinal cell differentiation ” . In The visual system from genesis to maturity , Edited by: Lent , R . 21 – 35 . Boston, MA : Birkhauser Inc .
- Andreazzoli , M . 2009 . Molecular regulation of vertebrate retina cell fate . Birth Defects Research Part C Embryo Today , 87 : 284 – 295 .
- Barr-Nea , L and Barishak , RY . 1970 . Tissue culture studies of the embryonal chicken retina . Investigative Ophthalmology , 9 : 447 – 457 .
- Bytyqi , AH and Layer , PG . 2005 . Lamina formation in the Mongolian gerbil retina (Meriones unguiculatus) . Anatomy and Embryology (Berlin) , 209 : 217 – 225 .
- Dellman , H-D . 1993 . “ Text book of veterinary histology ” . In , 4th , Philadelphia, PA : Lea & Febiger .
- Gorovits , R , Ben-Dror , I , Fox , LE , Westphal , HM and Vardimon , L . 1994 . Developmental changes in the expression and compartmentalization of the glucocorticoid receptor in embryonic retina . Proceedings of the National Academy of Sciences USA , 91 : 4786 – 4790 .
- Ishii , Y , Weinberg , K , Oda-Ishii , I , Coughlin , L and Mikawa , T . 2009 . Morphogenesis and cytodifferentiation of the avian retinal pigmented epithelium require downregulation of Group B1 Sox genes . Development , 136 : 2579 – 2589 .
- Mortell , A , Giles , J , Bannigan , J and Puri , P . 2003 . Adriamycin effects on the chick embryo . Pediatric Surgery International , 19 : 359 – 364 .
- Pittack , C , Grunwald , GB and Reh , TA . 1997 . Fibroblast growth factors are necessary for neural retina but not pigmented epithelium differentiation in chick embryos . Development , 124 : 805 – 816 .
- Smirnov , EB and Puchkov , VF . 2004 . Characteristics of cellular proliferation in the developing human retina . Neuroscience and Behavioral Physiology , 34 : 643 – 648 .
- Weller , C , Lindstrom , SH , De Grip , WJ and Wilson , M . 2009 . The area centralis in the chicken retina contains efferent target amacrine cells . Visual Neuroscience , 26 : 249 – 254 .
- Zareen , N , Khan , MY and Ali Minhas , L . 2009 . Derangement of chick embryo retinal differentiation caused by radiofrequency electromagnetic fields . Congenital Anomalies , 49 : 15 – 19 .