Abstract
Native species of Sardinian brown trout (Salmo trutta) have declined drastically because of the introduction of non-native salmonids, overharvesting, and habitat degradation. One approach to conservation of declining species is to establish new populations through repopulation with genetically certified fish. In this study, samples of brown trout from seven rivers in Sardinia were analysed to look for the presence of pure individuals of the endemic Sardinian trout. Analyses of the mitochondrial DNA control region and the LDH-C1* gene revealed the presence of non-endemic and hybrid individuals in four of the seven rivers analysed. In addition, we reared a stock of native Sardinian trout at the experimental fish farm of Sadali and had them reproduce artificially. This experiment provided more knowledge about the incubation phase, hatching, reabsorption of the vitelline sac and the initial phases of growth, which can be used to develop more optimal management of this resource.
Introduction
The brown trout, Salmo trutta L., belongs to the genus Salmo and the family Salmonidae. Among all of the freshwater species that originated in the Palaearctic region, this one has the greatest geographical distribution (Nelson Citation1994). The species is characterized by considerable morphological and eco-ethological variability, which makes its systematic allocation particularly difficult to define. Existing taxonomic studies based on morphological data have described several dozen species belonging to S. trutta (Behnke Citation1986; Elliot Citation1989; Kottelat & Freyhof Citation2007).
Sardinian rivers are home to the endemic brown trout and two introduced non-endemic species, one of North Atlantic origin (S. trutta), and the other of North American origin (Oncorhynchus mykiss) (Massidda Citation1995; Sabatini et al. Citation2006). From analysis of mitochondrial DNA (mtDNA) sequences, at least five principal evolutionary lineages have been identified in Europe, and they are referred to as the Salmo trutta complex (Bernatchez Citation2001). Based on the origin of the first identified haplotype, the following evolutionary lineages were identified: Atlantic (At), Danubian (Da), Marmoratus (Ma), Adriatic (Ad), Mediterranean (Me) (Bernatchez et al. Citation1992). Currently in Sardinia, mtDNA analysis has revealed the presence of two different evolutionary lineages of S. trutta: the endemic lineage (Ad) and the non-endemic lineage (At) (Bobbio et al. Citation1996; Bernatchez Citation2001; Sabatini et al. Citation2006).
For many years, massive stocking of several Sardinian rivers with specimens of North Atlantic origin was conducted. The release of these invasive fish into Sardinian rivers could have modified the distribution of endemic salmonid populations and caused genetic polluting effects (Gandolfi et al. Citation1991). In fact, given the relative ease with which the various forms of the genus Salmo hybridize, in many areas of Sardinia entire populations of endemic trout have disappeared and have been replaced by hybrids or allochthonous trouts (Massidda Citation1995).
Currently, the endemic trout are considered to be very important due to the reduction in their population numbers and the serious danger of extinction. In Sardinia, there is a fishing ban on endemic brown trout (called Salmo trutta macrostigma in this decree) in all of the island's rivers (Decree of the Assessor of the ‘Defence of the Environment’ 10.05.1995 n. 412). Furthermore, S. t. macrostigma is included in the Annex II of the Habitat 92/43/CEE Directive among the ‘animal and plant species of Community interest whose conservation requires the designation of special areas of conservation’.
The precarious position of endemic trout makes it urgent to control the native populations and possibly to conduct restocking programmes using genetically certified endemic individuals. The present study describes the results of a first attempt at reproducing genetically selected individuals of native Sardinian trout in captivity. The capture and selection of brooders that were reared at the Sadali fish farm made it possible to perform this first artificial reproduction experiment, with the aim of producing fry for reintroduction into the rivers of Sardinia. This study is the first to focus on the reproduction of genetically certified individuals of the endemic Sardinian trout. Ultimately, repopulation actions that would increase the distribution of native forms would reduce the risks of loss of autochthonous biodiversity and would allow the recovery of populations that are adapted to the environmental conditions of Sardinian rivers.
Materials and methods
Study site and biological material
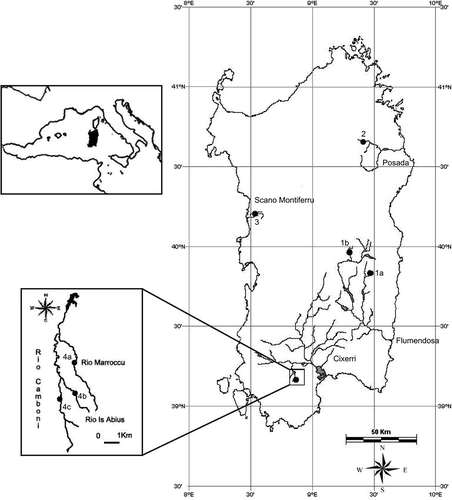
To identify residual populations of native Sardinia brown trout that have not hybridized with non-native Salmo trutta, sampling was conducted between 2005 and 2007 (). A total of 106 individuals were collected and tissue was extracted for genetic analysis.
Table I. Stream, river basin, date, coordinates, water temperature, oxygen concentration, number of samples
The areas searched for possible brooders included seven rivers located in four different river basins in Sardinia (). The analysed rivers were chosen on the base of information collected from various studies conducted around the island that described the distribution of endemic populations (Pomini Citation1940; Cottiglia Citation1968; Massidda Citation1995; Massidda et al. Citation1996; Sabatini et al. Citation2006). The trout were caught by electric fishing, placed in a tank and anaesthetized with tricaine methane sulphonate (MS-222, 100 mg l–1 water). From a total of 106 trout a tissue sample was cut, generally a small portion of the anal fin (approximately 0.5 cm2), and used for the genetic analysis. The fish were then placed inside containers with well-oxygenated water and released in the same place where they were captured.
Genetic analysis
DNA was extracted from the tissue samples and used to sequence the mitochondrial DNA and the nuclear LDH-C1* genes as described in Sabatini et al. (Citation2006). In particular, the extreme 5' end of the mtDNA control region (D-loop) was PCR-amplified using the primers L15998-PRO (Alvarado Bremer Citation1994; Ostellari et al. Citation1996) with thermal conditions: 94°C for 5 min, 35 cycles (94°C for 30 s, 52°C for 45 s, 72°C for 1 min and 72°C for 7 min). The obtained sequences were then aligned using ClustalW contained in the MEGA4 software (Tamura et al. Citation2007) and compared with sequences from the GenBank database for the principal evolutionary lineages of S. trutta (At, Atlantic; Ad, Adriatic; Ma, Marmoratus; Da, Danube; Me, Mediterranean) (Bernatchez et al. Citation1992).
A portion of the nuclear gene for lactate dehydrogenase LDH-C1* was amplified using the primers Ldhxon3F and Ldhxon4R (McMeel et al. Citation2001). The amplified portion (440 bp) was digested with restriction enzyme BslI. After digestion, DNA was scored via agarose gel electrophoresis to ascertain the presence of the *100 or *90 allele. This latter allele is found only in North West Europe, and it is the allele that has been fixed in the majority of bred trout (McMeel et al. Citation2001).
Individuals with the mitochondrial haplotype Ad and the genotype LDH-C1* homozygote *100/*100 were considered to be ‘native Sardinia Brown trout’, whereas those with the mitochondrial haplotype At and nuclear genotype *90/*90 were regarded as ‘pure Atlantic’. The other combinations of mitochondrial haplotypes and nuclear genotypes were considered to be ‘hybrids’.
Artificial reproduction
Twenty possible brooders were collected from the sites where endemic populations of Sardinian brown trout were present. Individuals that were considered to be suitable for reproduction were transported to the fish farm of Sadali and placed in round troughs. The brood stock was marked to distinguish the sexes using a labelling system that utilizes a colour jet injected subcutaneously. Fish farm of Sadali is fed by spring water (from the source of Funtana Manna), which offers optimum conditions for rearing trouts because the temperature of the water is almost constant (ranging between 12 and 13°C) and the concentration of oxygen falls between 8 and 9 mg l–1.
In December 2006, brooders (hybrids of S. trutta from the river basin of the Flumendosa) were subjected to artificial spawning. These wild specimens were kept in the Sadali fish farm to test their survival capability and reproductive capacities in captivity. Only one year later in December 2007, it was possible to spawn selected Sardinian trout (known from the genetic analyses to have Ad and *100/*100 genotypes). All of the equipment was carefully washed in a solution of acetic acid to eliminate risks of cross-contamination between the brooders. Before being manipulated for the extraction of eggs, the fish were anaesthetized with MS-222 (100 mgl–1 water) to minimize their stress and to make it easier for operators to handle them. The stripped eggs were photographed and measured using image analysis software. The mean diameter (D) of eggs was calculated following to Coward and Bromage (Citation1999) as:
where d 1 = long diameter and d 2 = short diameter.
The dry method was used for fertilization. Males were stripped in the same way as females. Once the eggs were collected, they were placed in a bowl, to which milt was added. The eggs and the milt were mixed thoroughly for 15–20 min, and water was added to activate the sperm (Giordani & Melotti Citation1984). The stripped brooders were immediately placed in large containers where there was a constant renewal of fresh water. In this way, they were able to completely recover from the anaesthesia before being returned to the tanks. The fertilized eggs were carefully rinsed and placed in the hatching troughs. To verify that each female had successfully spawned, a portion of the eggs was taken and photographed. Furthermore, a non-vital egg count was performed at intervals from 4 to 6 h to evaluate mortality rates. Later, for each group of eggs, we verified hatching times and the time required to reabsorb the vitelline sac (expressed as degrees per day; DD = Days × T°C).
Results
The genotype analysis revealed the presence of non-native individuals and hybrids in four out of the seven streams analysed (94% from the Ermolinus River, 71% from the Altana River, 100% from the Mannu river of Scano Montiferru and 100% from the Su Fruscu River of Girgini forest). In fact, in the Girgini sample, only invasive trout were found (mitochondrial haplotype At, GenBank Accession Number FJ765235) and/or hybrids together with three rainbow trout Oncorhyncus mykiss (GenBank Accession Number FJ765234). In the Camboni River and its tributaries (Is Abius and Marroccu), which belong to the Cixerri hydrographic basin, only Ad, 100*/100* trout populations were found. In particular, the genetic analyses revealed the presence of two Ad mitochondrial haplotypes (GenBank Accession Number FJ765232 = Ad1; FJ765233 = Ad2), which differed by only one nucleotide substitution ().
Table II. Numbers of the stations on the map, Stream, river basin, LDH-C1* genotypes and mtDNA haplotypes of Sardinian trout; in bold, individuals ‘endemic Sardinian trout’
The absence of non-endemic genes in the water courses of the Camboni River and its two tributaries suggests that no repopulation actions have occurred using allochthonous fish. It is possible that artificial barriers, such as crossings, fords, and the artificial lake Medau Zirimilis, could have blocked the journey of allochthonous fish, thereby helping to conserve the genetic purity of these populations.
The three rivers of the hydrographic basin of the Cixerri were chosen for the collection of suitable individuals for reproduction according to the results of the genetic analysis. To avoid compromising the health of the population, only 10 individuals were collected from the Is Abius River and transferred to the Sadali fish farm. Genetic analysis confirmed that these specimens were endemic trout.
During the first year of spawning, the wild trout had difficulty feeding, particularly if the food consisted of artificial pellets. This confirms their difficulty in surviving under hatchery conditions. Therefore, the trout were initially fed with living food, which included worms, insects, and larvae. From the second year of spawning onwards, they were fed with the same food generally given to trout in fish farms. In the period preceding reproduction (from November onwards), the largest individuals were separated and placed into different tanks according to sex and stock, and they were constantly monitored to verify their maturity stage. Females were considered to be mature if they had a swollen abdomen with everted genital papillae, and males were considered to be mature if they had milt. Once mature, the fish were stripped. Eggs were extracted by exerting gentle pressure on the abdomen of the females.
At the end of December 2007, the induced reproduction was initiated by crossing three males and three females from the pool of brooders stocked in the fish farm of Sadali. The timing of the maturity of the adults and the reproduction stages were the same as those observed in other regions of the Mediterranean for both the native trouts (Ad *100/*100) and the other types (Nicola & Almòdovar Citation2002; Alp et al. Citation2003; Berrebi Citation2008). However, in contrast to the timing for trout from Lake Fibreno in Lazio (Gandolfi et al. Citation1991), the reproductive period did not extend beyond the month of January.
The three females produced a total of 765 eggs. Fertilization of the eggs of one female individual with the milt of one male took place on 28 December 2007 and resulted in approximately 335 fertilized eggs, which were incubated in a horizontal trough (01 Tray A). On the same day, 430 eggs from the other two females were fertilized with the milt of the two males and incubated in another horizontal trough (01 Tray B). Of particular interest, however, is that a 20-cm long trout was able to produce an average of ∼280 eggs given the trout size.
Photos of 100 eggs were analysed and showed that egg diameter ranged from 3.9 and 5.1 mm, with an average value of 4.85 mm (s.d. ± 0.36). The eggs, which were laid into the trough, hatched after 24–28 days, with an average hatching time of 325 degrees/day (Tmean = 12.5). Microscopic analysis of the eggs of the native brown trout showed the appearance of eyes during the second week. At the time of hatching, the mortality rate in Tray A was much higher than that in Tray B, with only 28.6% of vital fry. In Tray B, mortality was low, with a good percentage of hatching (81.4%) ().
Table III. Production of hatched eggs in the two trays A, B
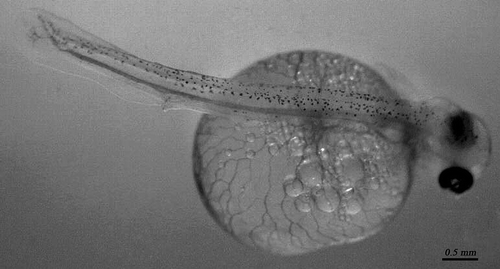
The fry had an average length of 11.54 mm (± s.d. 2.38). They were weak and delicate, with a head that protruded out of the vitelline sac. The eyes were large and occupied most of the head. The body was slender at the tail end, where the initial signs of developing caudal and anal fins were visible. The pectoral fins were clearly visible, whereas the ventral, dorsal, and anal fins were composed of small, transparent lamellae. The pigment consisted exclusively of black melanophores, with radial dendrites that were most visible on the head and back of the body. The large spherical vitelline sac was strongly vascular and orange in colour ().
The vitelline sac reabsorbed within the first 20 days following hatching, with an average reabsorption time of 250 degrees/day. After reabsorption of the vitelline sac, 218 fry remained and were ready to be fed with granular food. The genetic analyses carried out on 13 of these individuals confirmed the presence of the two haplotypes found in their parents (mitochondrial haplotype Ad1 or Ad2, and nuclear genotype 100*/100*).
Growth in the first few weeks after hatching was greatest in the first days of embryonic development, and then it dropped to more or less zero after reabsorption of the vitelline sac. During the feeding phase, the fry had difficulty in feeding, and at the sixth week they measured an average of 18.84 mm (± s.d. 1.04). Two years after hatching in the fish farm, 22 endemic trout born in captivity had survived. Although this result is numerically quite low, it represents a small stock of autochthonous trout bred in captivity.
Discussion
Up to the beginning of the twentieth century, the endemic Sardinian trout was the only abundant and dominant salmonid in Sardinia. Since the 1960s, however, an evident progressive reduction of its presence in the original areas began (Cottiglia Citation1968). To correct this progressive reduction in the abundance of the species and to balance exploitation due to fishing, at the end of the 1960s repopulation actions were intensified. Repopulation was conducted using non-endemic material that was selected and produced by broodfish; this was a common practice in Sardinia that began in the early 1900s. The invasive trout caused competition and hybridisation, which led endemic populations to contract and, in many cases, to disappear (Gandolfi et al. Citation1991). Cross-breeding of fish from the native population with fish released into the rivers during stocking programmes may have promoted the loss of important genes required for adaptation to the local environment (Vasemägi et al. Citation2005). The endemic populations had particular genetic and morphological characteristics that had developed throughout a long history of evolution (Sabatini et al. Citation2006). Currently in Sardinia, the endemic non-hybridized trout can be found in a very limited number of districts in where they are confined to areas of high altitude (Massida et al. 1996).
Precise information regarding the classification of brown trout in Sardinia and the rest of the Mediterranean Basin is lacking due to the ambiguous and complex nature of its systematics. In fact, many authors have frequently changed the recent description of new species (Delling & Doadrio Citation2005; Kottelat & Freyhof Citation2007). It is difficult to distinguish pure and hybrid individuals using only the analysis of morphological and meristic characteristics due to the extremely high plasticity of the phenotypes. Thus, the genetic approach is the only way to answer questions about the distribution of probable autochthonous stock of the genus Salmo in the Mediterranean (Lorenzoni et al. Citation2004).
Many authors use the name Salmo macrostigma to describe the native Sardinian trout populations (Pomini Citation1940; Cottiglia Citation1968; Gandolfi et al. Citation1991; Massidda Citation1995; Zerunian Citation2002). The native brown trout from Sardinia has a colour similar to that described for the first time in Algeria by Duméril (Citation1858). He observed prominent parr marks, black and red spots on the sides, and a large black patch on the gill cover area, but these characteristics also occur in numerous other perimediterranean Salmo populations (Kottelat & Freyhof Citation2007). Following Bernatchez et al. (Citation1992) and Bernatchez (Citation2001), we consider that all brown trout belong to only one species and that the name Salmo trutta is a valid name for all. Therefore, as shown by our analysis, the Sardinian trouts can be identified as Salmo trutta, belonging to the Adriatic lineage.
The rivers analysed, which were chosen based on reports of recent sightings of the species, showed a high level of genetic introgression; four of the seven rivers (Rio Ermolinus, Rio Su Fruscu, Rio Altana and Rio Mannu) were compromised, which reflects a real reduction in the genetic integrity of the endemic trout in the basins of the Mediterranean area; this fact has been reported previously by several authors (Gandolfi et al. Citation1991; Poteaux & Berrebi Citation1997; Nonnis Marzano et al. Citation2003; Caputo et al. Citation2004; Aparicio et al. Citation2005; Sabatini et al. Citation2006; Schöffman & Sušnik Citation2007; Splendiani et al. Citation2007). The data presented in this paper confirmed the presence of two evolutionary lineages in Sardinia: the endemic haplotype (Ad) and the non-endemic haplotype (At, originated from hatchery brown trout of Atlantic origin). It is noteworthy that the Mediterranean lineage (Me) is absent in all of the Sardinian rivers sampled, in contrast to results from Corsica (Berrebì Citation1995).
Our study of the early phases of development, from egg fertilization up to the initial growth phases of the fry, cannot be compared with other studies in literature, given that the present study represents the first artificial reproduction experiment for the endemic brown trout of Sardinia. Pomini (Citation1940) previously suggested that these animals are particularly adapted to the extreme ecological conditions of the rivers on the island.
The spawning season for native brown trout in Sardinian waters seemed to coincide with the observations several authors made of fish from perimediterranean areas. The experimental breeding of genetically certified endemic individuals of Sardinian trout has produced promising results, which offer important indications for an optimum management of this resource, including detailed information about the incubation phase, hatching, reabsorption of the vitelline sac, and growth under culture conditions at fish farms. Furthermore, we pointed out the difficulties in breeding wild individuals of native brown trout. Despite these difficulties, for the first time we were able to successfully reproduce in captivity genetically characterized brood stock of native brown trout caught in Sardinia. Our knowledge of the genetic structure of the Sardinian trout populations allowed us to propose an appropriate restocking program. This program has grown from an experimental status to become an integral part of endemic trout management in Sardinia. The conservation project for these native strains should be pursued through development and enhancement of conservation areas.
References
- Alp , A , Kara , C and Büyükçapar , M . 2003 . Reproductive biology of brown trout, Salmo trutta macrostigma Dumeril 1858, in a tributary of the Ceyhan River which flows into the eastern Mediterranean Sea . Journal of Applied Ichthyology , 19 : 346 – 351 .
- Alvarado Bremer , JR . 1994 . Assessment of morphological and genetic variation of the swordfish (Xiphias gladius Linnaeus): Evolutionary implication of allometric growth and of the patterns of the nucleotide substitution in the mitochondrial genome , Toronto : PhD thesis. Graduate Department of Ecology, Canada: University of Toronto .
- Aparicio , E , García-Berthou , E , Araguas , RM , Martínez , P and Marcía-Marín , JL . 2005 . Body pigmentation pattern to assess introgression by hatchery stocks in native Salmo trutta from Mediterranean streams . Journal of Fish Biology , 67 : 931 – 949 .
- Behnke , RJ . 1986 . Brown trout . Trout , 27 : 42 – 47 .
- Bernatchez , L . 2001 . The evolutionary history of brown trout (Salmo trutta L.) . interred from phylogeographic, nested clade and mismatch analyses of mitochondrial DNA variation. Evolution , 55 : 351 – 379 .
- Bernatchez , L , Guyomard , R and Bonhomme , F . 1992 . DNA sequence variation of the mitochondrial control region among geographically and morphologically remote European brown trout Salmo trutta population . Molecular Ecology , 1 : 161 – 173 .
- Berrebi , P . 1995 . “ Etude gènètique des truites de Corse ” . In Laboratoire Génome et Populations, Université de Montpellier pour Parc Naturel Régional de Corse
- Berrebi P. 2008. Activity report. Life program Conservation of the trout macrostigma in Corsica. Available http://www. lifemacrostigma.org/ (http://www. lifemacrostigma.org/)
- Bobbio , I , Cannas , R , Cau , A , Deiana , AM , Duchi , A , Gandolfi , G and Tavaglini , J . 1996 . Variabilità mitocondriale in trote con particolare riferimento alle forme macrostigma . Atti del VI Convegno Nazionale A.I.I.A.D. Varese Ligure. pp. , : 42 – 49 .
- Caputo , V , Giovannotti , M , Nisi Cerioni , P , Caniglia , ML and Splendiani , A . 2004 . Genetic diversity of brown trout in central Italy . Journal of Fish Biology , 65 : 403 – 418 .
- Cottiglia , M . 1968 . La distribuzione dell'ittiofauna dulciacquicola in Sardegna . Rivista di Idrobiologia , 7 : 63 – 116 .
- Coward , K and Bromage , NR . 1999 . Spawning periodicity, fecundity and egg size in laboratory-held stocks of a substrate-spawning tilapiine, Tilapia zillii (Gervais) . Aquaculture , 171 : 251 – 267 .
- Delling , B and Doadrio , I . 2005 . Systematics of the trouts endemic to Moroccan lakes, with description of new species (Teleostei: Salmonidae) . Ichthyological Exploration of Freshwaters , 16 : 49 – 64 .
- Duméril , M . 1858 . Note sur une truite d'Algérie (Salar macrostigma, A. Dum.) . Comptes rendus hebdomadaires des séances de l'Académie des Sciences , 47 : 160 – 162 .
- Elliot , JM . 1989 . Wild brown trout Salmo trutta: An important national and international resource . Freshwater Biology , 21 : 1 – 5 .
- Gandolfi , G , Zerunian , S , Torricelli , PM and Marconato , A . 1991 . I Pesci delle Acque Interne Italiane , 617 Roma : Ministero dell'Ambiente, Unione Zoologica Italiana .
- Giordani , G and Melotti , P . 1984 . Elementi di acquacoltura , 1st , Vol. 6 , 121 – 169 . Bologna : Edagricole .
- Kottelat , M and Freyhof , J . 2007 . Handbook of European freshwater fishes , 401 – 430 . Cornol, CH : Publications Kottelat .
- Lorenzoni , M , Maio , G and Nonnis Marzano , F . 2004 . Present knowledge on the native trout populations in Italy: Needs of an integrated approach . Journal of Freshwater Biology Quaderni ETP , 33 : 1 – 12 .
- Massidda , P . 1995 . Salmo (trutta) macrostigma in Sardegna . Biologia ambientale , 5 : 40 – 43 .
- Massidda , P , Sabatini , A , Davini , M , Conti , G , Loddo , G and Cau , A . 1996 . Nuovi dati sulla distribuzione dell'ittiofauna d'acqua dolce in Sardegna . Atti del VI Convegno Nazionale Associazione Ittiologi Acque Dolci. Varese Ligure. pp. , : 239 – 246 .
- McMeel , OM , Hoby , EM and Ferguson , A . 2001 . Partial nucleotide sequences, and routine typing by polymerase chain reaction fragment length polymorphism, of the brown trout (Salmo trutta) lactate dehydrogenase, LDH-C1*90 and 100* alleles . Molecular Ecology , 10 : 29 – 34 .
- Nelson , JS . 1994 . Fishes of the world , 3rd , New York, NY : John Wiley & Sons .
- Nicola , GG and Almòdovar , A . 2002 . Reproductive traits of stream-dwelling brown trout Salmo trutta in contrasting neighbouring rivers of central Spain . Freshwater Biology , 47 : 1353 – 1365 .
- Nonnis Marzano , F , Corradi , N , Papa , R , Tagliavini , J and Gandolfi , G . 2003 . Molecular evidence for introgression and loss of genetic variability in Salmo (trutta) macrostigma as a result of massive restocking of Apennine populations (Northern and Central Italy) . Environmental Biology of Fishes , 68 : 349 – 356 .
- Ostellari , L , Bargelloni , L , Penzo , E , Patarnello , P and Patarnello , T . 1996 . Optimization of single-strand conformation polymorphism and sequences analysis of the mitochondrial control region of Pagellus bogaraveo (Sparidae, Teleostei): rationalized tools in fish population biology . Animal Genetics , 27 : 423 – 427 .
- Pomini , FP . 1940 . Ricerche sul Salmo macrostigma . Bollettino di Pesca, Idrobiologia e Pescicoltura , 16 : 3 – 63 .
- Poteaux , C and Berrebi , P . 1997 . Intégrité génomique et repeuplements chez la truite commune du versant méditerranéen . Bulletin Francais de la Peche et de la Pisciculture , 344/345 : 309 – 322 .
- Sabatini , A , Orrù , F , Cannas , R , Serra , P and Cau , A . 2006 . Conservation and management of Salmo (trutta) macrostigma in Sardinian freshwaters: First result of genetic characterization . Journal of Freshwater Biology , 34 : 335 – 340 .
- Schöffman , J and Sušnik , S . 2007 . Phylogenetic origin of Salmo trutta L. 1758 from Sicily, based on mitochondrial and nuclear DNA analyses . Hydrobiologia , 575 : 51 – 55 .
- Splendiani , A , Giovannotti , M , Nisi Cerioni , P , Caniglia , ML and Caputo , V . 2007 . Phylogeographic inferences on the native brown trout mtDNA variation in central Italy . Italian Journal of Zoology , 73 : 179 – 189 .
- Tamura , K , Dudley , J , Nei , M and Kumar , S . 2007 . MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) Software Version 4.0 . Molecular Biology and Evolution , 24 : 1596 – 1599 .
- Vasemägi , A , Cross , R , Paaver , T , Koljonen , ML and Nilsson , J . 2005 . Extensive immigration from compensatory hatchery releases into wild Atlantic salmon population in the Baltic Sea: Spatio-temporal analysis over 18 years . Heredity , 95 : 6 – 83 .
- Zerunian , S . 2002 . Condannati all'estinzione? Biodiversità, biologia, minacce e strategie di conservazione dei Pesci d'acqua dolce indigeni in Italia , Edited by: Bologna . 220 Agricole .