Abstract
Spermatozoa from members of Hemerobiidae, Chrysopidae and Mantispidae (Arthropoda; Hexapoda: Neuroptera) have been examined by electron microscopy. In all species examined, the nucleus is surrounded by a nuclear envelope that in its anterior domain, fans out laterally into one (Chrysopidae) or two wings (Hemerobiidae and Mantispidae). Furthermore, the anterior sperm region is surrounded by external dense material. In Mantispidae, sperm dimorphism with two types of spermatozoa is also confirmed: paraspermatozoa (not fertilizing), provided with giant axoneme and mitochondrial derivatives, and euspermatozoa (fertilizing). Spermatozoa of Chrysopidae and Mantispidae are characterized by the lack of an acrosome while sperm cells of Hemerobiidae are provided with a bilayered acrosome. Spermatozoa from all the investigated species have axonemes of the conventional insect type, with a 9+9+2 microtubular pattern and with accessory tubules provided with 16 protofilaments. In all the examined taxa the intertubular material has the same localization also observed in all other previously analysed Neuroptera. The mitochondrial derivatives and the accessory bodies in the three families are also described. Hemerobiidae are characterized by the presence of a large groove of the plasma membrane along the right side of the anterior sperm region, which results in an eccentric position of the axoneme. Chrysopidae have large mitochondrial derivatives, which encircle the axoneme. The peculiar feature regarding the nuclear envelope was not seen in other members of neuropteroid insects. These data are discussed in the light of the phylogenetic relationships of the taxa examined.
Introduction
Neuropterida (Neuroptera sensu lato) comprise the hexapod orders Raphidioptera (two families), Megaloptera (two families) and the extremely heterogeneous Neuroptera (17 families) (Aspöck Citation2002). Although they are considered one of the key groups for reconstructing the groundplan and elucidating the evolution of Holometabola (Tsutsumi & Machida Citation2006), their phylogenetic relationships are still unclear.
Molecular studies of Neuropterida performed with nuclear and mitochondrial genes (Haring & Aspöck Citation2004), produced results congruent with previous morphological cladistic analyses; however, some unexpected discrepancies were also found. Megaloptera were confirmed to be the sister group of Neuroptera sensu stricto and Nevrorthidae as the sister group of the other Neuroptera families (Aspöck Citation2002).
Neuroptera represents the real challenge because of the high number of families with respect to Raphidioptera and Megaloptera. The monophyly of Neuroptera is based on the suctorial mouthparts of the larvae, the closure of the larval midgut, and the double-walled silken cocoon of the pupae.
Phylogenetic analyses of Neuroptera based on morphological characters allowed to identify three suborders within the taxon: Nevrorthiformia, Myrmeleontiformia, and Hemerobiiformia (Aspöck Citation1992, Citation1995, 2002). According to Aspöck (Citation2002), Nevrorthiformia include only the family Nevrorthidae; Hemerobiiformia includes up to 11 families, and Myrmeleontiformia the remaining five families. The relationships among Hemerobiiformia are still debated.
The results obtained with comparative analyses of the sperm structure of different insect orders (Jamieson et al. Citation1999) have confirmed that sperm have a valuable series of characters which can be useful to investigate the relationship between insect taxa. In previous works, a possible relationship between Neuropterida and Coleoptera has been suggested based on both the fine structure of the accessory tubules and the shape and position of the intertubular material in the sperm tail axoneme (Afzelius & Dallai Citation1994). More recently, it was confirmed that Coniopterygidae (Neuroptera, Hemerobiiformia) are not only an aberrant group for their morphological traits (Withycombe Citation1923, Citation1925; Meinander Citation1972) but also for their sperm structure (Zizzari et al. Citation2008). Moreover, it was found that members of Mantispidae are provided with two different sperm types: typical (euspermatozoa) and non-functional (paraspermatozoa) sperm, the latter exhibiting giant accessory tubules and large mitochondrial derivatives (Dallai et al. Citation2005; Zizzari et al. Citation2010). In this paper we describe the results we obtained on the sperm structure of some members of Hemerobiiformia: Hemerobiidae, Chrysopidae and Mantispidae, with the aim to contribute to the phylogeny of this interesting insect group, considered to be one of the basal lineages of Holometabola.
Materials and methods
Species
Chrysopidae
| • | Chrysopa formosa Brauer, 1850; Siena, Italy | ||||
| • | Chrysopa intima MacLachlan, 1898; Nagano, Japan | ||||
| • | Dichochrysa prasina (Burmeister, 1839); Siena, Italy | ||||
Hemerobiidae
| • | Hemerobius micans Olivier, 1792; Grosseto, Italy; Vintgar, Slovenija | ||||
| • | Wesmaelius subnebulosus (Stephens, 1836); Zannone island, Italy | ||||
Mantispidae
| • | Perlamantispa perla (Pallas, 1772); Majella, Italy | ||||
Light microscopy (LM)
For measurements of sperm length, free spermatozoa were obtained from adult males by dissecting their testes in 0.1 M phosphate buffer, pH 7.2, to which 3% sucrose was added (PB). Free spermatozoa were then mounted in 90% glycerol and photographed by interference-contrast with a Leica DMRB light microscope equipped with a Zeiss AxioCam MRc5 digital camera.
Transmission electron microscopy (TEM)
Testes and seminal vesicles were isolated from adult males by dissection in phosphate buffer solution (PB) 0.1 M pH 7.2 in which 3% of sucrose was added. Part of the material was fixed for 2 h at 4°C with 2.5% glutaraldehyde in PB. After rinsing with PB, the material was post-fixed for 1 h in 1% osmium tetroxide, rinsed again in PB, dehydrated in ethanol and embedded in Epon-Araldite. The remaining material was processed according to Dallai and Afzelius (Citation1990) using 1% tannic acid in glutaraldehyde fixation (but omitting osmic post-fixation), then en-block stained in 1% uranyl acetate and rinsed in PB.
Ultrathin sections obtained with a Reichert Ultracut II E ultramicrotome were routinely stained and then observed with a Philips CM 10 electron microscope operating at 80 kV.
Results
Hemerobiidae
Wesmaelius subnebulosus. Sperm are about 450 μm long, with two very thin anterior prolongations up to 40 μm long (). At the anterior region, a short (0.3 μm long) apparently bilayered acrosome is in continuity with the nucleus (). This latter has a quite unusual structure consisting of a dense cylindrical axial part, only 0.1 μm in diameter, which expands laterally into two 0.65 μm long electron-dense wings (). These two wings, 0.1 μm thick, are anteriorly in continuity with the two prolongations seen at the light microscope (). The unusual nuclear organization, as well as that of the flagellum, can be better understood by considering the development of the sperm components during spermiogenesis.
Figure 1. Hemerobiidae. Wesmaelius subnebulosus. A, Interference-contrast micrograph of the anterior region of a living sperm. Note the two thin prolongations; B, Longitudinally sectioned spermatozoa showing a bilayered acrosome (a), the nucleus (N) and the two prolongations of the apical region (arrowheads); C, Cross-sectioned sperm at the level of the nuclei (N). The condensed nuclear material is surrounded by a nuclear envelope that fans out laterally into two differently long thin wings (arrows). Note the dense material (asterisks) surrounding the sperm cells; D, Cross-section through testes showing early spermatids. At this level a layer of microtubules (mt) surrounds the nucleus which shows dense chromatin filaments; E, Cross-section through the posterior region of spermatids showing the nuclei (N) with a triangular shape; mt, microtubules; F, Cross-section through the anterior region of spermatids with the lenticular nuclei (N); m, mitochondria; ax, axoneme; G, Cross-section through an early spermatid showing the basal body provided with microtubule triplets and two mitochondrial derivatives (m).
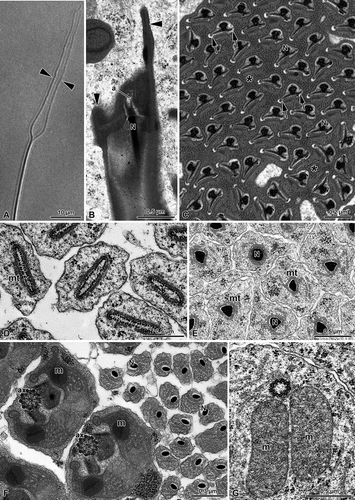
In the early spermatid, the nuclear cross-section is elliptic. It measures 1.0 × 0.2 μm and has the chromatin uniformly dispersed in short filaments. A layer of microtubules surrounds the nucleus (,E). During maturation, the nucleus progressively flattens and its chromatin condenses beneath the nuclear envelope (). The nuclei are embedded in a homogeneous finely granular material (), possibly derived from the cyst cell enveloping the sperm cells. The chromatin condensation proceeds until the nucleus becomes lenticular in a cross-section of the anterior sperm region, only 0.1 μm in its larger diameter (), while, more posteriorly, it has a triangular outline (). In the anterior region, the material surrounding the spermatid progressively becomes dense and then it fragments in small areas. At the end of this process, each sperm cell is surrounded by a layer of this dense material. This material will be lost when the sperm cells will reach the deferent duct lumen. In the early spermatids, the region beneath the nucleus consists of a typical basal body, provided with microtubule triplets and two expanded mitochondrial derivatives (). In nearly mature spermatids and later in the sperm cells, however, this region becomes very complex. The nucleus increases its diameter up to about 0.2 μm and is surrounded by a thin layer of dense material, possibly the centriole adjunct. Beneath this region, a bundle of nine microtubular doublets and nine single microtubules, embedded in a dense matrix, are visible (, ). This area corresponds to the basal body region. More posteriorly, two kidney-shaped small mitochondria are located close to the bundle of microtubules (, ). The mitochondrial derivatives increase their size and exhibit a crystallized region in their matrix. Further behind, the mitochondrial derivatives become large and elliptic, while the bundle of microtubules can be resolved in nine microtubule doublets and nine accessory tubules (, ). No central tubules are yet present, but the axonemal components are now orderly arranged in a circle to form the beginning of the axoneme (). Behind this region, a conventional 9+9+2 axoneme occurs, surrounded by a layer of dense material of the centriole adjunct; more posteriorly, it gives rise to two elongated accessory bodies (). Further posteriorly, the mitochondrial derivatives increase their size assuming in cross-section an unusual shape having two finger-like prolongations flanking the axoneme (, ). The two mitochondrial derivatives are in contact with their outer membranes in the region beneath the axoneme, which is surrounded by an expanded centriole adjunct material (, ). The central part of the mitochondrial matrix shows a crystallization exhibiting two adjacent regions separated by a dense band (,B, 3A,B).
Figure 2. Hemerobiidae. Wesmaelius subnebulosus. A,B, Cross-sections through several sperm tails, each showing a 9+9+2 axoneme (ax), the accessory bodies (ab) and two mitochondrial derivatives (m). Note the basal body region (bb) consisting of a bundle of microtubular elements embedded in a dense material; arrows indicate the plasma membrane grooves. N, nucleus.
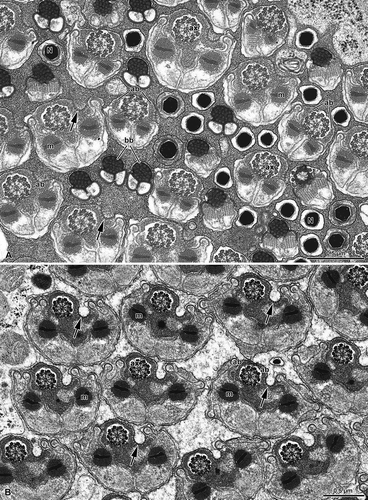
Figure 3. Hemerobiidae. A, Cross-sections through the sperm tails of Wesmaelius subnebulosus. Two mitochondrial derivatives (m) flank the axoneme (ax). Note that in the region close to the axoneme each mitochondrial derivative contains crystalline material (asterisks). This material shows a dense mid ridge (arrowheads). The centriolar region (c) consists of a bundle of microtubular elements embedded in a dense material; B, A membrane specialization is visible at the level of a groove beneath the plasma membrane (arrowheads). Note that the centriole adjunct material (ca) surrounds the axoneme (ax). The flagellum shows a 9+9+2 axonemal pattern with accessory tubules (at) provided with 16 protofilaments. Note that each mitochondrial derivative (m) has two finger-like projections and a crystallized material in the matrix (asterisk); C, Cross-section through the most posterior end of the sperm tail which contains only the axoneme (ax); D–G, Spermatozoa of Hemerobius micans cross-sectioned at the level of the nuclei (N). Note that they are embedded in a dense material and consist of a thin axial region and two long lateral wings (arrows); H, Cross-section through the sperm tails of H. micans showing a 9+9+2 axoneme (ax), two elongated accessory bodies (ab) and the two mitochondrial derivatives (m). Note the asymmetrical shape of the sperm tail.
The most peculiar trait of the anterior region of the sperm tail, however, is the eccentric position of the axoneme. In cross-section, the right side of the sperm flagellum shows a large groove of the plasma membrane, at the level of which a row of outer dense spots is visible (,B, 3A,B). A similar series of dense dots are also present beneath the plasma membrane. Such a membrane specialization is present for most of the flagellar length and it is interrupted only when the two mitochondrial derivatives reduce their size. The most posterior flagellar end consists of only the axoneme ().
Hemerobius micans shares with the previous species the peculiar structure of the nucleus, which consists of a thin axial region and two long lateral wings. The nuclear region is surrounded by a homogeneous dense material (–G). On the contrary, the sperm flagellum has a more conventional appearance, compared to W. subnebulosus sperm cell. The axoneme has always a 9+9+2 pattern; two elongated accessory bodies and two triangular-shaped mitochondrial derivatives are also present in the sperm flagellum (). As in the previous species, a large infolding of the plasma membrane is evident on the right side of the sperm flagellum, thus determining a clear asymmetry of the cross section ().
Chrysopidae
All the species studied here are characterized by a sperm structure similar to that previously described by Baccetti et al. (Citation1969), Dallai and Afzelius (Citation1993), and Afzelius and Dallai (Citation1994) in some species.
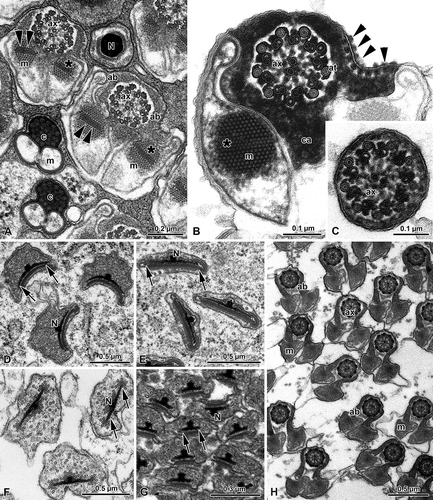
The peculiar features of the sperm cell are the lack of an acrosome and the presence of wide mitochondrial derivatives. The nucleus has a cylindrical shape, about 0.2 μm in diameter, tapering towards the anterior end (,B). In cross-section it forms two wings at the most anterior end, but a little behind they reduce to a single wing 0.38 μm long (,B). As usual, the sperm bundles are embedded in the cytoplasm of the cyst cell (,B). The anterior sperm region is surrounded by a crystalline structure, triangular in cross-section and adherent to the laminar expansions of the nuclear envelope, and by a layer of granules (). At sperm maturity, this latter material disappears, while the crystallized one remains adherent to the nuclear wings ().
Figure 4. Chrysopidae. A, Spermatozoa of Dichocrysa prasina and B, Chrysopa formosa cross-sectioned at the level of the nuclei (N). The cylindrical nucleus (N) shows the nuclear envelope that expands in a thin wing (arrows). The nucleus is surrounded by a great amount of dense paracrystalline material (p) and by a bundle of parallel rodlets (r); C,E, Cross-sectioned sperm tails of D. prasina and D, Chrysopa intima showing the two mitochondrial derivatives (m) that surround most of the axoneme (ax) and leave only a little space for the accessory bodies (ab). The flagellum shows a 9+9+2 axoneme. Note the bridges (arrowheads) connecting the axonemal doublets 2 and 5 to the mitochondrial derivatives.
The sperm flagellum shows a 9+9+2 axoneme. As already described by Dallai and Afzelius (Citation1993), and by Afzelius and Dallai (Citation1994), two bridges anchor the axonemal doublets number 2 and 5 to two opposite small and flattened vesicles (), that are remnants of the periaxonemal cistern surrounding the axoneme in the early spermatid. The accessory microtubules of the axoneme have 16 protofilaments in their tubular wall and the dense intertubular material retains the configuration typical of the order. The axoneme is located along the middle part of the sperm flagellum, in the space comprised between the two expansions of the mitochondrial derivatives and the accessory bodies (–E). The cross-sectioned sperm tail has a pronounced bilateral symmetry (–E). The two mitochondrial derivatives encircle most of the axoneme () with compact crystallized material in their core region (–E).
As previously described by Baccetti et al. (Citation1969), two accessory bodies are in contact with the mitochondrial derivatives and sometimes they can protrude from the outline of the sperm flagellum as two large feet. The plasma membrane facing the accessory bodies shows two linear densities of glycocalyx, possibly corresponding to membrane specializations.
Mantispidae
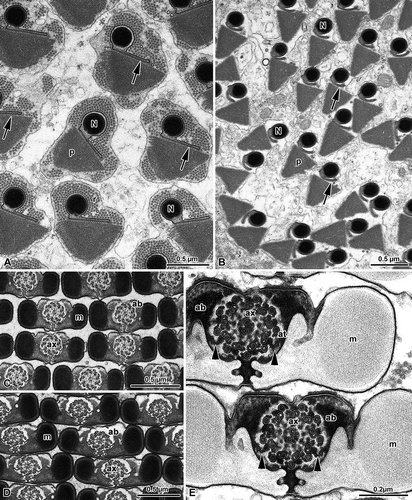
The sperm of this family are of two types: eu- and para-spermatozoa; with these latter characterized by giant sperm tails containing huge accessory tubules and mitochondrial derivatives filled with granular inclusions.
In the most anterior region of the euspermatozoa of P. perla, the nucleus is thin and shows two differently long wings, about 0.6 μm in cross-section ().
Figure 5. Mantispidae. Perlamantispa perla. A, Cross-sectioned sperm at the level of the nuclei (N). Each nucleus consists of a dense cylindrical axial part which expands laterally into two differently long thin wings (arrows). The nucleus is surrounded by a dense material; B, Cross-sectioned euspermatozoon showing long accessory bodies (ab) partially flanking the axoneme (ax). The flagellum has a 9+9+2 axoneme with accessory tubules (at) provided with 16 protofilaments in their tubular wall. Note the beak-like shape of the intertubular material (im) adhering to the accessory tubules; m, mitochondrial derivatives.
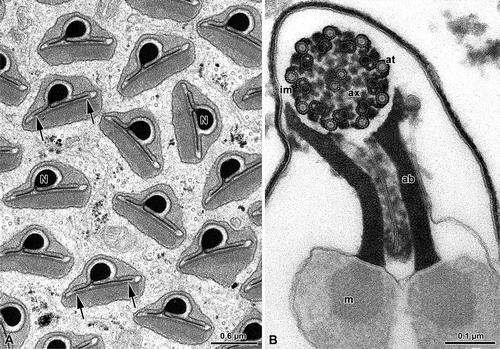
The main part of the sperm flagellum is characterized by an axoneme and by two mitochondrial derivatives, provided with a crystallized axis. Accessory bodies are represented by two small, dense structures located between the axoneme and the two mitochondrial derivatives. In the mature sperm flagellum the two mitochondrial derivatives become ovoidal in cross-section, while the accessory bodies into two elongate ribbon-like structures ().
Discussion
Neuropterida are a relatively small but greatly diversified ancient clade. Thus, it is not surprising to find different sperm models in this taxon. The sperm structure of members of the three families examined is characteristic of each taxon. The acrosome is small in the Hemerobiidae and is missing in Mantispidae and Chrysopidae. The peculiar shape of the large mitochondrial derivatives of Chrysopidae, a character already mentioned by Baccetti et al. (Citation1969), and the bridges connecting the axoneme to two cisterns adherent to the inner side of the mitochondrial derivatives (Dallai & Afzelius Citation1993; Afzelius & Dallai Citation1994) are autapomorphies of this taxon. In previous papers the sperm structure of Chrysopa carnea, C. prasina and C. formosa was described by Baccetti et al. (Citation1969), while details of the C. carnea axoneme were studied by Dallai and Afzelius (Citation1993), and Afzelius and Dallai (Citation1994). The preserved uniformity of morphological features of the different sperm structures independently from the species examined is remarkable, as it has already been emphasized by the quoted authors.
Similarly, the long and thin appendages present at the anterior tip of the hemerobiid Wesmaelius subnebulosus and the peculiar lateral groove associated to a membrane specialization in the anterior region of the sperm flagellum of the Hemerobiidae are features typical of this group.
Several mantispid species collected in different countries have been examined and two types of sperm have been always found (Dallai et al. Citation2005; Zizzari et al. Citation2010). This means that Mantispidae are characterized by functional sperm and giant paraspermatozoa, these latter probably playing a role in male competition (Simmons Citation2001). The axonemal structure with the particular arrangement of the intertubular material associated to the accessory tubules and to the outer surface of the microtubular doublets is typical of all Neuroptera; analogously, the large centriole adjunct material which expands along the flagellum to form two longitudinal pillars and the accessory bodies on both sides of the axoneme are characters shared by all Neuroptera.
In the last 10 years, different relationships among the Neuroptera families have been proposed based either on the cladistic analysis of some selected morphological characters (Aspöck et al. Citation2001), molecular data (Aspöck et al. Citation2003; Winterton Citation2003; Haring & Aspöck Citation2004), the genital sclerites (Aspöck & Aspöck Citation2008), or the morphology of the larva head (Beutel et al. Citation2010). In particular, morphological studies suggested that Mantispidae would be placed in a clade together with Berothidae, Rhachiberothidae and Dilaridae (the dilarid clade). Moreover, a sister-group relationship of Coniopterygidae + dilarid clade has been hypothesized (Aspöck et al. Citation2001; Aspöck & Aspöck Citation2008; Zimmermann et al. Citation2009; Beutel et al. Citation2010). On the contrary, Hemerobiidae and Chrysopidae would have to be placed in a separate clade. A relationship between Hemerobiidae and Chrysopidae was also hypothesized on the basis of the results of the study of the ‘silent songs’ produced by the abdominal vibration (Henry Citation1997). According to molecular studies, Hemerobiidae and Chrysopidae, together with Mantispidae and Berothidae (Winterton Citation2003) or with only Mantispidae (Haring & Aspöck Citation2004), should be assembled in a distinct clade, while Coniopterygidae should be placed in a separate taxon ().
Figure 6. Phylogenetic relationships based on holomorphological and molecular characters. The latter data are also supported by the spermatological results reported in the paper (from Haring & Aspöck Citation2004).
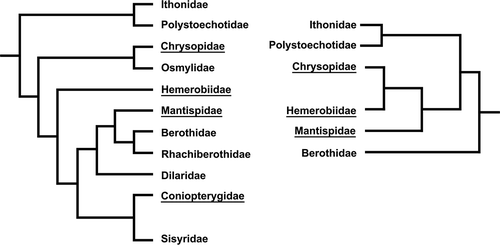
Spermatological data strongly corroborate these last results. The feature of the anterior nuclear region, in particular the shape of the nuclear envelope exhibiting the peculiar wing-like expansions, represents indeed an important character shared by chrysopid, hemerobiid and mantispid spermatozoa. Such a character might be considered a synapomorphy revealing the occurrence of a close relationship among these taxa. On the contrary, Coniopterygidae, a taxon closely related to Hemerobiiformia, are characterized by a flagellar axoneme provided with a 9+9+3 microtubular pattern with the accessory tubules with 13 protofilaments in their tubular wall, a number not shared by other neuropteran families (Zizzari et al. Citation2008). The family is retained to be aberrant not only regarding the sperm structure but also for several morphological traits (Meinander Citation1972).
Although sperm ultrastructure has provided useful contributions to the study of Neuropterida, phylogenetic relationships within Hemerobiiformia still remain the real challenge for future studies. Many more representatives of the neuropteroid taxa must be examined before a sound and convincing conclusion can be reached.
Acknowledgements
The authors wish to thank D. Mercati (University of Siena) for technical assistance. We are also grateful to K. Tsutsumi (University of Tsukuba) for providing Chrysopa intima. This research was supported by grants PRIN to R. Dallai.
References
- Afzelius , BA and Dallai , R . 1994 . Characteristics of the flagellar axoneme of Neuroptera, Coleoptera, and Strepsiptera . Journal of Morphology , 219 : 15 – 20 .
- Aspöck , U . 1992 . “ Crucial points in the phylogeny of the Neuroptera (Insecta) ” . In Current research in neuropterology. Proceedings of the Fourth International Symposium on Neuropterology, 1991 , Edited by: Canard , M , Aspöck , H and Mansell , MW . 63 – 73 . Toulouse, , France : Bagnères-de-Luchon .
- Aspöck , U . 1995 . Neue Hypothesen zum System der Neuropterida . Mitteilungen der Deutschen Gesellschaft für Allgemeine und Angewandte Entomologie , 10 : 633 – 636 .
- Aspöck , U . 2002 . Phylogeny of the Neuropterida (Insecta: Holometabola) . Zoologica Scripta , 31 : 51 – 55 .
- Aspöck , U and Aspöck , H . 2008 . Phylogenetic relevance of the genital sclerites of Neuropterida (Insecta: Holometabola) . Systematic Entomology , 33 : 97 – 127 .
- Aspöck , U , Aspöck , H and Haring , E . 2003 . Phylogeny of the Neuropterida – morphological evidence and the molecular advocatus diaboli . Entomologische Abhandlungen , 61 : 155 – 156 .
- Aspöck , U , Plant , JD and Nemeschkal , HL . 2001 . Cladistic analysis of Neuroptera and their systematic position within Neuropterida (Insecta: Holometabola: Neuropterida: Neuroptera) . Systematic Entomology , 26 : 73 – 86 .
- Baccetti , B , Dallai , R and Rosati , F . 1969 . The spermatozoon of Arthropoda. III. The lowest holometabolic insects . Journal de Microscopie , 8 : 233 – 248 .
- Beutel , RG , Friedrich , F and Aspöck , U . 2010 . The larval head of Nevrorthidae and the phylogeny of Neuroptera (Insecta) . Zoological Journal of the Linnean Society , 158 : 533 – 562 .
- Dallai , R and Afzelius , BA . 1990 . Microtubular diversity in insect spermatozoa. Results obtained with a new fixative . Journal of Structural Biology , 103 : 164 – 179 .
- Dallai , R and Afzelius , BA . 1993 . Substructure of the axoneme of pterygota insect spermatozoa. Phylogenetic considerations . International Journal of Insect Morphology and Embryology , 22 : 449 – 458 .
- Dallai , R , Lupetti , P , Osella , G and Afzelius , BA . 2005 . Giant sperm cells with accessory macrotubules in a neuropteran insect . Tissue and Cell , 37 : 359 – 366 .
- Haring , E and Aspöck , U . 2004 . Phylogeny of the Neuropterida: A first molecular approach . Systematic Entomology , 29 : 415 – 430 .
- Henry , CS . 1997 . “ Modern mating systems in archaic Holometabola: Sexuality in neuropterid insects ” . In The evolution of mating systems in insects and arachnids , Edited by: Choe , JC and Crespi , BJ . 193 – 210 . Cambridge : Cambridge University Press .
- Jamieson , BGM , Dallai , R and Afzelius , BA . 1999 . “ Insects ” . In Their spermatozoa and phylogeny , New Hampshire, , U.S.A : Science Publishers . Enfield
- Meinander , M . 1972 . A revision of the family Coniopterygidae (Planipennia) . Acta Zoologica Fennica , 136 : 1 – 357 .
- Simmons , LW . 2001 . “ Sperm competition and its evolutionary consequences in the insects ” . In Monographs in behaviour and ecology , Princeton, NJ : Princeton University Press .
- Tsutsumi , K and Machida , R . 2006 . Embryonic development of a snakefly, Inocellia japonica Okamoto (Insecta: Neuroptera, Raphidiodea) . Proceedings of Arthropodan Embryological Society of Japan , 41 : 37 – 45 .
- Winterton , SL . 2003 . Molecular phylogeny of Neuropterida with emphasis on the lacewings (Neuroptera) . Entomologische Abhandlungen , 61 : 158 – 160 .
- Withycombe , CL . 1923 . Notes on the biology of some British Neuroptera (Planipennia) . Transactions of the Entomological Society of London , 20 : 501 – 594 .
- Withycombe , CL . 1925 . Some aspects of the biology and morphology of the Neuroptera with special reference to the immature stages and their possible phylogenetic significance . Transactions of the Entomological Society of London , 15 : 303 – 411 .
- Zimmermann , D , Klepal , W and Aspöck , U . 2009 . The first holistic SEM study of Coniopterygidae (Neuroptera) – Structural evidence and phylogenetic implications . European Journal of Entomology , 106 : 651 – 662 .
- Zizzari , ZV , Lupetti , P , Mencarelli , C and Dallai , R . 2008 . Sperm ultrastructure and spermiogenesis of Coniopterygidae (Neuroptera, Insecta) . Arthropod Structure and Development , 37 : 410 – 417 .
- Zizzari , ZV , Machida , R , Tsutsumi , K , Reynoso-Velasco , D , Lupetti , P and Dallai , R . 2010 . “ Ultrastructural studies on euspermatozoa and paraspermatozoa in Mantispidae (Insecta, Neuroptera) ” . In Tissue and Cell Vol. 42 , 81 – 87 .