Abstract
A morphological and ultrastructural study of the spermatheca of some species of Orthoptera Tettigoniidae was carried out to understand the role of this female organ in the reproductive biology because no literature exists about it in this insect group. In all the examined species, the spermatheca is of ectodermal origin and is composed of a seminal receptacle, mainly involved in the collection and storage of the spermatozoa, and of a spermathecal duct. In both these organs, the epithelium of the wall is made up of two different cell types: cuticle-forming cells, underlying the cuticular intima, and gland cells. Both of these cell types have secretory features that differ among the species and also within the same species, in relation to the tracts examined. In particular, the ultrastructure of the distal tract of the spermathecal duct indicates a more marked secretory activity than in the other tracts of the duct. This activity is often accompanied by ultrastructural aspects, suggesting a lysis activity in both the epithelium and the upper cuticle. Based on these findings, it is hypothesized that the seminal receptacle and the spermathecal duct have different functional roles, despite having a similar general structural organization.
Introduction
In addition to the gonads, the insect reproductive system includes a considerable number of various secondary structures that are essential for reproduction; in fact, these structures take part in the transfer and protection of gametes. One of the main roles is performed by the spermatheca, which is the organ of the female reproductive system responsible for sperm collection during copulation, sperm storage and their release for fertilization. Furthermore, the spermatheca has also been recognized as the place where morphological and biochemical changes of the male gamete occur, making it ready for fertilization (Longo et al. Citation1993).
The insect spermatheca is a complex anatomic system of ectodermal origin that shows notable differences in number, shape and structure among the various species. In general, however, it is composed of a seminal receptacle, whose main function is to collect and store sperm, and a spermathecal duct, of various lengths, opening into a bursa copulatrix or a recess in the vagina (Snodgrass Citation1937; Smith Citation1968; Chapman Citation1969; Grassè Citation1977).
Even though numerous observations have been made on the morphology and structure of the insect spermatheca, data concerning Othoptera Ensifera are referred only for few species (Melis & Dallai Citation1963, 1966); in addition, no studies exist on the ultrastructural organization of the Tettigoniidae spermatheca. In order to extend our knowledge of the insect spermathecal systems, investigations were made on the morphology and ultrastructure of the spermatheca in some species of Orthoptera Tettigoniidae, to provide a better understanding of its structure and role in the reproductive biology of this insect group.
Materials and methods
Surveys were carried out on the spermathecas of sexually mature females of four species of Orthoptera Tettigonioidea: Platycleis intermedia (Serville), Platycleis grisea (Fabricius), Tessellana tessellata (Charpentier), and Sepiana sepium (Yersin), belonging to the Tettigoniidae family and collected in various locations in eastern Sicily, Italy.
Observations were carried out on the various tracts of the genital spermatheca of each insect.
For in toto observations concerning the morphology of the spematheca, insects were dissected in Ringer solution and the spermathecas were observed using a stereomicroscope; for histological investigations, the spermathecas were then fixed in Carnoy's or in Bouin's liquid, dehydrated in an alcohol series and then included in Histowax. The 5–7 µm sections were stained using Haematoxylin–Eosin and Heidenhain's Azan. No significant differences were found in the samples of the same species treated with the different fixatives.
For transmission electron microscopy (TEM) investigations, the insects were fixed in 2.5% glutaraldehyde in 0.1 M Na–cacodilate buffer at pH 7.4 for 4 h at room temperature. The samples were then washed several times in the same buffer, post-fixed with 2% osmium tetroxide in the same buffer for 1 h at room temperature. They were then dehydrated in ethyl alcohol, immersed in propylene oxide and included in Embed 812. The ultrathin sections were collected on copper and rodium grids (200/300 mesh) and contrasted with uranyl acetate and lead citrate, according to Reynolds (Citation1963).
Results
In this study the species Platycleis intermedia was used as a model; for the other species only the different characteristics, compared to the model species, will be reported.
I. Spermatheca of Platycleis intermedia (Serville)
Morphology and histology
The spermatheca is composed of a large seminal receptacle and a spermathecal duct. The seminal receptacle, sac-shaped, is 5 mm in length and 3 mm in width at its widest point. The spermathecal duct is tubular, winding, which when extended is about 2–3 mm in length with a calibre of about 0.3 mm.
In order to facilitate our investigation and description, the spermathecal duct was divided, for all the examined species, into three tracts. The distal tract nearest to the receptacle is called the connecting tract or ‘neck’, followed by the intermediate and the proximal tract, which opens into the vagina.
The general organization of the wall of the spermatheca is similar in the seminal receptacle and in the various tracts of the spermathecal duct. Histologically, from the internal to the external layer, it consists of a pseudostratified epithelium, surrounded by the cuticular intima, which delimits the lumen of the organ (). The epithelium lies on a thin, fibrillar basal lamina and is delimited by a thick, connective-muscular sheath. These components gradually thicken from the distal to the proximal tract of the duct, with the consequent reduction of the organ lumen.
Figure 1. Platycleis intermedia, cross-section of seminal receptacle. c, cuticle; e, epithelium; L, lumen of seminal receptacle; mu, muscle. Haematoxylin–Eosin. Scale bar: 50 mm.
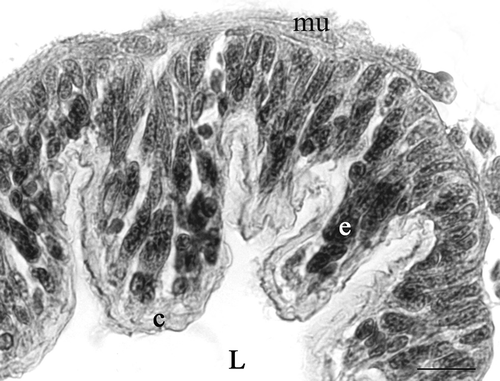
Since the epithelium of the seminal receptacle forms more or less prominent folds, its thickness is not constant along the same section. It can have a maximum thickness of about 53 µm. These folds reduce in thickness along the course of the spermathecal duct, until they completely disappear in the proximal tract.
The epithelia of the spermathecal duct, and that found at the level of the seminal receptacle, show a similar ultrastructural organization exhibiting two adjacent cell types: cuticle-forming cells and gland cells, which are not in contact with the intima ().
Figure 2. Diagram of the ultrastructural organization of the epithelium of the seminal receptacle and spermathecal duct of Platycleis intermedia. 1, cuticle-forming cell; 2, gland cell. bl, basal lamina; ci, cuticular intima; g, Golgi bodies; gr, electron-dense granules; m, mitochondria; mt, microtubules; n, nucleus; r, reservoir; RER, rough ergastoplasmic reticulum; v, vesicle.
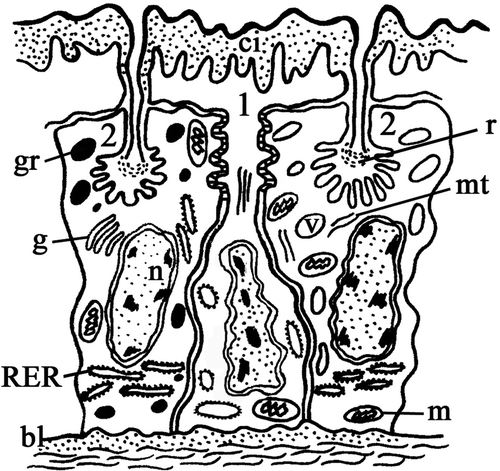
The cuticle-forming cells have a thin body and their nucleus, containing numerous chromatin granules, is elongated along the main axis of the cell; it is generally in a central or basal position.
The gland cells have the shape of a truncated pyramid with their oval or spherical nucleus in a basal position and containing few dispersed chromatin granules.
Ultrastructure
Seminal receptacle. The cuticle-forming cells and the gland cells are tightly packed (, ). The contiguous membranes are rectilinear, with the exception of the apical region where they form deep interdigitations with septate junctions (, arrows). The cuticle-forming cells have a heterochromatic nucleus with numerous indentations, a perinuclear cistern with numerous dilations and the cytoplasm contains a rough ergastoplasmatic reticulum (RER) with dilated cisterns and numerous vesicles of varying size with heterogeneous content (). The Golgian areas are not clearly recognizable. The microtubules run parallel to the main axis of the cell both individually and grouped together (). In the apical region of these cells sac-like dilations are formed which contain a finely granular material, below which there are mitochondria of varying shapes (). Similarly to the spermatheca of other insects, the cuticular intima of Platycleis intermedia, about 4.5-µm thick, consists of an endocuticle with heterogeneous aspect and a thin granular epicuticle composed of an outer compact and intensely osmiophilic thin layer (about 47 nm) delimiting the organ lumen (, arrow).
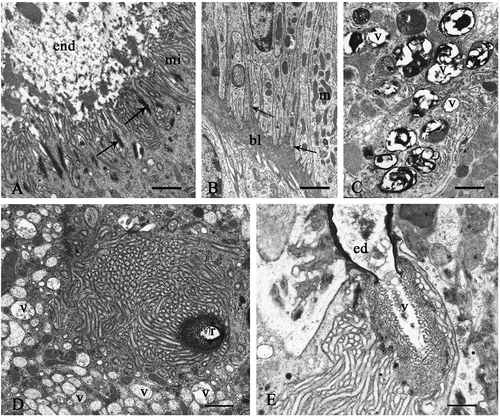
Figure 3. Platycleis intermedia, seminal receptacle. A, the cuticle-forming cells (cc) and the gland cells (gc) are tightly packed. The perinuclear cistern appears dilated (arrow); RER, rough ergastoplasmic reticulum; n, heterochromatic nucleus; v, vesicles. B, the contiguous membranes form deep interdigitation with septate junctions (arrows); C, the microtubules (mt) in the cuticle-forming cells; D, the cuticle-forming cells around the apical region form sac-like dilations (little square). Inset: detail of the dilations in the little square; c, cuticle; gc, gland cell; gr, electron-dense granules; m, mitochondria; mi, microvilli of the end-apparatus; n, nucleus. E, apical region. Arrow: osmiophilic layer of epicuticle; end, endocuticle; ep, granular layer of epicuticle. F, gland cell. RER, rough ergastoplasmic reticulum is mainly made up of flattened and parallel cisterns. Scale bars: A, 92 nm. B, 1920 nm. C, 140 nm. D, 3390 nm. E, 480 nm. F, 870 nm.
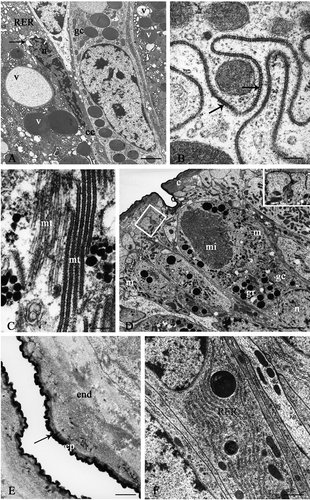
The second type of cells or gland cells never reach the organ lumen; their large oval nucleus, in a basal region, is euchromatic and shows a clear nucleolus. The cytoplasm contains numerous elongated mitochondria and extended ergastoplasmic areas, which are mainly made up of flattened and parallel cisterns (). The Golgi complexes are not very evident. In the cytoplasmic region between the nucleus and the excretory pole, there is a high concentration of secretory granules with compact and electron-dense contents. The apical region displays a large invagination of the plasma membrane, which delimits the extracellular cavity known as the ‘reservoir’, which is more or less dilated in relation to the secretory activity. In correspondence to the ‘reservoir’ the plasma membrane forms numerous and prominent microvilli which are tightly packed and radially arranged around the cavity.
The reservoir possesses a single end-apparatus composed of microvilli and electron-dense material (felt-work) (, ). This material is made up of numerous fibrils, which run parallel to each other and perpendicular to the microvilli (). The cytoplasm surrounding the reservoir is particularly rich in mitochondria, electron-dense granules and electron-transparent vesicles. The efferent duct, of cuticular origin, opens into the reservoir and the material elaborated from the gland cells passes through this duct and is released into the lumen of the organ. The efferent duct is short, straight and perpendicular to the cuticular surface. The duct wall consists solely of epicuticle and is thinner at the reservoir level (, arrowhead), thickens immediately next to it (, arrow) and remains constant throughout its course.
Figure 4. Platycleis intermedia. A and B, seminal receptacle. A, the wall of the efferent duct consists solely of an epicuticle (ep) that is thinner at the reservoir (headarrow) and immediately after thickens and remains constant throughout its course (arrow); fw, felt-work. B, basal portion. Arrow, sediment of basal lamina; bl, basal lamina; e, epithelium; mu, muscular layer. C and D, connecting tract of the spermathecal duct: cuticle-forming cells. C, end, endocuticle; m, mitochondria; s, septate junctions. D, ae, apical expansions; v, vesicles with heterogeneous content. Scale bars: A, 650 nm. B, 1920 nm. C, 870 nm. D, 1510 nm.
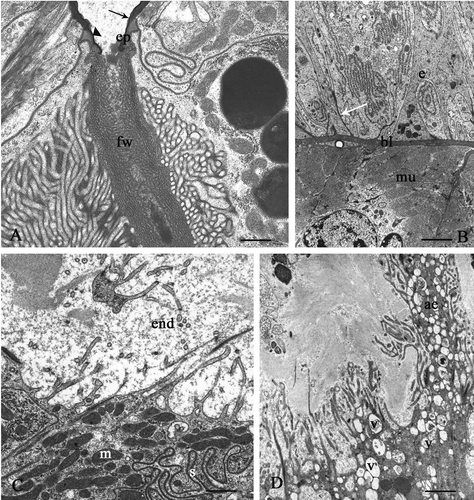
All of the cells lie on a basal lamina surrounded by a thick connective-muscular layer (). The finely fibrillar basal lamina is mainly rectilinear, with some sediments which penetrate into the entire epithelial layer (, arrow).
Spermathecal duct. The general organization of the epithelium is similar to that of the seminal receptacle. The two cytotypes are narrower and higher; in their apical region, the contiguous membranes form deep interdigitations with wide systems of septate junctions ().
In particular, in the connecting tract, the cuticle-forming cells show some peculiarities. Some of them have irregular apical microvilli penetrating into the endocuticle, underneath which there are numerous mitochondria (); other cells, instead, have expansions variously extended with vesicles having heterogeneous content ().
The cuticular intima, which is thicker than that in the seminal receptacle, has an endocuticle with a strongly heterogeneous structure. Efferent ducts, with an essentially rectilinear course, pass through the cuticular intima and their content is granular.
The gland cells have an oval nucleus with small lumps of chromatin dispersed in the carioplasm. They also have a marked secretory activity, whereas this activity is low in the intermediate and in the proximal tracts. Furthermore, in the proximal tract the cuticle-forming cells have regular and tightly packed apical microvilli; the microtubule attachment plates (, arrows) are numerous between the contiguous cells and the endocuticle is strongly heterogeneous (). In this tract, the basal region of the cell, rich in mitochondria, form deep infoldings in which the basal lamina penetrates (, arrows).
Figure 5. A and B: Platycleis intermedia spermathecal duct. A, proximal tract, apical region of the cuticle-forming cells. Arrows, points of attachment of microtubules; end, endocuticle; mi, microvilli. B, basal region. Arrows, infoldings of the plasma membrane; bl, basal lamina; m, mitochondria. C–E: Platycleis grisea, seminal receptacle. C, gland cell. v, vesicles with heterogeneous content; D, vesiscles (v) with low electrondense content near to central reservoir (r); E, ed, efferent duct; v, vesicles with heterogeneous content. Scale bars: A, 1120 nm. B, 1920 nm. C, 870 nm. D, 870 nm. E, 650 nm.
II. Platycleis grisea (Fabricius)
The piriform seminal receptacle is 6.5 mm long and 3 mm wide at its widest point. The spermathecal duct has a constant calibre of 0.3 mm.
Ultrastructurally, the epithelium displays a general organization similar to that described above for Platycleis intermedia. In some gland cells, there are numerous vesicles with highly heterogeneous content (), probably of lysosomal origin. Vesicles, with low electron-dense content, are gathered in the cytoplasm near the central reservoir (). The content of the efferent duct is heterogeneous with electron-transparent vesicles of varying sizes ().
In gland cells, the RER is well developed throughout all the cytoplasm; it consists of dilated and flattened cisterns often parallel to each other.
The examined ultrastructural characteristics of the spermathecal duct are similar to those already described for P. intermedia.
III. Sepiana sepium (Yersin)
The spermatheca shows a piriform seminal receptacle, 3 mm long and 2 mm wide, and a tubular spermathecal duct, 3 mm long and 0.25 mm wide.
The wall of this organ has a pseudostratified folded epithelium; a cuticular intima, 3 µm thick, overlies the epithelium.
Ultrastructurally, the cytoplasm of the cuticle-forming cells is rich in free ribosomes, groups of microtubules, which run parallel to the main axis of the cell and vesicles of varying size with heterogeneous content. Numerous mitochondria are gathered in the apical area beneath irregular microvilli.
The cuticular intima consists of a fibrillar endocuticle and an epicuticle with small granules. It is surmounted by a thin, highly osmiophilic, layer.
The voluminous gland cells have a vesicular nucleus in the basal region, with few chromatin granules. The RER is widespread, the mitochondria are numerous and the Golgi complex is not evident. The reservoir is particularly dilated and delimited by sparse microvilli; its content is finely granular (). The cytoplasm around the reservoir displays vesicles with a finely granular material mixed with another more compact and electron-dense one ().
Figure 6. Sepiana sepium. A, seminal receptacle. Arrow, cuticle; ed, efferent duct; L, lumen; r, reservoir; v, vesiscles with heterogeneous content. B, proximal tract of the spermathecal duct. Cuticle-forming cells. Arrow, osmiophilic layer of the epicuticle; end, endocuticle; r, reservoir. C, arrows, break of the osmiophilic layer of the epicuticle; L, lumen; sp, sperms. D, the cytoplasm of the gland cells has numerous vesicles (v) with a low electron-dense content. E and F Tessellana tessellata, seminal receptacle. E, vesicles (v) in the cytoplasm around the reservoir (r); ed, efferent duct; m, mitochondria; n, nucleus. F, gland cells. g, Golgi bodies; r, reservoir; v, vesicle with a granular content opening into the reservoir. Scale bars: A, 1510 nm. B, 1920 nm. C, 1120 nm. D, 1920 nm. E, 2000 nm. F, 480 nm.
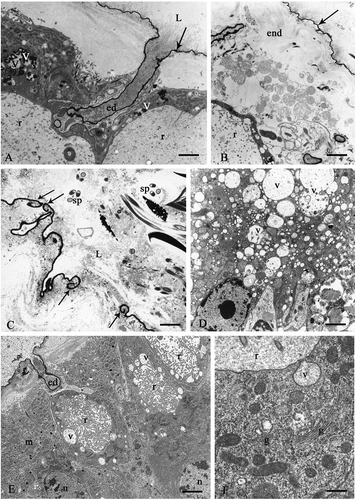
The rectilinear efferent duct displays a content consisting of very thin, irregular and tightly packed fibrils. A similar material is found inside the lumen of the organ near the cuticle (, arrow).
The general ultrastructural organization of the spermathecal duct is not substantially different from that of the seminal receptacle.
However, in the connecting tract, some areas have peculiar ultrastructural characteristics involving both the epithelium and the upper cuticular intima. In these areas, the cuticle-forming cells undergo partial or total lysis: their cytoplasm is highly vacuolized, the apical microvilli are reduced or completely absent. The cuticular intima loses its typical organization except in the highly osmiophilic superficial layer of the epicuticle (, arrow). The epicuticle is open in several tracts and folds back towards the epithelium (, arrows). The lumen is rich in isolated, often altered, sperm (). Furthermore, in these regions, the cytoplasm of the gland cells has numerous vesicles, which vary in size and have a low electron-dense content ().
IV. Tessellana tessellata (Charpentier)
The sac-like seminal receptacle is about 5 mm long and 2 mm wide. The cuticular intima is 3–4 µm thick. The rectilinear spermathecal duct is about 2 mm long.
From the TEM observations, the cytoplasm of the cuticle-forming cells appears rich in free ribosomes and vesicles with an electron-dense content.
The microtubules are grouped together and run parallel to the main cell axis. The numerous mitochondria are more abundant in the apical region, under the microvilli ().
The cuticular intima has a fibrillar endocuticle with fibrils loosely arranged near the microvilli and its epicuticle, containing very small granules, is surmounted by a very thin irregular and highly osmiophilic layer, as in the other species.
The gland cells have a voluminous nucleus, in the basal region, with few chromatin granules and an evident nucleolus. In the apical portion of the cell there is a very dilated reservoir surrounded by long microvilli (). The cytoplasm around the reservoir is rich in vesicles, with a granular content, some of which seem to open into the reservoir between the microvilli ().
The RER is in the paranuclear zone, where it forms several flattened parallel cisterns. The Golgi complex is evident and consists of sacculi and vesicles, with a low electron-dense content (). The secretion of the rectilinear efferent duct is granular and electron-dense ().
The epithelial cells lie on a thin basal lamina consisting of packed fibrils, which penetrates deeply between the contiguous cells.
There are no substantial differences in the ultrastuctural organization of the three tracts of the spermathecal duct between T. tessellata and P. intermedia. However, in the connecting tract, as described for Sepiana, the cuticle-forming cells have an extended rough endoplasmatic reticulum and marked secretory activity. None the less, many of these cells show clear signs of degeneration as evidenced by the highly vacuolized cytoplasm and by the reduction or complete absence of the apical microvilli. The cell boundaries between the cuticle-forming cells and the gland cells are not often easily distinguishable.
Discussion
Anatomically, our observations show a relatively uniform morphology of the seminal receptacle and the spermathecal duct of the spermatheca, in agreement with data present in the literature for other species belonging to Orthoptera Ensifera.
The general morphological homogeneity of the spermatheca of the Tettigoniidae examined is also accompanied by histological and ultrastructural uniformity; the wall of this organ gradually thickens in the spermathecal duct. The epithelium consists of two different cytotypes: the cuticle-forming cells and the gland cells. However, in the spermatheca of other species of Ensifera examined, Gryllus domesticus, Liogryllus bimaculatus (Gryllidae) (Sathe & Joshi Citation1988) and Dolicopoda schiavazzi (Melis & Dallai Citation1963), the epithelium consists only of cuticle-forming cells; in the genus Gryllotalpa (Grillotalpidae) (Melis & Dallai Citation1966; Sathe & Joshi Citation1988), elements specialized in the gland function in same tracts of the spermathecal duct were observed, whereas the epithelium of the seminal receptacle consisted only of cuticle-forming cells.
Further differences between the above-mentioned Orthoptera Ensifera and our species revealed a more efficient division of the functions of the epithelial cells: the gland cells elaborate the spermathecal secretion, whereas the cuticle-forming cells mainly perform the secretion of the cuticular intima and of the epicuticle of the efferent ducts.
In some Orthoptera Acrididoidea and Phasmatodea (Viscuso et al. Citation1994) this latter function is carried out by a third cytotype in the spermathecal epithelium: the ‘duct cell’. The duct cell is considered a specialized cuticle-forming cell due to its cytological characteristics and functions (Noirot & Quennedey Citation1974; Quennedey Citation1998). Instead, in the Tettigonioidea and Grilloidea, the spermathecal epithelium lacks any other further specializations of the cuticle-forming cells; thus suggesting a greater primitiveness of these groups with respect to Acridoidea.
In the species we examined, the gland cells are never in contact with the cuticular intima and their general organization is identifiable as class 3 according to the Noirot and Quennedey (Citation1974) subdivision regarding the ectodermic glands of insects.
The secretion elaborated by the gland cells differs in its aspect between different species. Only in P. intermedia does this secretion consist of uniformly compact and electron-dense granules.
Secretion inside the reservoir seems to take place by the fusion of the vesicles with the membrane, which delimits the reservoir; this is partly similar in Periplaneta (Gupta & Smith Citation1969).
Both invaginations of the basal plasma membrane of the epithelial cells and numerous mitochondria, in the terminal tract of the spermathecal duct, might imply the involvement of these cells in the re-absorption process (probably from the haemocoel), as already noted in other insects (Blum et al. Citation1962). However, it is also possible that the invagination systems, in the species we examined, could be correlated more with the ionic transport from the haemocoel, as already referred in other tissues of insects (Anderson & Harvey Citation1966; Berridge & Oschman Citation1969), rather than the re-absorption of macromolecules.
Ultrastructural analysis shows that the gland cells of the spermatheca, in the same specimen, can have different functional stages, above all if, on the one hand, we consider the gland cells of the connecting tract of the spermathecal duct (which are usually more active) and, on the other hand, the gland cells of the other duct tracts that do not show signs of any marked synthesis activity. However, this does not exclude the fact that in the latter tracts the gland cell activity, which is usually limited, can increase at specific functional moments (for example, when sperms are transported, etc.).
The general structural organization of the cuticle-forming cells is similar to that seen in other insects. Throughout the spermathecal tracts, the presence, in the apical region, of mitochondria that are grouped beneath numerous microvilli, suggests that these cells have a re-absorption role. Similar cytological aspects already seen in the epithelial cells of the spermatheca in Periplaneta americana (Gupta & Smith Citation1969), Tenebrio molitor (Happ & Happ Citation1975), Apis mellifera (Dallai Citation1975) and Melanoplus sanguinipes (Ahmed & Gillot Citation1982), seem to be characteristic of cells involved in the ionic transport process (Berridge & Oschman Citation1972). For example, in A. mellifera, between the cells of the spermathecal wall and the lumen of the organ, there is a different ionic concentration maintained by active transport (Gessner & Gessner Citation1976).
In the cuticle-forming cells, groups of microtubules, perpendicular to the surface of the organ, assure resistance to mechanical strain of the epithelium during the transit of the gametes. Moreover, in addition to the cytoskeletric system, this role may be carried out by numerous junction systems, such as cellular interdigitation and septate junctions in the lateral plasma membrane.
The cuticular intima of the spermatheca could carry out different functions: first of all, it constitutes of a type of ‘endoskeleton’ which supports the organ, keeping the lumen of the various tracts open; it could also have a function of ‘isolating’ the spermathecal epithelium from the secretions in the lumen, preventing cells coming into direct contact with them.
The last function of the intima is of particular importance in some cases; in the Acridoidea, for example, the lytic activity, carried out by the spermathecal secretions, is used for the demolition of the spermatophore, transiting in the spermatheca, and of the spermatozoa when, inside the seminal receptacle, they undergo a process of remanagement, with their partial or total demolition (Longo et al. Citation1993). However, in the Acridoidea examined, this lytic activity does not involve any direct participation of the wall cells of the organ, unlike what has been noted in the spermatheca of Rhacocleis annulata (Tettigoniidae), where the activity of lysis of the sperm occurs in the connecting tract of the spermathecal duct, in a progressive sequence of events primarily involving the cells of the wall (Viscuso et al. Citation1996a).
In the species here examined, the ultrastructural aspects of the connecting tract in the spermathecal duct show a secretory activity of the gland cells, which is more marked than in other tracts of the duct. In particular, in T. tessellata, the secretory activity of the cuticle-forming cells and the gland cells is accompanied by an evident cytoplasm vacuolization; even in S. sepium, peculiar aspects of lysis of the epithelium and the cuticular intima have been seen, largely similar to what has already been described for the same region in R. annulata (Viscuso et al. Citation1996a).
It is known that in the genital tracts of various insect species the high secretory activity of some cells is often accompanied by a cytoplasmic vacuolization (Hamon et al. Citation1982; Viscuso et al. Citation1985, 1996a, Citation1996b, Citation2005), which can precede the lysis of these cells (Riemann Citation1973; Viscuso et al. Citation1985). Nevertheless, when this lysis seems to involve different parts of the genital tract at the same time, it could indicate the end of the reproductive period of an individual. In the species examined, instead, vacuolization of the epithelial cells was constantly found in a specific region of the spermathecal duct (connecting tract) where, in some cases, as was stated before, also a lysis activity was found.
Taking this into consideration, the lack of evidence of lytic activity in the same tract of the other species examined, where the secretory activity is even more marked, could be related to the functional moment.
It is on the basis of this evidence and these considerations that we could hypothesize, as for R. annulata, that in the connecting tract of the duct, at least in some Tettigoniidae, there is a lysis activity of the epithelium aimed at the rapid capture and consequent lysis of the surplus of spermatozoa transferred by the male during copulation.
However, lysis could also be a mechanism making room for sperm from a new mating; in insects, which copulate repeatedly, in fact, the sperm from the most recent mating are biologically advantageous for egg fertilization (Walker Citation1980).
The above-mentioned hypothesis is also supported by the remark that in the Orthoptera Tettigoniidae there is no specific anatomical structure comparable to a fertilization chamber able to collect the spermatozoa that will fertilize the egg and from where they would be eventually removed before further mating, as, however, was found in other insects such as Diptera (Marchini et al. Citation2001). The anatomical situation of the Tettigoniidae could thus justify the presence of a specific tract of the spermathecal duct that is able to regulate spermatic material.
A lysis activity involving both epithelium and cuticular intima is already known in the genital tracts of other insects: in the spermatheca of the Apidae Melipona bicolor; indeed, this activity has been related to a spermiophagic function performed by some cells of the organ wall (Da Cruz-Landim Citation2002). A lysis activity of the epithelium which involves the cuticular intima has also been described in Isopoda Oniscidea: in the oviduct of Porcellio laevis, indeed, the epithelium and the cuticular intima undergo lysis phenomena due to a secretory activity of the oviduct glands; this lysis activity facilitates egg release from the ovary and sperm entrance into the seminal receptacle (De Luca et al. Citation1987; Longo et al. Citation1998).
Recent research has shown that, in the Tettigoniidae, the feather-shaped spermatodesms from the spermatophore with the male accessory gland secretions are sent to the seminal receptacle where they are stored inside the spermatodoses (Viscuso et al. Citation2002). These are small, sclerified capsules with a particular morphology depending on the species (Viscuso et al. Citation2002; Vahed Citation2003), which are found within the seminal receptacle, following and/or at the same time as the transfer of the male gametes.
Even if there is a lack of demonstrative data on the genesis of the spermatodoses, Vahed (Citation2003) has hypothesized that the secretion of the male accessory glands is responsible for its genesis. Although we agree with this hypothesis, nevertheless it is probable that the secretion from the spermathecal epithelium also contributes to the genesis of the spermatodoses.
In conclusion, from the results obtained it would seem reasonable to hypothesize that, in the examined species, the seminal receptacle and the spermathecal duct are different, from the functional point of view, despite showing similarities in their structural organization. Indeed, only the spermathecal duct and, in particular, the connecting tract would appear to control and regulate the gametes transferred by the male during copulation in order to confine fertilization activity exclusively to spermatozoa within the seminal receptacle.
Instead, the seminal receptacle would be predominantly responsible for the genesis of spermatodoses, for their storage and, at the same time, it could play a role in the release of single sperms from the feather-shaped spermatodesms, before they interact with the female gamete.
Acknowledgements
This research was supported by a MIUR grant, Scientific research programs, co-financing: ‘Structural and molecular studies on insect reproduction’.
References
- Ahmed , L and Gillot , C . 1982 . The spermatheca of Melanoplus sanguinipes (Fabr.). I. Morphology, histology and histochemistry . International Journal of Invertebrate Reproduction , 4 : 281 – 295 .
- Anderson , E and Harvey , WR . 1966 . Active transport by the Cecropia midgut. II. Fine structure of the midgut epithelium . The Journal of Cell Biology , 31 : 107 – 134 .
- Berridge , MJ and Oschman , JL . 1969 . A structural basis for fluid secretion by Malpighian tubules . Tissue and Cell , 1 : 247 – 272 .
- Berridge , MJ and Oschman , JL . 1972 . Transporting epithelia , New York, NY : Academic Press .
- Blum , MS , Glowska , Z and Taber , III S . 1962 . Chemistry of the drone honey bee reproductive system. II. Carbohydrates in the reproductive organs and semen . Annals of the Entomological Society of America , 55 : 135 – 139 .
- Chapman , RF . 1969 . The insect: Structure and function , London : The English Universities Press .
- Da Cruz-Landim , C . 2002 . Spermiophagy in the spermatheca of Melipona bicolor Lepeletier, 1836 (Hymenoptera, Apidae, Meliponini) . Anatomia, Histologia Embryologia , 31 : 339 – 343 .
- Dallai , R . 1975 . Fine structure of the spermathecal gland of Apis mellifera . Journal of Insect Physiology , 21 : 89 – 109 .
- De Luca , V , Longo , G , Sottile , L , La Spina , G and Viscuso , R . 1987 . Scanning electron microscopy and histochemistry of the reproductive female system and sperm storage in Porcellio laevis Latreille (Isopoda, Oniscoidea) . Acta Embryologiae et Morphologiae Experimentalis n.s. , 8 : 243 – 255 .
- Gessner , B and Gessner , K . 1976 . Inorganic ions in spermathecal fluid and their transport across the spermathecal membrane of the queen bee Apis mellifera . Journal of Insect Physiology , 22 : 1469 – 1474 .
- Grassè , PP . 1977 . Traitè de Zoologie, Tome VIII , 680 Paris, New York, Barcelone, Milan : Masson .
- Gupta , BL and Smith , DS . 1969 . Fine structural organization of the spermatheca in the cockroach Periplaneta americana . Tissue and Cell , 1 : 295 – 324 .
- Hamon , C , Biemont , JC and Chauvin , G . 1982 . Ultrastructure et fonction sécrétrice des cellules de la paroi des oviductes lateraux chez Acanthoscelides obtectus Say (Coleoptera: Bruchidae) . International Journal of Insect Morphology & Embryology , 2 : 327 – 339 .
- Happ , GM and Happ , CM . 1975 . Fine structure of the spermatheca of the mealworm beetle (Tenebrio molitor L.) . Cell and Tissue Research , 162 : 253 – 269 .
- Longo , G , Musmeci , R , Privitera , R and Sottile , L . 1998 . Ultrastructural organization of seminal receptacle and sperm storage in Porcellio laevis Latreille (Crustacea: Isopoda Oniscidea) . Tissue and Cell , 30 : 464 – 474 .
- Longo , G , Sottile , L , Viscuso , R , Giuffrida , A and Privitera , R . 1993 . Ultrastructural changes in sperm of Eyprepocnemis plorans (Charpentier) (Orthoptera: Acrididae) during storage of gametes in female genital tract . Invertebrate Reproduction and Development , 24 : 1 – 6 .
- Marchini , D , Del Bene , G , Falso , LF and Dallai , R . 2001 . Structural organization of the copulation site in the medfly Ceratitis capitata (Diptera: Tephritidae) and observations on sperm transfer and storage . Arthropod Structure and Development , 30 : 39 – 54 .
- Melis , G and Dallai , R . 1963 . Studio istochimico ed ultrastutturale delle vie genitali di un Ortottero Gryllacride . Redia , 48 : 193 – 216 .
- Melis , G and Dallai , R . 1966 . Istochimica ed ultrastruttura delle vie genitali femminili di Grillotalpa grillotalpa L . Redia , 50 : 163 – 186 .
- Noirot , C and Quennedey , A . 1974 . Fine structure of insect epidermal glands . Annual Review of Entomology , 19 : 61 – 80 .
- Quennedey , A . 1998 . “ Insect epidermal gland cells: Ultrastructure and morphogenesis ” . In Microscopic anatomy of invertebrates, insects , Edited by: Harrison , FW and Locke , M . Vol. 11A , 177 – 207 . London : Wiley-Liss .
- Reynolds , ES . 1963 . The use of lead citrate at high pH as an electron opaque stain in electron microscopy . The Journal of Cell Biology , 17 : 208 – 212 .
- Riemann , JG . 1973 . Ultrastructure of the ejaculatory duct region producing the male housefly accessory material . Journal of Insect Physiology , 19 : 213 – 223 .
- Sathe , AA and Joshi , PV . 1988 . Comparative study on the spermatheca of some Orthopteran insects . Cytologia , 53 : 347 – 352 .
- Smith , DS . 1968 . Insect cells: Their structure and function . Edinburgh: Oliver and Boyd. pp. , : 223 – 266 .
- Snodgrass , RE . 1937 . The male genitalia of Orthopteroid insects . Smithsonian Miscellaneous Collections , 96 : 1 – 107 .
- Vahed , K . 2003 . Structure of spermatodoses in shield-back bushcrickets (Tettigoniidae, Tettigoniinae) . Journal of Morphology , 257 : 45 – 52 .
- Viscuso , R , Barone , N , Sottile , L and Narcisi , L . 1996a . Spermiolytic activity of the epithelium of the spermathecal duct of Rhacocleis annulata Fieber (Orthoptera: Tettigoniidae) . International Journal of Insect Morphology and Embryology , 25 : 135 – 144 .
- Viscuso , R , Brundo , MV and Sottile , L . 2002 . Mode of transfer of spermatozoa in Orthoptera Tettigoniidae . Tissue and Cell , 34 : 337 – 348 .
- Viscuso , R , Brundo , MV and Sottile , L . 2005 . Ultrastructural organization of the seminal vesicles of Baculum thaii (Phasmida, Phasmatidae) during sexual maturity . Italian Journal of Zoology , 72 : 113 – 119 .
- Viscuso , R , Longo , G and Sottile , L . 1985 . Ultrastructural modifications in the ejaculatory duct epithelium of Eyprepocnemis plorans (Charp.) (Orthoptera : Acrididae) during sexual maturation . International Journal of Insect Morphology and Embryology , 14 : 163 – 177 .
- Viscuso , R , Sottile , L and Narcisi , L . 1994 . Ultrastructural characteristics of the epithelium of the spermatheca and copulatory bursa of Baculum thaii Haus (Phasmatodea) . European Archives of Biology , 105 : 19 – 31 .
- Viscuso , R , Sottile , L and Narcisi , L . 1996b . Ultrastructural and histochemical characteristics of the lateral oviducts of Baculum thaii Haus. (Phasm.) . European Journal of Morphology , 34 : 271 – 283 .
- Walker , WF . 1980 . Sperm utilization strategies in non-social insects . The American Naturalist , 115 : 780 – 799 .