Abstract
Mild stresses are known to retard progressive decline in survival with age. The process wherein mild stresses exhibit a beneficial role is termed hormesis. The two mild stresses which are gaining interest in the present scenario of aging research are repeated mild heat shock (RMHS) and histone deacetylase inhibitors (HDACi). Our interest was to know how the unique laboratory-evolved short- and long-lived cytoraces of nasuta-albomicans complex of Drosophila would respond to these stresses. Differential response by these cytoraces to RMHS and HDACi was observed. The lifespan of short-lived cytoraces, SL-1 and SL-2, extended more remarkably than other races in response to both RMHS and HDACi, whereas two of the long-lived cytoraces, LL-1 and LL-2, have not shown significant response to HDACi, even though they showed mild response to RMHS. The LL-3 and LL-4 cytoraces have not behaved similarly for all the three hormesis treatments as LL-1 and LL-2 cytoraces. These findings specify that there is a race-specific response developed in each system, and the reason we predict for such plasticity would be their genetic background. These cytoraces which evolved through hybridization have unique genome introgression and recombination, through which they might have acquired a race specific aging pathways, which in turn, played a crucial role in their differential response to stresses.
Introduction
Interracial hybridization is considered to be an important facet in speciation. Such interracial hybridization was carried out in our laboratory between Drosophila nasuta nasuta (coorg), 2n = 8 and Drosophila nasuta albomicans (Okinawa), 2n= 6, which are morphologically identical, cross-fertile and karyotypically dissimilar chromosomal races belonging to the nasuta subgroup of the immigrans group of Drosophila (Wilson et al. Citation1969; Kitagawa et al. Citation1982). They were isolated from each other by more than 3000 miles and were allopatrically distributed, and in nature, no hybrids were found. The interracial hybridization between them resulted in the formation of hybrids with ‘karyotypic mosaicism’, which declined over generations, and during this process there was selective elimination of some of the parental chromosomes, while some of the parental chromosomes were retained. Finally, at around F20 generations, the karyotypic variability disappeared and four new strains of karyotypically stabilized hybrid populations evolved namely, cytoraces 1, 2, 3 and 4 (Ramachandra & Ranganath Citation1986, 1990). Further interracial hybridization with different combinations was carried among these four newly evolved cytoraces along with their parental races, which resulted in the creation of 12 more karyotypically stabilized new cytoraces, namely cytoraces 5–16 (Ramachandra & Ranganath Citation1996). Each of these cytoraces is composed of recombined genomes of the parental races, contain chromosomes of both D. n. nasuta and D. n. albomicans and differ in their karyotypic composition. These 16 newly created cytoraces along with their parental races constitute a new assemblage, the ‘nasuta-albomicans complex (NAC) of Drosophila’ (Ramachandra & Ranganath Citation1996). As this complex inhabited the same area, and more importantly a common set of environmental conditions, Tanuja et al. (Citation1998) had suggested a new terminology called ‘allo-sympatric’ and artificial hybrid zone in the environs of the laboratory. Since their evolution, the NAC of Drosophila have been subjected to different assessments such as fitness, morphophenotypes (Harini & Ramachandra Citation2000, Citation2003), isozymes (Aruna & Ranganath Citation2004), RAPD (Random Amplified Polymorphic DNA) and ISSRs (Inter Simple Sequence Repeat) (Nagaraja et al. Citation2004) to understand raciation.
Figure 1. Mean lifespan ± S.E of control, RMHS, BuA and TSA treated males (a) and females (b) of all the eight members of nasuta-albomicans complex of Drosophila under study.
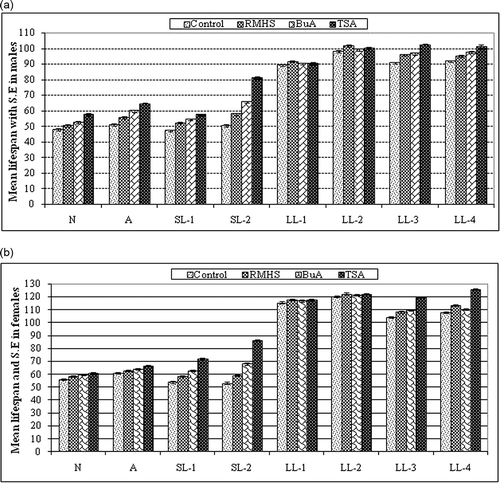
Figure 2. Survivorship (lx) of control and RMHS treated males (a) and females (b) of all the members of nasuta-albomicans complex of Drosophila. All the eight races with initial C indicate control and without C is RMHS treated.
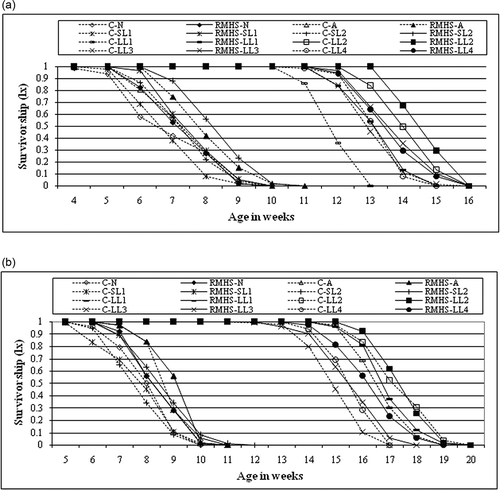
Figure 3. Survivorship (lx) of control and BuA treated males (a) and females (b) of all the members of nasuta-albomicans complex of Drosophila. All the eight races with initial C indicate control and without C is BuA treated.
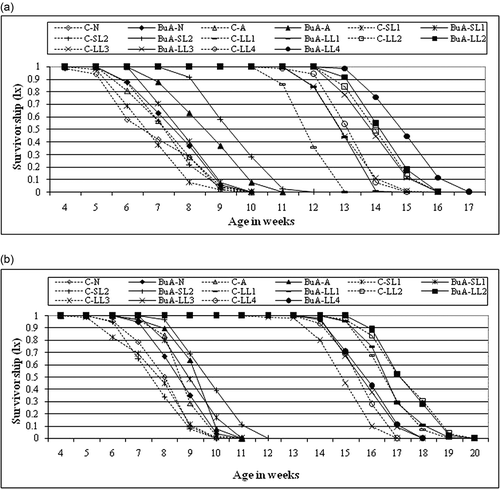
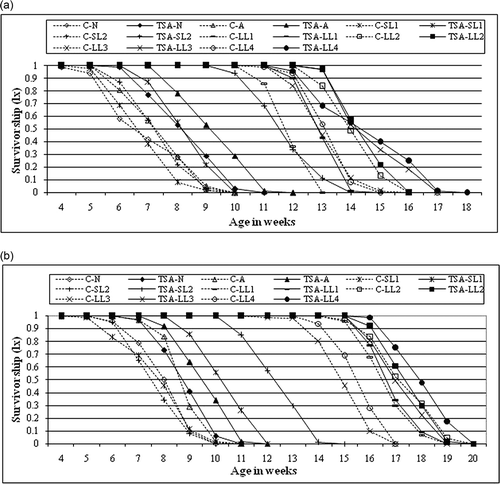
Figure 4. Survivorship (lx) of control and TSA treated males (a) and females (b) of all the members of nasuta-albomicans complex of Drosophila. All the eight races with initial C indicate control and without C is TSA treated.
The assessment of lifespan in the members of NAC of Drosophila was carried when they were passing through 650 generations and differences observed among them. Out of 16 cytoraces, cytoraces 3 and 15 lived a shorter lifespan than the others, whereas four cytoraces – cytorace 2, cytorace 9, cytorace 11 and cytorace 16 – showed significantly longer lifespans than other cytoraces (Ranjini & Ramachandra Citation2009). This was again confirmed by two more replicate lifespan assessments and crossing experiments between two short- and two long-lived cytoraces. Thus, as they share a unique lifespan of being short- and long-lived, they remain a potent system to understand the different dynamics of aging with evolutionary and genetic background.
Aging can be defined as a gradual process in which accumulation of molecular damage occurs, causing detrimental changes in the homeostasis of the body. Approaches such as environmental stresses that modulate aging are gaining interest. Hormesis is one such mechanism wherein stressors improve the functional ability of organisms at low doses and modulate aging. Hormetic effects are widely used in aging research and so far studied model organisms are D. melanogaster, Caenorhabditis elegans and rodents (Le Bourg Citation2009). Mild stress is being treated as a modulator of aging and longevity by exposing individuals to one or more short-term, sub-lethal treatments (Masoro Citation2000; Minois Citation2000; Cypser & Johnson Citation2003; Hercus et al. Citation2003; Rattan Citation2004). Stresses that have been reported to delay aging and prolong longevity include exercise (Holloszy et al. Citation1985), temperature shock, irradiation like UV-, gamma-, and X-rays (Calabrese & Baldwin Citation2000), heavy metals, pro-oxidants (Brown et al. Citation2006), cold stress (Le Bourg Citation2007), acetaldehyde, alcohols, hypergravity (Le Bourg et al. Citation2000; Le Bourg 2009), and calorie restriction/dietary restriction (Mair et al. Citation2005; Partridge et al. Citation2005; Ranjini & Ramachandra Citation2009). Other than calorie restriction, the two mild stresses which had reported to extend the lifespan of D. melanogaster are repeated mild heat shock (RMHS) and histone deacetylase inhibitors (HDACi) (Hercus et al. Citation2003; Zhao et al. Citation2005). Maynard Smith (Citation1958) was one of the first to report that an exposure to heat stress for a short period increases the lifespan of female D. subobscura. Zhao et al. (Citation2005) observed increased lifespan of isofemale lines of D. melanogaster in response to repeated mild heat shock. The effect of mild heat shock (37°C for 1 h at 4 days of age) on longevity was also studied by Scannapieco et al. (Citation2007) in two sibling species of Drosophila, D. buzzatii and D. koepferae, and reported that D. buzzatii was longer-lived than D. koepferae. The repeated mild treatments will continue to induce a beneficial response by upregulating stress-induced gene expression which in turn enhances longevity, whereas treatments only late in life will not induce beneficial response (Khazaeli et al. Citation1997; Le Bourg et al. Citation2000; Hercus et al. Citation2003; Olsen et al. Citation2006).
It is important to investigate the genetic and physiological changes in animals brought upon by alteration in the level of histone acetylation, which can be studied using HDACi. These are known to enhance the acetylation resulting in increased recruitment of DNA-binding transcription factors, which in turn leads to increase in the expression of particular genes and also decreases in the expression of other downstream genes (Marks et al. Citation2001; Richon & O'Brien Citation2002). HDACi have received attention as potential therapeutic drugs for several diseases (Kramer et al. Citation2001; Marks et al. Citation2001). The general effect of HDACi such as cytotoxicity, differentiation, inhibition of proliferation, induction of apoptosis are often seen in cell lines, and also in extending the lifespan of Drosophila (Chang & Min Citation2002; de Ruijter et al. Citation2003; Ovakin & Heikkila Citation2003; Zhao et al. Citation2005). The influence of RMHS, HDACi–Trichostatin A (TSA) and sodium butyrate (BuA) on the lifespan of short- and long-lived isofemale lines of D. melanogaster was studied by Zhao et al. (Citation2005). They reported that short-lived lines were influenced by all three treatments and showed extended lifespan, whereas no obvious effect was observed in the lifespan of long-lived lines with BuA treatment. However, the lifespan of long-lived lines increased with RMHS and continuous TSA treatment, but the percent increase was low compared to short-lived lines. This study was taken as base to study the degrees of variation in the influence of RMHS and two HDACi on the lifespan of our unique laboratory-evolved short- and long-lived cytoraces. Due to their stabilized karyotype, these cytoraces have their own value in evolutionary studies, as it would have taken millions of years to evolve such unique cytoraces in nature which have taken only a few generations to produce them in the environs of laboratory.
Our aim revolved around the following questions: Do our laboratory-evolved karyotypically stabilized short- and long-lived cytoraces show similar responses to the RMHS and HDACi as those of the studies of Zhao et al. (Citation2005) on isofemale lines of D. melanogaster in extending their lifespan? Have these cytoraces, which are passing through 650–700 generations of raciation, acquired different qualities which would be support for their speciation? What could be considered as a more evolutionary relevant stress scenario that could lead to a change in the lifespan of both short- and long-lived cytoraces?
Materials and methods
Fly stocks
| 1. | Drosophila nasuta nasuta (N) (Coorg, India), | ||||
| 2. | Drosophila nasuta albomicans (A) (Okinawa strain, Texas collection, USA, 3045.11), | ||||
| 3. | Cytorace 2 (Long-lived: LL-1) (Ramachandra & Ranganath Citation1986), | ||||
| 4. | Cytorace 3 (Short-lived: SL-1) (Ramachandra & Ranganath Citation1990), | ||||
| 5. | Cytorace 9 (Long-lived: LL-2) (Ramachandra & Ranganath Citation1996), | ||||
| 6. | Cytorace 11 (Long-lived: LL-3) (Ramachandra & Ranganath Citation1996), | ||||
| 7. | Cytorace 15 (Short-lived: SL-2) (Ramachandra & Ranganath Citation1996), and | ||||
| 8. | Cytorace 16 (Long-lived: LL-4) (Ramachandra & Ranganath Citation1996). | ||||
All fly stocks were maintained separately in half-pint bottles with wheat cream agar medium and yeast grains at 22 ± 1°C with ∼70% humidity and 12D:12L cycle. For each experiment, eggs of approximately the same age (± 4 h) of the above-mentioned eight members of NAC of Drosophila were collected using the modified procedure of Delcour (Ranganath & Krishnamurthy Citation1974). Eggs were collected on acetic acid–alcohol media, and with sterilized micro needles, transferred to half-pint bottles, each containing the same amount of wheat cream agar medium seeded with yeast. Each half-pint bottle contained 100 eggs of a single race and 5 replicates were maintained separately for each of the race for the following three experiments. To perform these experiments, the protocol of Zhao et al. (Citation2005) was employed with slight modifications.
Experiment on repeated mild heat shock (RMHS)
For this experiment, from five replicate bottles, the unmated males and virgin females of each of the eight races were collected separately and 10 flies of each sex were transferred to a standard culture media vial (25 mm × 76 mm). After 3 days, these flies were separately exposed to 32°C for 1 h and the treatment was continued once every 3 days until all the flies died. Simultaneously, the lifespan of each fly was recorded once every 3 days. In total, 10 replicate assessments were made for both males and females separately. The control experiment was also carried with same number of replicates and was maintained at 22 ± 1°C.
Experiment on histone deacetylase inhibitors (HDACi)
Two HDACi, sodium butyrate (BuA) and Trichostatin A (TSA), were used in the present study. BuA belongs to a short-chain fatty acid derived from bacterial metabolism of dietary fiber in the colon (de Ruijter et al. Citation2003; Rahman et al. Citation2003). For the BuA experiment, from five of the above-mentioned half-pint bottles, ∼50 third-instar larvae were transferred to Petri dishes along with the media and treated with physiological saline (130 mM NaCl, 4.7 mM KCl and 1.9 mM CaCl2) and 10 mM BuA for 5 h and then transferred back to a fresh half-pint culture media bottle. The eclosed unmated males and virgin female flies were then collected separately and transferred to a standard culture media vial (25 mm × 76 mm). After 3 days, 10 unmated males and virgin females were transferred to a fresh culture media vial and provided with 10 mM of BuA. Once every two days, flies were transferred to a fresh culture media vial provided with 10 mM of BuA and the lifespan recorded until all the flies died. Ten such replicate assessments were carried out for males and females separately. The control experiment was also carried out without BuA treatment with same number of replicates.
TSA is a fermentation product of Streptomyces. It belongs to hydroxamic acids. The ability of TSA to inhibit HDAC was reported for the first time by Yoshida et al. (Citation1990). The TSA experiment was conducted similarly as BuA except replacing BuA with TSA with 10 μM concentration.
Statistical analysis
Kaplan Meier analysis was used to compare the survival of control flies with treated flies of eight races. Log-rank test was used to compare the survival distribution of control flies with treated flies. The hazard ratio is an estimate of the ratio of the hazard rate in the treated versus the control group (Spruance et al. Citation2004), which indicates the time to the endpoint reduced by treatment. The survival curves show age on the x-axis and the portion of all individuals surviving on the y‐axis. Both tests were done using MedCalc software (version 10.4.3; http://www.medcalc.be/). Survivorship (lx ) is a measure of the proportion of individuals which survive to the beginning of age category x. It was estimated as lx = nx /n 0, where nx is the number of individuals in the study population which survive to the beginning of age category x, and n 0 = N, the total population size. This was measured in both control and treated flies of all the eight races under study.
Bonferroni correction is a statistical method adapted to reduce the error rate in the multiple comparisons of given data (Rice Citation1989). One-way ANOVA was applied between sex, races, interaction and treatments by using SPSS version 10.0. Applying Bonferroni correction, the level of significance ‘p’ was fixed at 0.0125.
Results
RMHS effect on lifespan
RMHS increased the survivability of flies with different degrees in the eight members of the nasuta-albomicans complex of Drosophila. Mean lifespan of males increased slightly, by 2.5% in LL-1, 3.4% in LL-2, 5.6% in LL-3, 3.7% in LL-4, 5.2% in D. n. nasuta, 8.8% in D. n. albomicans, and moderately, by 10.3% in SL-1 and 14% in SL-2 (). In females, the extension of lifespan was 2%, 1.5%, 3.7%, 4.8%, 8.6%, 11.8%, 4% and 2.9% in LL-1, LL-2, LL-3, LL-4, SL-1, SL-2, D. n. nasuta and D. n. albomicans, respectively (b). In both males and females, the survivability was higher in SL-2 than any other races (a, b). Log-rank test revealed that RMHS increased the lifespan of all races significantly except males of D. n. nasuta and females of LL-2. Hazard ratio and 95% confidence interval (CI) were comparatively higher and lower in males of SL-2 and females of D. n. nasuta, respectively, than any other races (). In both control () and RMHS treatment (), males and females of LL-2 were considered the longest survived race with the percent of flies surviving up to the 15th and 19th weeks, respectively, whereas both males and females of D. n. nasuta showed the lowest survivability compared to other races (a, b).
Table I. Comparison of lifespan of control flies with repeated mild heat shock (RMHS)-treated laboratory-evolved flies of Drosophila by log-rank test, where n = 100 and df = 1
Table II. Distribution of percent survivability in control males and females of all the eight members of the nasuta-albomican s complex of Drosophila.
Table III. Distribution of percent survivability in repeated mild heat shock (RMHS)-treated males and females of all eight members of the nasuta-albomicans complex of Drosophila.
One-way ANOVA for RMHS treatment among the races revealed the following:
| a. | D. n. nasuta showed significant differences with all the races except with males and females of SL-1 and females of SL-2; | ||||
| b. | D. n. albomicans showed significant differences with all other races except males of SL-1 and males and females of SL-2; | ||||
| c. | LL-1 as well as LL-3 showed nonsignificant differences with males of LL-4; | ||||
| d. | SL-1 showed nonsignificant differences with females of SL-2. The analysis between the sexes in each race for RMHS treatment revealed significant differences in all races except SL-2. | ||||
HDACi BuA effect on lifespan
BuA increased the survivability in SL-2 more than any other races when compared to control. However, BuA slightly increased the lifespan of males and females of LL-3 (7.2%, 5.16%) and LL-4 (6.4%, 2%). The effect on lifespan was moderate in D. n. nasuta by 9.5% in males and 6.8% in females, and in D. n. albomicans by 17% in males and 5.3% in females. This effect was striking in both males and females of short-lived cytoraces SL-1 and SL-2, with 14.7%, 16.9% and 29.7%, 28%, respectively (a, b). Log-rank test indicated that BuA significantly increased the lifespan of D. n. nasuta, D. n. albomicans, SL-1, SL-2 LL-3 and LL-4; however, it did not increase the lifespan of LL-1 and LL-2. Hazard ratio and 95% CI were higher in females of SL-2 and lower in females of LL-2 than any other races (). In BuA treatment (), males of LL-4 and females of LL-2 were considered the longest survived races, with the highest percentage of flies surviving up to the 16th and 19th weeks, respectively. Both males and females of D. n. nasuta showed the lowest survivability compared to other races (a, b).
Table IV. Comparison of lifespan of control flies with sodium butyrate (BuA)-treated laboratory-evolved flies of Drosophila by log-rank test where n= 100 and df = 1
Table V. Distribution of percent survivability in sodium butyrate (BuA)-treated males and females of all eight members of the nasuta-albomicans complex of Drosophila.
One-way ANOVA BuA among the races revealed that all comparisons showed significant differences, except males of D. n. nasuta vs. SL-1, LL-2 vs. LL-3, LL-2 vs. LL-4, and LL-3 vs. LL-4. In females, nonsignificant differences were observed between D. n. nasuta and SL-1, D. n. albomicans and SL-1, and LL-3 and LL-4. The analysis between the sexes in each race for BuA treatment revealed significant differences in all races except SL-2.
HDACi TSA effect on lifespan
TSA increased the mean lifespan of two long-lived cytoraces, LL-3 (males 12.6%, females 14.6%) and LL-4 (males 10.57%, females 16.1). A remarkable increase in the lifespan was recorded in short-lived cytoraces, SL-1 (males 21.25%, females 33%) and SL-2 (males 60.4%, females 62.5%). TSA treatment also increased the lifespan of D. n. nasuta (males 19.63%, females 9.0%) and D. n. albomicans (males 26.4%, females 8.8%) considerably (a, b). TSA was not able to significantly increase the lifespan of LL-1 and LL-2 compared to D. n. nasuta, D. n. albomicans, SL-1, SL-2 and LL-3 LL-4. Hazard ratio and 95% CI were higher in males of SL-2 and lower in females of LL-2 than any other races (). Among all races, the percent survivability was higher in SL-2 than control in both males and females. ANOVA revealed nonsignificant difference in the survivability of LL-1 and LL-2 when compared with control. Both males and females of LL-4 () were considered to be the longest-surviving race with the highest percentage of flies surviving up to the 17th and 19th weeks, respectively. Both males of SL-1 and females of D. n. nasuta showed the lowest survivability (a, b). One-way ANOVA for TSA treatment among the races revealed that all comparisons showed significant differences, except males and females of D. n. nasuta vs. SL-1, LL-2 vs. LL-3, LL-2 vs. LL-4, and LL-3 vs. LL-4. The analysis between the sexes showed significant differences in all other races except D. n. albomicans.
Table VI. Comparison of lifespan of control flies with Trichostatin A (TSA)-treated laboratory-evolved flies of Drosophila by log-rank test where n=100 and df=1
Table VII. Distribution of percent survivability in Trichostatin A (TSA)-treated males and females of all eight members of the nasuta-albomicans complex of Drosophila.
One-way ANOVA among all the above three treatments in each sex of each race separately revealed the following. (a) Both males and females of SL-2, only males of D. n. albomicans, and females of SL-1 showed significant differences among all the three treatments. (b) In both males and females of LL-2 and only in females of LL-1, nonsignificant differences were observed among all the three treatments. (c) In males of D. n. nasuta and SL-1, and in both males and females of LL-3 and LL-4, significant differences were observed between treatments RMHS and TSA as well as BuA and TSA. (d) In D. n. nasuta and D. n. albomicans significant differences were observed between RMHS- and TSA-treated females.
Discussion
Evolution of short- and long-lived cytoraces in the environs of laboratory is considered to be one of the key reports in the hybridization experiment in Drosophila (Ranjini & Ramachandra Citation2009). These karyotypically stabilized cytoraces have always been the favorite system for studying the dynamics of aging, as they share differential lifespan. Earlier reports on validation of short- and long-lived cytoraces by crossing experiments confirmed their evolution and thus established a potent experimental model to study longevity (Ranjini & Ramachandra Citation2009). Ever since their evolution, they are subjected to various assessments including different mild stresses (Le Bourg et al. Citation2009), which are known to extend lifespan in different organisms. One such well-known mild stress, dietary restriction, showed remarkable differences in SL-1, SL-2, LL-1, LL-2, LL-3 and LL-4 cytoraces (Ranjini & Ramachandra Citation2009). Significant extension in the dietary-restricted lifespan of short-lived cytoraces SL-1 and SL-2 has been observed when compared to standard diet, whereas the long-lived cytoraces LL-1, LL-2, LL-3 and LL-4 which lived longer than any other races in the standard diet did not extend their lifespan significantly (Ranjini & Ramachandra Citation2009). This has encouraged further studies using other stressors such as RMHS and HDACi, which are well studied in extending lifespan in isofemale lines of D. melanogaster (Zhao et al. Citation2005).
Hormesis is known to increase the functional ability of an organism at low doses (Calabrese Citation2008a). Sacher (Citation1977) opined that hormetic effects are unlikely to occur in the healthy active individual, and are more likely to be significant in the ill or depressed animal. In contrast to this, Minois (Citation2000) stated that mild stressors have beneficial effects on longevity and stress resistance. Later, it was extensively studied and definitely shown that hormetic effects are not observed only, or mainly, in ill or depressed animals, but rather in animals experiencing living conditions promoting high longevity (Le Bourg et al. Citation2000). In the present study, D. n. nasuta, D. n. albomicans, SL-1 and SL-2 have shown greater response to the hormetic effects with extended lifespan than the other races. Two of the long-lived cytoraces, LL-3 and LL-4, have also responded positively. Interestingly, the other two long-lived cytoraces, LL-1 and LL-2, even being considered long-lived have not shown positive response to hormetic effect.
Calabrese (Citation2008b) reported the dose–response relationships with hormesis, and stated that the maximum stimulatory response generally was only approximately 30–60% greater than the control value. Zhao et al. (Citation2005) have reported that the mean lifespan of short-lived isofemale lines extended to 25.8% by RMHS and BuA, and 24.4% by TSA treatment and in long-lived lines to 11.5% by RMHS, 15.6% by TSA and no obvious effect by BuA. In our study, the SL-1 and SL-2 cytoraces extended mean lifespan by 8.6–14% in RMHS, 14.7–29.7% in BuA and 21.2–62.5% in TSA treatment, whereas the LL-1 and LL-2 cytoraces extended mean lifespan by 1.5–3.4% in RMHS, 0.9–1% in BuA and 1.3–1.9% in TSA treatment. Other races extended their lifespan in between the above two groups of cytoraces.
Zhao et al. (Citation2005) also reported nonsignificant differences among treatments. In contrast to this, in a few of the cytoraces we observed significant differences among treatments at different combinations; to highlight, SL-2 in particular has shown significant differences among treatments in males and females. The SL-1 and SL-2 cytoraces responded greatly in extending their lifespan in all the above three treatments and more prominently in TSA. In our earlier report, interestingly, in response to dietary restriction SL-1 and SL-2 behaved similarly by extending their lifespan significantly and all the four long-lived cytoraces LL-1, LL-2, LL-3 and LL-4 also behaved similarly by not extending their lifespan, while in the present study, long-lived cytoraces have not responded similarly to RMHS and HDACi, wherein, LL-3 and LL-4 response is different from LL-1 and LL-2 response to these treatments. This implies that there is plasticity in the racial response to different stresses.
Baack and Rieseberg (Citation2007) reported in plants the impact of hybridization on genomic stability, which can result in genomic changes including alterations to gene expression, chromosomal structure and genome size. We opine that differential response of our short- and long-lived cytoraces for RMHS and HDACi is due to their strong genetic background. These short- and long-lived cytoraces are introgressed genomes and have undergone different degrees of recombination that have influenced evolutionary trajectory of differential response to RMHS and HDACi. Here, one could surmise that these inhibitors might have rescued the deacetylated complexes, which are carried in the SL-1 and SL-2 cytoraces. Through recombination, these karyotypically stabilized introgressed cytoraces might have acquired a new haplotype blocks, which in turn lead to the establishment of ‘private/specific’ gerontogenic pathway for different stresses. Thus, the laboratory-evolved short- and long-lived cytoraces with their unique qualities becomes an important model to understand the complexity of aging.
Acknowledgements
The authors wish to thank Prof. H. A. Ranganath for his help, encouragement, and support; the Chairman of our department for providing facilities and also Dr. Mahesh G. for his consistent help in providing related papers which were not accessible for us. This work was supported by DST, Government of India.
References
- Aruna , S and Ranganath , HA . 2004 . Isozymes and genetic divergence in the nasuta-albomicans complex of Drosophila . Current Science , 86 : 1017 – 1023 .
- Baack , EJ and Rieseberg , LH . 2007 . A genomic view of introgression and hybrid speciation . Current Opinion in Genetics and Development , 17 : 513 – 518 .
- Brown , MK , Evans , JL and Luo , Y . 2006 . Beneficial effects of natural antioxidants EGCG and α±-lipoic acid on life span and age-dependent behavioral declines in Caenorhabditis elegans . Pharmacology Biochemistry and Behavior , 85 : 620 – 628 .
- Calabrese , EJ . 2008a . Converging concepts: Adaptive response, preconditioning, and the Yerkes–Dodson law are manifestations of hormesis . Ageing Research Review , 7 : 8 – 20 .
- Calabrese , EJ . 2008b . Hormesis: Why it is important to toxicology and toxicologists . Environmental Toxicology and Chemistry , 27 : 1451 – 1474 .
- Calabrese , EJ and Baldwin , LA . 2000 . The effects of gamma rays on longevity . Biogerontology , 1 : 309 – 319 .
- Chang , KT and Min , KT . 2002 . Regulation of lifespan by histone deacetylase . Ageing Research Reviews , 1 : 313 – 326 .
- Cypser , JR and Johnson , TE . 2003 . Hormesis in Caenorhabditis elegans dauer-defective mutants . Biogerontology , 4 : 203 – 214 .
- de Ruijter , AJM , van Gennip , AH , Caron , HN , Kemp , S and van Kuilenburg , ABP . 2003 . Histone deacetylases (HDACs): Characterization of the classical HDAC family . Biochemical Journal , 370 : 737 – 749 .
- Harini , BP and Ramachandra , NB . 2000 . Racial divergence in abdominal bristles among the parental races and the newly evolved cytoraces of nasuta-albomicans complex of Drosophila . Indian Journal of Experimental Biology , 38 : 1263 – 1266 .
- Harini , BP and Ramachandra , NB . 2003 . Evolutionary experimentation through hybridization under laboratory condition in Drosophila: Evidence for recombinational speciation . BMC Evolutionary Biology , 3 : 1 – 19 .
- Hercus , MJ , Loeschcke , V and Rattan , SIS . 2003 . Lifespan extensión of Drosophila melanogaster through hormesis by repeated mild heat stress . Biogerontology , 4 : 149 – 156 .
- Holloszy , JO , Smith , EK , Vining , M and Adams , S . 1985 . Effect of voluntary exercise on longevity of rats . Journal of Applied Physiology , 59 : 826 – 831 .
- Khazaeli , AA , Tatar , M , Pletcher , SD and Curtsinger , JW . 1997 . Heat-induced longevity extension in Drosophila. I. Heat treatment, mortality, and thermotolerance . Journal of Gerontology Series A. Biological Sciences and Medical Sciences , 52 : B48 – B52 .
- Kitagawa , O , Wakahama , K , Fuyama , Y , Shimada , Y and Takanashi , E . 1982 . Genetic studies of the Drosophila nasuta subgroup, with notes on distribution and morphology . Japanese Journal of Genetics , 57 : 113 – 141 .
- Kramer , OH , Gottlicher , M and Heinzel , T . 2001 . Histone deacetylase as a therapeutic target . Trends in Endocrinology and Metabolism , 12 : 294 – 300 .
- Le Bourg , E . 2007 . Positive effects of exposure to cold at young age on longevity, aging and resistance to heat or cold shocks in Drosophila melanogaster . Biogerontology , 8 : 431 – 444 .
- Le Bourg , E . 2009 . Hormesis, aging and longevity . Biochimica et Biophysica Acta , 1790 : 1030 – 1039 .
- Le Bourg , E , Minois , N , Bullens , P and Baret , P . 2000 . A mild stress due to hypergravity exposure at young age increases longevity in Drosophila melanogaster males . Biogerontology , 1 : 145 – 155 .
- Mair , W , Piper , MDW and Partridge , L . 2005 . Calories do not explain extension of life span by dietary restriction in Drosophila . PLoS Biology , 3 : 1305 – 1311 .
- Marks , P , Rifkind , RA , Richon , VM , Breslow , R , Miller , T and Kelly , WK . 2001 . Histone deacetylases and cancer: Causes and therapies . Nature Reviews Cancer , 1 : 194 – 202 .
- Masoro , EJ . 2000 . Hormesis is the beneficial action resulting from the response of an organism to a low-intensity stressor . Human Experimental Toxicology , 19 : 340 – 341 .
- Maynard Smith , J . 1958 . The effects of temperature and of egg-laying on the longevity of Drosophila subobscura . Journal of Experimental Biology , 35 : 832 – 842 .
- Minois , N . 2000 . Longevity and aging: beneficial effects of exposure to mild stress . Biogerontology , 1 : 15 – 29 .
- Nagaraja , Nagaraju , J and Ranganath , HA . 2004 . Molecular phylogeny of the nasuta subgroup of Drosophila based on 12S rRNA, 16S rRNA and CoI mitochondrial genes, RAPD and ISSR polymorphisms . Genes and Genetic Systems , 79 : 293 – 299 .
- Olsen , A , Vantipalli , MC and Lithgow , GJ . 2006 . Lifespan extension of Caenorhabditis elegans following repeated mild hormetic heat treatments . Biogerontology , 7 : 221 – 230 .
- Ovakin , DH and Heikkila , JJ . 2003 . Effect of histone deacetylase inhibitors on heat shock protein gene expression during xenopus development . Genetics , 36 : 88 – 96 .
- Partridge , L , Piper , MDW and Mair , W . 2005 . Dietary restriction in Drosophila . Mechanisms of Aging and Development , 126 : 938 – 950 .
- Rahman , M , Kukita , A , Kukita , T , Shobuike , T , Nakamura , T and Kohashi , O . 2003 . Two histone deacetylase inhibitors, trichostatinA and sodium butyrate, suppress differentiation into osteoclasts but not into macrophages . Blood , 101 : 3451 – 3459 .
- Ramachandra , NB and Ranganath , HA . 1986 . The chromosomes of two races: Drosophila nasuta nasuta and Drosophila albomicana: IV. Hybridization karyotype repatterning . Chromosoma , 93 : 243 – 248 .
- Ramachandra , NB and Ranganath , HA . 1990 . The chromosomes of two Drosophila races: Drosophila nasuta nasuta and Drosophila nasuta albomicana: V. Introgression and the evolution of new karyotypes . Zeitschrift Fur Zoologische Systematik und Evolutionsforschung , 28 : 62 – 68 .
- Ramachandra , NB and Ranganath , HA . 1996 . Evolution of nasuta-albomicans complex of Drosophila . Current Science , 71 : 515 – 517 .
- Ranganath , HA and Krishnamurthy , NB . 1974 . Developmental studies in Drosophila nasuta. III. Developmental obstinacy of the eggs laid after starvation . Experientia , 30 : 312 – 313 .
- Ranjini , MS and Ramachandra , NB . 2009 . Evolution of short-lived and long-lived races of Drosophila in the environs of laboratory . Indian Journal of Gerontology , 23 : 381 – 398 .
- Rattan , SIS . 2004 . Aging, anti-aging, and hormesis . Mechanism of Aging Development , 125 : 285 – 289 .
- Rice , WR . 1989 . Analyzing table of statistical tests . Evolution , 43 : 223 – 225 .
- Richon , VM and O'Brien , JP . 2002 . Histone deacetylase inhibitors: A new class of potential therapeutic agents for cancer treatment . Clinical Cancer Research , 8 : 662 – 664 .
- Sacher , GA . 1977 . “ Life table modification and life prolongation ” . In Handbook of the biology of aging , Edited by: Finch , CE and Hayflick , L . 582 – 638 . New York, NY, Van Nostrand : Reinhold Company .
- Scannapieco , AC , Sorensen , JG , Loeschcke , V and Norry , FM . 2007 . Heat-induced hormesis in longevity of two sibling Drosophila species . Biogerontology , 8 : 315 – 325 .
- Spruance , SL , Reid , JE , Grace , M and Samore , M . 2004 . Hazard ratio in clinical traits . Antimicrobial Agents and Chemotherapy , 48 : 2787 – 2792 .
- Tanuja , MT , Ramachandra , NB and Ranganath , HA . 1998 . Creation of a hybrid zone in Drosophila with ‘allo-sympatric’ races . Current Science , 75 : 1116 – 1117 .
- Wilson , FD , Wheeler , MR , Harget , M and Kambysellis , M . 1969 . Cytogenetic relations in the Drosophila nasuta subgroup of species . University of Texas Publications , 6918 : 207 – 254 .
- Yoshida , M , Kijima , M , Akita , M and Beppu , T . 1990 . Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin . Journal of Biological Chemistry , 265 ( 17 ) : 174 – 17 . 179
- Zhao , Y , Sun , H , Lu , J , Li , X , Chen , X , Tao , D , Huang , W and Huang , B . 2005 . Lifespan extension and elevated hsp gene expression in Drosophila caused by histone deacetylase inhibitors . Journal of Experimental Biology , 208 : 697 – 705 .