Abstract
Microvasculature, anatomy and histomorphology of the gallbladder of the adult pipid frog, Xenopus laevis (Daudin) were studied by light (LM) and scanning electron microscopy (SEM) of tissue preparations and vascular corrosion casts. The thin wall of the gallbladder consisted of columnar epithelium, lamina propria, a fibromuscular layer, subserosa and serosa. A few diverticula but no epithelial folds were present. Up to five cystic arteries arose from the adjacent right hepatic artery and approached the gallbladder via its neck region. One to two cystic veins departed from the bottom and drained into the nearby abdominal vein. Cystic arteries were of the pinnate-type and embraced the gallbladder with their branches. Terminal arterioles gave rise to wide subepithelial capillaries. Transition distances from arteries to veins were short. In general, three to four layers of blood vessels built the vascular bed of the gallbladder wall. The wide subepithelial capillaries established an interface well adapted (1) to allow capillary circulation over a wide range of filling states of the gallbladder and (2) to serve the exchange processes taking place between stored bile and the blood vascular system via the microvillous columnar epithelium.
Introduction
The gallbladder serves to concentrate and store bile before bile is discharged via the bile duct system into the duodenum. Why a gallbladder is present in most animals but absent in others is still under discussion (Gilloteaux Citation1997; Oldham-Ott & Gilloteaux Citation1997).
Presently, the development of the gallbladder (Gilbert Citation2003) along with the molecular basis of the differentiation of its epithelial lining from endoderm is well known (Zorn & Wells Citation2007). Also well known in mammals and man is its macroscopic and microscopic anatomy (Schreiber Citation1939, Citation1942; Walraff Citation1969; Oldham-Ott & Gilloteaux Citation1997), innervation (Mawe et al. Citation1997), mechanism of bile discharge (Schreiber Citation1939), pathologies (Nakanuma et al. Citation1997), and its blood vascular system (Gordon Citation1967; Ohtani Citation1981, Citation1988; Polyzonis et al. Citation1989; Caggiatti et al. Citation1990, Citation1992; Aharinejad & Lametschwandtner Citation1992a; Nakanuma et al. Citation1997; Ohtani et al. Citation1997; Jackowiak & Lametschwandtner Citation2005; Jackowiak Citation2006; Jackowiak et al. Citation2007).
Little is known about the microvascular anatomy of the gallbladder in lower vertebrates. Presently we know that in anuran amphibians the gallbladder is supplied by either one or two cystic arteries which branch off the right hepatic artery, and that one or two cystic veins drain it into the abdominal vein (Xenopus laevis Daudin; Millard Citation1941; Rana esculenta; Gaupp Citation1899). Only one short note is published on the microvascularization of the gallbladder of the adult pipid frog, Xenopus laevis (Häring Citation2004).
In the present study we use scanning electron microscopy of vascular corrosion casts (Murakami Citation1971) and correlative light microscopy and demonstrate for the first time the microvasculature of an anuran gallbladder in greater detail. Specifically, we show that this microvasculature by virtue of its architecture allows for a sufficiently high intrinsic blood circulation over a wide range of filling states of the gallbladder.
Materials and methods
Stereomicroscopic dissection
Three adult males of the South African Clawed Toad, Xenopus laevis Daudin (body weights: 75.0, 78.0 and 108.7 g; snout–vent lengths (SVL): 80, 85 and 105 mm) were killed by submersion into an overdose of tricaine methansulfonate (MS 222; Sigma Chemicals, St. Louis, MO). The abdominal cavity was opened by a paramedian cut, and after a bilateral anterior and posterior laterally directed incision the abdominal wall was flipped laterally and pinned down. Then gross arterial supply and venous drainage of the gallbladder, its superficial microvasculature, and the bile duct system were exposed under stereomicroscopic control (Stereomicrosope Wild M 651; Wild, Heerbrugg, Switzerland) and documented (digital camera system Nikon DS-5-L1).
The gallbladder was excised in the two smaller animals, submerged in fixative (described below), and cut meridionally into two halves. While submerged, the fixed bile was carefully removed with forceps and the luminal surfaces of the halves of the gallbladder were cleaned by a gentle jet of fresh fixative to allow for a detailled scanning electron microscope (SEM) inspection. Further details are provided below.
Scanning electron microscopy of critical point dried gallbladders
After stereomicroscopical inspection and documentation the two halves of one gallbladder were fixed further by immersion in fresh 2.5% buffered glutaraldehyde (0.15 M cacodylate; pH 7.4; 4°C, overnight), washed in 0.2 M cacodylate buffer (3 × 20 min), postfixed in buffered (0.1 M cacodylate) osmium textroxide (2%; 2 h; 20°C), washed in 0.2 M cacodylate (3 × 20 min), and stored overnight in ethanol (70%; 4°C). Finally, specimens were dehydrated in a graded series of ethanol (80, 90, 96 and 100%; three passages each), and critical point dried via carbon dioxide. Then one of the two dried halves of the gallbladder was fractured meridionally by pulling with two forceps. Finally, specimens were mounted to specimen stubs with colloidal silver, sputtered with gold, and examined in the scanning electron microscope (XL-30; FEI-Company, Eindhoven, Netherlands or Stereoscan 250, Cambridge, UK) at 20 kV.
Histomorphology
Gallbladders of two adult animals (body weights: 46 and 126 g; SVL: 55 and 110 mm) were studied. Animals were killed by immersion in an overdose of an aqueous solution of MS 222 (0.5 %) and first perfused via ventricle–arterial trunk with Amphibian Ringer's solution (20°C; for recipe see Adam & Czihak Citation1964) using manual pressure. After clear reflux from the opened atria 10 ml of Bouin's solution (Adam & Czihak Citation1964) were injected for fixation of the animals. Then gallbladder, cystic duct and common bile duct were excised and postfixed in fresh fixative (20°C). After dehydration in a graded series of ethanol, specimens were embedded in paraplast, sectioned transversely and longitudinally (7 µm), and stained (Goldner's trichrome stain; Romeis Citation1989). Micrographs taken with a digital camera (Axiocam) were imported to Photoshop 7.0 (Adobe) and brightness and contrast were adjusted when necessary.
Vascular casting
For vascular casting, 13 adult animals (body weights: 46–126 g; SVL: 55–110 mm) were used. Animals were killed by immersion in an overdose of an aqueous solution of MS 222 (0.5 %) and first perfused via ventricle–arterial trunk with Amphibian Ringer's solution (20°C; for recipe see Adam & Czihak Citation1964) using manual pressure. After clear reflux from the opened atria 10 ml Mercox CL-2B (Ladd Research Inc.; Burlington, VT, USA) diluted with monomeric methylmethacrylate (4 + 1, v + v) were injected with manual pressure. After polymerization of the injected resin, whole animals were tempered (water bath; 60°C, 12 h), macerated (KOH; 7.5%, 12–24 h), rinsed (tap water), cleaned (5% formic acid; 10 min), rinsed (distilled water), frozen (distilled water), and freeze-dried (Lyovac GT2; Leybold Heraeus, Cologne, Germany). In dry specimens, gallbladders were exposed by removal of the overlaying right lobe of the liver, and specimens were mounted by the ‘conductive bridge method’ (Lametschwandtner et al. Citation1980). Mounted specimens were evaporated with carbon and gold, sputtered with a thin layer of gold and examined in the scanning electron microscope (Stereoscan 250, Cambridge Ltd, UK; accelerating voltage: 10 kV). After the microvascular anatomy of the gallbladders had been documented in-situ, gallbladders were removed, mounted onto new stubs, coated, and previously hidden areas were documented. Finally, gallbladders were removed from the specimen stubs, frozen in distilled water, and sectioned transversely, longitudinally, tangentially or horizontally with a mini wheel-saw placed in the chamber of a cryo-microtome (Lametschwandtner & Lametschwandtner Citation1992). Sectioned ice-embedded specimens were allowed to thaw in distilled water, cleaned, and refrozen in bidistilled water for subsequent freeze-drying (Lyovac GT 1, Leybold-Hereaus; Cologne, Germany). Further steps in specimen preparation were as described above. For further details on SEM and vascular corrosion casting see Aharinejad and Lametschwandtner (Citation1992b), Motta et al. (Citation1992), and Lametschwandtner et al. (Citation2006).
Results
Anatomy
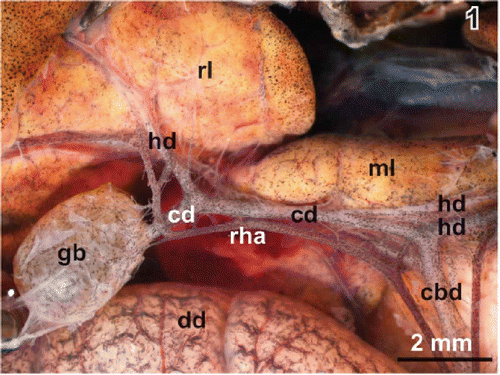
In adult Xenopus laevis, the gallbladder is located at the medial border of the right lobe of the liver close to the base of the heart ventricle. Depending on how full it is, the gallbladder is ball-, ovoid- or club-shaped and 4–5 mm in diameter (). A bottom, a body, and a neck region can be discerned ( and ). The cystic duct is joined first by the right hepatic duct, then by the median and left hepatic ducts ( and ).
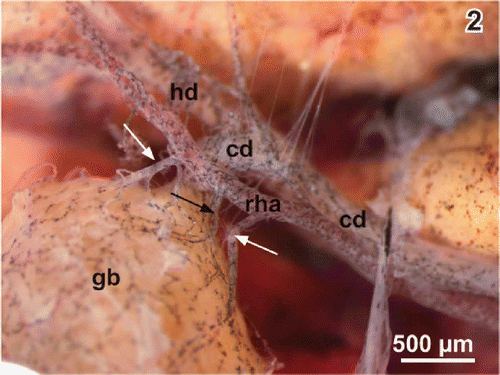
Figure 1. Gallbladder and bile duct system in adult male Xenopus laevis Daudin. Stereomicroscopy. Ventral view. Note the right hepatic artery (rha) which abuts the cystic arteries. cbd common bile duct, cd cystic duct, dd duodenum, gb gallbladder, hd hepatic duct, ml median lobe of liver, rl right lobe of liver.
Gross arterial supply and venous drainage
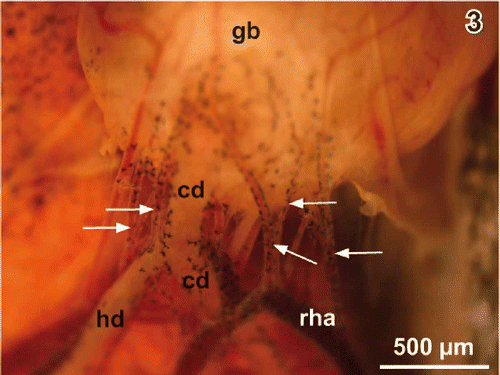
The right hepatic artery supplies the gallbladder. When this artery passes the gallbladder neck region it abuts one to five cystic arteries towards the gallbladder (Figures ). Cystic arteries often bifurcate when they approach the serosal side of the neck region (). One to two cystic veins leave the bottom region and drain the gallbladder into the nearby abdominal vein.
Histomorphology
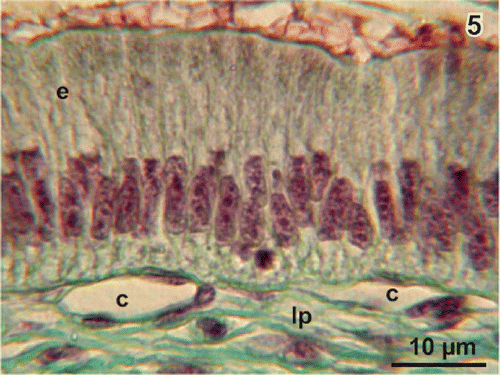
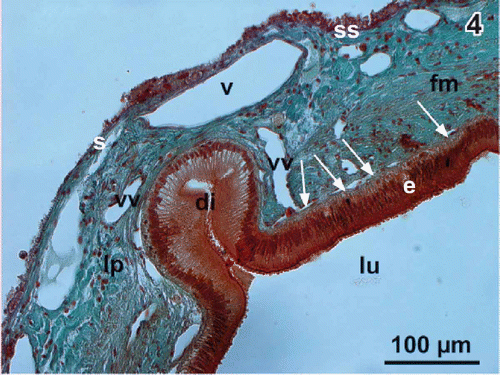
The wall of the gallbladder of adult Xenopus laevis consists (from inside to outside) of epithelium, lamina propria, fibromuscular layer, subserosa, and serosa (). Wide vessels locate in the lamina propria while the capillary bed lies directly beneath the epithelium (). The epithelium consists of a few roundish basal cells with a round nucleus and slender columnar epithelial cells with a longish nucleus located in the basal half of the cell (). Cell nuclei of columnar cells show several nucleoli. Subepithelial capillaries have a conspicuous flat profile and locate directly beneath the basal lamina ().
Figure 4. Histomorphology of the gallbladder wall. Light microscopy. Transverse section (10 µm; Goldner staining). Note the flat capillaries beneath the columnar epithelium (arrows) and a diverticulum (di) extending into the wall. e columnar epithelium, fm fibromuscular layer, lu lumen, lp lamina propria, s serosa, ss subserosa, v vein, vv venule.
Luminal surface structure
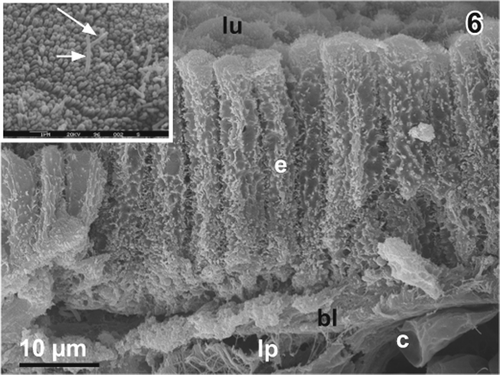
Medium-powered scanning electron micrographs of critical point dried fractured gallbladders confirm the histomorphological findings and show that the lateral surfaces of the columnar epithelial cells are strongly ruffled (). Apical cell surfaces are slightly convex, polygonal in shape and have diameters around 3.3 µm (). They usually have a dense coating with ∼20 nm thick microvilli. Locally, a varying number of longer microvilli with diameters around 16 nm or even one or two centred long microvilli can be found (, arrows). Conspicious epithelial folds are missing.
Figure 6. Columnar epithelium of the gallbladder of adult male Xenopus laevis Daudin. Scanning electron micrograph of a critical point dried fractured gallbladder. Note the strong ruffling of the lateral surfaces of the columnar epithelial cells (e). Inset. Polygonal apical surface of a columnar epithelial cell with a dense coat of short microvilli and two long central microvilli (arrows). bl basal lamina, c capillary, lp lamina propria, lu lumen of gallbladder.
Microvascular architecture
One to five cystic arteries approach via the neck region and give off branches bilaterally on their course towards body and bottom (
Figure 7. Microvascular anatomy of the gallbladder in adult Xenopus. Vascular corrosion cast. Ventral aspect. SEM micrograph. Anterior is to the left, medial is on top. Arteries are red, veins are blue. av abdominal vein, ca cystic artery, cv cystic vein, dd duodenum, gb gallbladder, hd hepatic duct, ml median lobe of liver, pv portal vein. Inset a. Cystic artery (ca) with side branch. Note the imprints of a flow divider (arrows). Inset b. Cystic artery (ca) with small side branch representing a terminal arteriole (ta). Note the narrowing at the origin of the terminal arteriole caused by an intimal cushion (arrow).
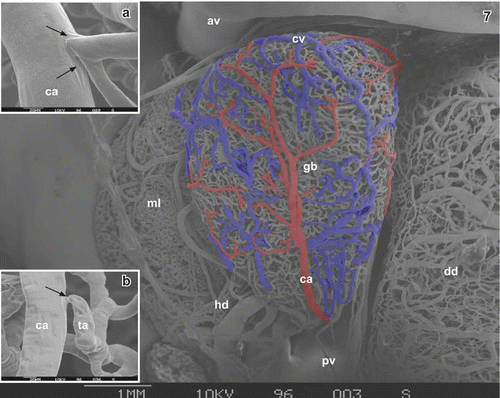
Figure 8. Same as , but gallbladder rotated 180° anticlockwise along its long axis to show its dorsal aspect. b bottom, bo body, ca cystic artery, cv cystic vein, ne neck. Asterisks mark ‘conductive bridges’.
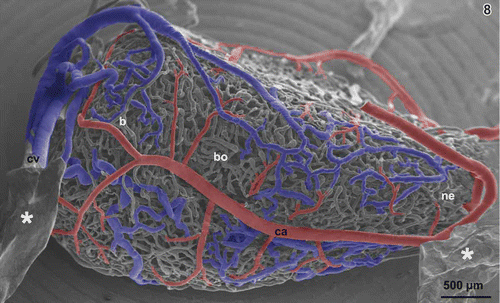
Figure 9. Transition zone of three terminal arterioles (ta) to postcapillary venules (vv)(dashed lines). Serosal view. Arteries are red. Note that interposed capillaries (c) are few and short. Locally, terminal arterioles (large arrows) seem to continue directly as venules. lu lumen of gallbladder.
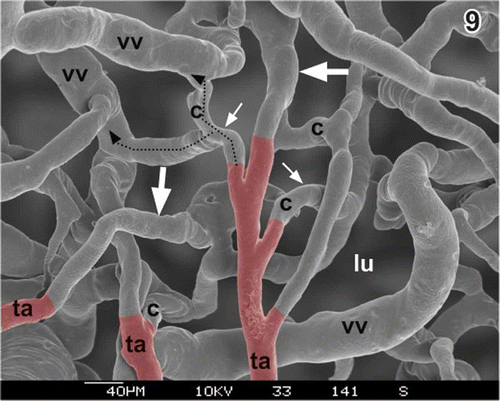
Figure 10. Short terminal arteriole (ta) without narrowing at the origin. Arteries are red. c capillary, ca cystic artery, lu lumen of gallbladder, vv venule.
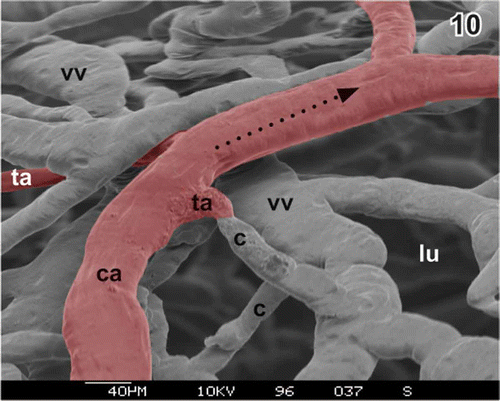
The subepithelial vascular network is made up of relatively wide flat capillaries, which form a wide meshwork with venules interposed between (). Occasionally, outbulgings of the capillary bed are present outlining diverticula extending into the gallbladder wall (; compare with ). Subepithelial capillaries drain either via adjacent venules or directly into postcapillary venules located somewhat deeper in the lamina propria. Some cast venules have holes of varying sizes and shapes, which can be interpreted as signs of ongoing intussuceptive microvascular growth (Figures ). Vascular sprouts imposing as blind-ending vessels with fine tapering endings are few ( and ). They arise from subepithelial venules. Venules of the lamina propria join at the bottom of the gallbladder and form one or two cystic veins () and drain into the nearby abdominal vein.
Figure 11. Microvascular pattern of the subepithelial vascular bed at the bottom of the gallbladder. Luminal view. Cystic artery (ca) and its branches are red, part of cystic vein (cv) is blue. Note the venules (vv) interposed between capillaries (c) and venules forming kind of ‘venular ring’ (outlined in blue). Asterisks mark ‘conductive bridges’.
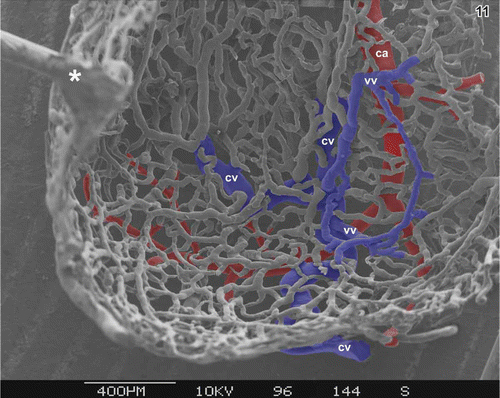
Figure 12. Venule (vv) of the lamina propria with a small hole (arrow) indicating ongoing intussusceptive microvascular growth. Serosal view. c capillary, lu lumen of gallbladder.
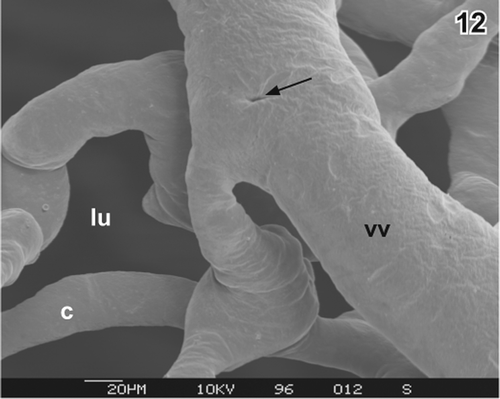
Figure 13. Same as , but intussusception (arrows) in a more advanced state. c capillary, lu lumen of gallbladder, vv venule.
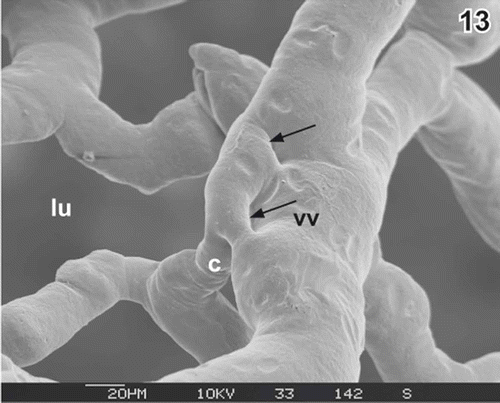
Figure 14. Vascular sprouts (sp) and ongoing intussusception (arrows) at a venule (vv) in the lamina propria. Serosal view. lu lumen of gallbladder.
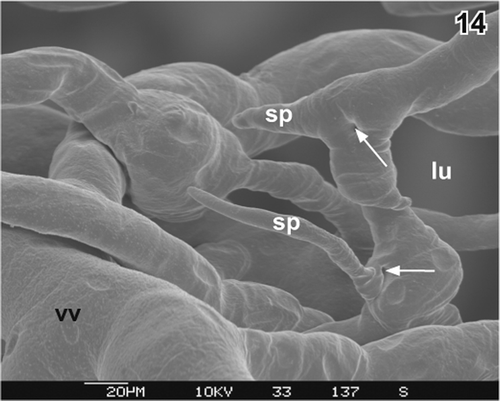
Figure 15. Vascular sprout (sp) arising from a subepithelial venule (vv). Serosal view. lu lumen of gallbladder.

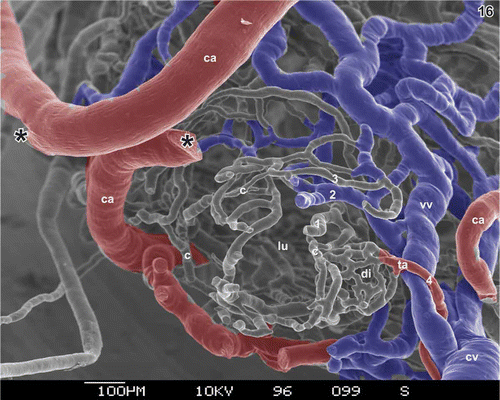
Throughout the wall of the gallbladder three to four layers of blood vessels are present ().
Figure 16. Vascular pattern of the transition region from the cystic duct to the neck of the gallbladder. Lumen (lu) seen from the site of the (removed) cystic duct. Note the wide (blue) venules (vv) located in the subserosa, the subepithelial capillaries (c), and the dense capillary bed of a diverticulum (di) fed by a terminal arteriole (ta). ca cystic artery, cv cystic vein. Asterisks mark the site where the branch of the cystic artery (ca) broke apart. 1–4 indicate number of vascular layers within the wall of the gallbladder.
Discussion
Scanning electron microscopy of the luminal surface of the gallbladder of Xenopus laevis demonstrated that (1) the epithelium lacked prominent folds, and (2) epithelial cells had one or two long microvilli with diameters of ∼16 nm. Up to now, such microvilli, which protrude above the dense short microvilli coat of the gallbladder epithelial cells, were neither reported in anuran amphibians (Grzycki & Nowakowski Citation1967; Azanza et al. Citation1989; Oldham-Ott & Gilloteaux Citation1997) nor in urodelian ones (Oldham-Ott & Gilloteaux Citation1997). Interestingly, a similar, extremely tall microvillus per epithelial cell was demonstrated sofar in the Armadillo lizard only (Oldham-Ott & Gilloteaux Citation1997). The functional importance of the tall microvillus remains open.
The pattern of the arterial supply with the cystic arteries coming from the hepatic artery and the cystic arteries entering via the neck region clearly reflects the development of the gallbladder as a derivative of the draining duct of the liver diverticulum (Gilbert Citation2003). This pattern obviously ensures that the entire gallbladder is well supplied with oxygenated blood while the venous drainage from the bottom region via the cystic veins into the abdominal vein facilitates reabsorbed substances to be transported via the left branch of the abdominal vein directly to the left liver lobe and via the right branch of the abdominal vein into the portal which drains into the right liver lobe.
The microvascular patterns of the subepithelial capillary bed of the gallbladder of adult Xenopus show three striking differences if compared with those in mammals. In mammals, (1) capillaries are much thinner, (2) capillary mesh sizes are much smaller, and (3) venules always stay beneath capillaries and are never interposed between capillaries like they are in adult Xenopus. As a consequence the surface of the subepithelial vascular bed of the gallbladder, which serves the exchange of solutes and electrolytes between luminal bile and blood, is much smaller in Xenopus than it is in mammals.
Acknowledgements
This work was supported by the Fonds zur Förderung der wissenschaftlichen Forschung, Projekt P-19050 and the Stiftungs- und Förderungsgesellschaft der Paris-Lodron-Universität Salzburg (to A.L.). The authors thank OR Dr. W. D. Krautgartner for providing excellent working conditions in the SEM facility.
References
- Adam , H and Czihak , G . 1964 . Arbeitsmethoden der makroskopischen und mikroskopischen Anatomie , Stuttgart : G. Fischer Verlag .
- Aharinejad , S and Lametschwandtner , A . 1992a . Microangioarchitecture of the Guinea pig gallbladder and bile duct as studied by scanning electron microscopy of vascular corrosion casts . Journal of Anatomy , 181 : 89 – 100 .
- Aharinejad , S and Lametschwandtner , A . 1992b . Scanning electron microscopy of vascular corrosion casts , Wien : Springer Verlag .
- Azanza , MJ , Aisa , J , Junquera , C and Castiella , T . 1989 . The autonomic innervation of the liver and gallbladder of Rana ridibunda . Histology and Histopathology , 4 : 405 – 410 .
- Caggiatti , A , Macchiarelli , G , Nottola , SA , Vizza , E and Familiari , G . 1992 . Scanning electron microscopy of the rabbit gallbladder mucosal microvasculature . Journal of Anatomy , 180 : 275 – 280 .
- Caggiatti , A , Nottola , SA , Vizza , E and Macchiarelli , G . 1990 . “ The rabbit gallbladder microvascular bed as studied by SEM of vascular corrosion casts ” . In Abstract Book of the IX International Symposium on Morphological Sciences, Nancy, Jarville: Vagner 23 – 24 .
- Gaupp , E . 1899 . Anatomie des Frosches. Zweite Abtheilung. Lehre vom Nerven und Gefässsystem. 2 Auflage , Braunschweig : F. Vieweg und Sohn. pp. 548 .
- Gilbert , SF . 2003 . Developmental biology , 7th , Sunderland, MA : Sinauer Associates. pp. 838 .
- Gilloteaux , J . 1997 . Introduction to the biliary tract, the gallbladder, and gallstones . Microscopy Research and Technique , 38 : 547 – 551 .
- Gordon , KCD . 1967 . A comparative study of the distribution of the cystic artery in man and other species . Journal of Anatomy , 101 : 351 – 359 .
- Grzycki , S and Nowakowski , A . 1967 . The histochemical observation of monoamine oxidase (MAO) in the gall bladder epithelium of the frog and mouse . Zeitschrift fur Mikroskopisch-Anatomische Forschung , 77 : 611 – 616 .
- Häring , P . 2004 . Die Gefäßarchitektur der Leber von Xenopus laevis Daudin , Inauguraldissertation. Universität Basel .
- Jackowiak , H . 2006 . “ Morphology and angioarchitecture of the gallbladder and extrahepatic bile tract in selected species of carnivores. Thesis for Habilitation ” . In In Polish (with English summary and English figure legends) , Akademia Rolnicza im. Augusta Cieszkowskiego W Poznaniu. Poznan 2006. pp. 125 .
- Jackowiak , H , Godynicki , S and Lametschwandtner , A . July 2007 . The angioarchitecture of the human gallbladder in the cholecystitis – A preliminary study on the vascular corrosion cast , July , 7 – 12 . Salzburg : Proceedings of the International Workshop on Advances in Vascular Casting .
- Jackowiak , H and Lametschwandtner , A . 2005 . Angioarchitecture of the rabbit extrahepatic bile ducts and gallbladder . The Anatomical Record Part A: Discoveries in Molecular, Cellular and Evolutionary Biology , 286 : 974 – 981 .
- Lametschwandtner , A and Lametschwandtner , U . 1992 . “ Historical review and technical survey of vascular casting and scanning electron microscopy ” . In Scanning electron microscopy of vascular casts: Methods and applications , Edited by: Motta , PM , Murakami , T and Fujita , H . 1 – 11 . Boston : Kluwer .
- Lametschwandtner , A , Lametschwandtner , U , Radner , C and Minnich , B . 2006 . Spatial growth and pattern formation in the small intestine microvascular bed from larval to adult Xenopus laevis: A scanning electron microscope study of microvascular corrosion casts . Anatomy and Embryology (Berl) , 211 : 535 – 547 .
- Lametschwandtner , A , Simonsberger , P and Adam , H . 1980 . On the prevention of specimen charging in scanning electron microscopy of vascular corrosion casts by attaching conductive bridges . Mikroskopie , 36 : 270 – 273 .
- Mawe , GM , Talmage , EK , Cornbrooks , EB , Gokin, Zhang , L and Jennings , LJ . 1997 . Innervation of the gallbladder: Structure, neurochemical coding, and physiological properties of Guinea pig gallbladder ganglia . Microscopy Research and Technique , 39 : 1 – 13 .
- Millard , N . 1941 . The vascular anatomy of Xenopus laevis (Daudin) . Transactions of the Royal Society of South Africa , 28 : 387 – 439 .
- Motta , PM , Murakami , T and Fujita , H . 1992 . Scanning electron microscopy of vascular casts: methods and applications , Boston : Kluwer Academic Publishers .
- Murakami , T . 1971 . Application of the scanning electron microscope to the study of the fine distribution of the blood vessels . Archivum Histologicum Japonicum , 32 : 445 – 454 .
- Nakanuma , Y , Hoso , M , Sazen , T and Sasaki , M . 1997 . Microstructure and development of the normal and pathological biliary tract in humans, including blood supply . Microscopy Research and Technique , 38 : 552 – 570 .
- Ohtani , O . 1981 . “ Microcirculation studies by the injection-replica method with special reference to the portal circulation ” . In Three dimensional microanatomy of cells and tissues surfaces , Edited by: DiDio , LJA , Motta , PM and Allen , DJ . 51 – 70 . New York, NY : Elsevier .
- Ohtani , O . 1988 . “ The microvascularization of the liver, the bile duct and the gallbladder ” . In Biopathology of the liver , Edited by: Motta , PM . 83 – 88 . Dordrecht : Kluwer Academic Publisher .
- Ohtani , O , Lee , MH , Wang , QX and Uchino , S . 1997 . Organization of the blood and lymphatic vessel microvasculature of the gallbladder in the Guinea pig: A scanning electron microscopic study . Microscopy Research and Technique , 38 : 660
- Oldham-Ott , CK and Gilloteaux , G . 1997 . Comparative morphology of the gallbladder and biliary tract in vertebrates: Variation in structure, homology in function and gallstones . Microscopy Research and Technique , 38 : 571 – 595 .
- Polyzonis , MB , Tsikaras , P , Hytiroglou , P and Agios , A . 1989 . Further observations on the vascular system of the gallbladder in man . Bulletin de'l Association des Anatomistes (Nancy) , 73 : 25 – 28 .
- Romeis , B . 1989 . Mikroskopische Technik. 17 , Urban and Schwarzenberg : neubearb. Aufl. München .
- Schreiber , H . 1939 . Zum Bau und Entleerungsmechanismus der Gallenblase . Anatomischer Anzeiger , 87 : 257 – 275 .
- Schreiber , H . 1942 . Das Muskellager der menschlichen Gallenblasenwand im Vergleich zu der vierfüßiger Säuger , 91 – 150 . Zeitschrift für Anatomie und Entwicklungsgeschicte .
- Walraff , J . 1969 . “ Die Leber – Gallengangsystem, Gallenblase und Galle ” . In Handbuch der mikroskopischen Anatomie des Menschen , 384 Verdauungsapparat. Atmungsapparat. Part IV .
- Zorn , AM and Wells , JM . 2007 . Molecular basis of vertebrate endoderm development . International Review of Cytology , 259 : 49 – 111 .