Abstract
Armadillos constitute a neotropical endemic mammalian group in the American continent and the main diversity is distributed in Argentina. Several species of this family are in danger of extinction. Blood collection in armadillos is difficult because of their anatomy. In the present work, in latex-perfused material, the rete mirabile of the coccygeal artery of the tail was recognized as an appropriate venipuncture site for sterile blood collection. From cadaveric material, histological sections of the tail of six species of five genera of armadillos were used to determine the diameter and the distance to access the main artery of the rete mirabile ventrodorsally. Field studies were carried out in wild animals. A total of 232 blood samples were obtained from the following species: Chaetophractus villosus (70), C. vellerosus (105), Zaedyus pichiy (15), Euphractus sexcinctus (8), Dasypus hybridus (25), and Tolypeutes matacus (9). The animals were restrained with the thumbs pressing the abdomen ventrolaterally and the rest of the fingers holding the carapace. Punctures were carried out between the first and second rings of the tail with a 21-gauge needle. Alternatively, an immobilization device was designed to allow sampling by a single operator. Blood cell integrity was evaluated through histological analysis and ulterior development in lymphocyte culture. We concluded that the rete mirabile of the tail of armadillos is an effective venipuncture site for field studies that allows obtaining sterile blood samples, avoiding animal mortality, high-risk venipuncture sites, anesthetics and excessive stress.
Introduction
Xenarthra is an order of neotropical mammals with a large morphological variety, composed of 31 species that are mainly distributed in Argentina (Vizcaino & Loughry Citation2008). The extant species of this order are the armadillos, sloths and anteaters. The armadillos, which belong to the dasypodidae family, are an endemic group from the American continent presenting a great diversity of body size, habits and geographic distribution. Several species of this family, such as the giant armadillo (Priodontes maximus), are in danger of extinction (IUCN Citation2006). They are a model of interest in many fields because of exclusive aspects of their anatomy (Wetzel Citation1985b; Galliari et al. Citation2009), physiology (Roig Citation1969, Citation1970; Galbreath Citation1985; Storrs & Burchfield Citation1985; Cetica et al. Citation1993, Citation1997, Citation2005; Casanave & Affanni Citation1994; Affanni et al. Citation2001), biology (Vizcaino & Loughry Citation2008) and their relatively antique biogeographic position in South America (Wetzel Citation1985a).
Some aspects of their peculiar anatomy, along with their fossorial habits, determine that the collection of blood samples in the field results extremely difficult. Some of these aspects are their thick skin, the presence of bony dermal plates, the large quantity of adipose tissue and their short limbs (Nowak Citation1999). Even though different blood collection techniques have been described in the nine-banded armadillo (Dasypus novemcinctus), all of them have been carried out in captivity and in some cases using anesthesia (Herbst & Webb Citation1988). Blood collection in field armadillos has been described briefly in some species by Deem and Fiorello (Citation2002). In spite of this, the cardiac puncture is frequently used for blood sample collection (Casanave et al. Citation2006). To our knowledge, there are no studies about the injure impact of cardiac puncture in armadillos, although it has been demonstrated to be a detrimental procedure that can shorten the life in other species (Moore Citation1983), and therefore its application is controversial. On the other hand, the obligatory use of anesthesia generates another disadvantage of the method (Gannon & Sikes Citation2009) and makes its use inadvisable when a large population of samplings is required.
The quality of a blood sample is conditioned by: (1) the great diversity of body size in armadillos and the correlation between the maximal collection volume, the size of blood vessels and the weight of the animal (Belcher & Harriss Citation1957); (2) necrotic factors and biological contaminants and also the preservation of the cell viability and morphology, which are extremely important in cell culture; (3) the use of anesthesia that can interfere in the experimental use of the blood (Siebert et al. Citation2007).
Due to the importance of the aspects mentioned above, the present work aimed to: (1) develop a method of field blood collection in six species of armadillos of different body size, through anatomo-histological studies; (2) develop an efficient immobilization device for small armadillos; (3) test the efficiency of the blood collection method through histological analysis and obtain viable lymphocytes capable of dedifferentiating and dividing in cell culture.
Materials and methods
One male Chaetophractus villosus, killed for previous studies, was perfused with latex (Maeda et al. Citation1999). After that, the principal arteries and veins of the sacrococcygeal region were dissected and identified under a Nikon stereoscopic microscope using 10× magnification.
In animals from museum collections (), fixed in formaldehyde 4%, anatomic dissections and transverse sections between the first and the second concentric rings of the tail were carried out. Before their inclusion in paraffin, pieces were decalcified with formic acid 25% and sodium citrate 16%. Histological sections of 5 µm were stained as a routine with hematoxylin and eosin (HE) and photographed using a Sony Cybershot DSC P93 camera mounted on a Nikon stereoscopic microscope. Microscopic details were observed and captured with a Leica camera DFC300 mounted on a Leica microscope. Histo-morphological analyses, including measurements, were carried out with the program Adobe Photoshop CS2 9.0.
Table I. Number of specimens used for anatomo-histological studies, field blood collection and lymphocyte culture
A total of 232 animals () were sampled in the field. Animals were captured and restrained manually by two operators. One operator held the animal firmly with the abdomen upwards (dorsal decubite), placing the thumbs on the ventro-lateral region at the abdomen and the rest of the fingers on the carapace (). The other operator held the tail with one hand and collected the blood sample with the other (). A bag made of cloth that covers the animal's head was used in order to avoid increase of animal resistance.
Figure 1. a, Restraint in Zaedyus pichiy; b, blood collection in C. villosus from the tail between the first and the second rings. Scale bar: 3 cm.
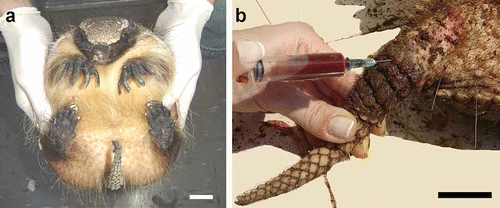
The proper level of restraint was established as the one that allows obtaining the blood sample at the first instance lasting 3 min maximum in order to avoid excessive stress in the animal. The peculiar anatomy of Tolypeutes matacus allows it to acquire a defensive posture that impairs access to blood vessels. In order to prevent this, the animal must be surprised when walking.
Sterile syringes of different calibers (3, 5, 10 ml) charged with sodium heparin at a final concentration of 5 IU/ml and 21-gauge needles were used. Maximum extraction volume was 10% of the volemia (considered as 77 ml/kg of animal body weight) (Belcher & Harriss Citation1957). The puncture site was cleaned with 70º ethanol. The puncture was carried out at a middle point between the first and the second rings of the tail (). Due to the presence and overlapping of bony dermal plates in all the species of armadillos studied, a puncture angle of 25˚ had to be used when the tail was straight. More accuracy was required when sampling D. hybridus due to the dense overlapping of bony dermal plates. Due to the fast blood coagulation in armadillos (Casanave et al. Citation2006), once the sample was obtained, the sample was gently shaken with one hand, while a smooth but firm pressure was exerted with the other on the puncture zone, in order to cause hemostasis and avoid the generation of a hematoma.
Sample quality was evaluated by: (1) cell morphology in blood smears stained with the May–Grünwald–Giemsa method (Strumia Citation1963); (2) leukocyte concentration and viability, in Newbauer counting chamber, using Trypan Blue stain (Tennant Citation1964); (3) lymphocyte cultures (Moorhead et al. Citation1960) (n = 222) considering successful the ones that showed a transformation index of 80% after 96 h of culture (Luaces Citation2007).
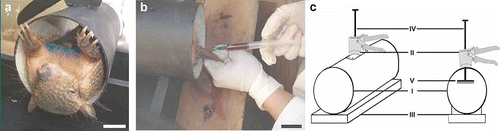
An immobilization device for the species of small armadillos was designed from anatomic measurements of body length and width taken from 37 animals of Chaetophractus vellerosus (–c). The device was built with a polyvinyl chloride pipe, 22 cm in length and 10.5 cm in diameter, a wooden base where the pipe was mounted, a gradable piston and a movement mechanism to hold the animal firmly according to its size (). The movement mechanism was obtained using the trigger and the piston rod of a caulking gun. The pipe was drilled with an 8-mm bit in the middle of its long axis. The piston rod was introduced through the hole and welded to an iron plate of 12×5 cm. The iron plate was lined with EVA (ethyl vinyl acetate) foam on the surface that contacts with the animal (). The device was effective for the proper immobilization of small armadillos (C. vellerosus and Z. pichiy) () and allowed sampling by a single operator ().
Figure 2. a, Individual of C. vellerosus in immobilization device; b, blood collection by a single operator using immobilization device; c, schematic drawing of immobilization device. I: Polyvinyl chloride pipe, II: trigger, III: wooden base, IV: rod, V: iron plate lined with EVA foam. Scale bar: 3 cm.
Results
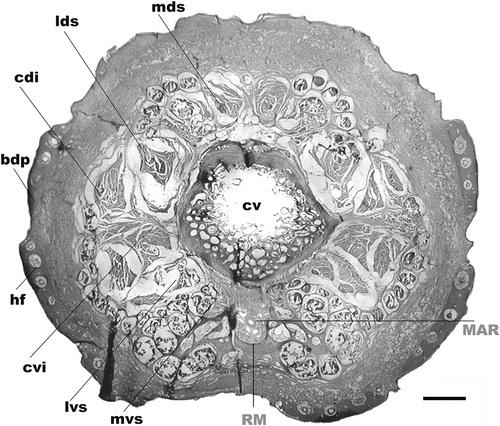
Anatomic dissections in one C. villosus perfused with latex revealed that the sacral artery originates the coccygeal artery of medial and deep location under the coccygeal vertebral bodies toward the caudal region. At the level of the base of the tail, the coccygeal artery branches off in a group of arteries that flow strikingly regularly, parallel side by side, with a group of veins. This peculiar organization named rete mirabile was previously described in the armadillo D. novemcinctus by Arruda and Arruda (Citation1999) ().
Figure 3. Tail transverse section of Chaetophractus villosus. HE staining. Scale bar: 2.5 mm. RM: rete mirabile of the medial coccygeal artery. MAR: main artery of the rete mirabile, cv: coccygeal vertebrae, bdp: bony dermal plate, mvs: medial ventral sacrocaudal muscle, lvs: lateral ventral sacrocaudal muscle, cvi: caudal ventral intertransverse muscle, cdi: caudal dorsal intertransverse muscle, lds: lateral dorsal sacrocaudal muscle, mds: medial dorsal sacrocaudal muscle, hf: hair follicle.
Histological studies of the transverse sections of the tail of armadillos evidenced the presence of a rete mirabile in all the animals studied (–d). Histology of Zaedyus pichiy was similar to that shown in C. vellerosus (data not shown). The rete mirabile was constituted of arteries and veins of mid-caliber and a larger central artery (main artery of the rete mirabile, MAR). Two veins of latero-dorsal location were observed (–d). The diameters of the MAR, latero-dorsal veins and the approximate distance to access the MAR ventro-dorsally are shown in .
Figure 4. Tail transverse sections of the following armadillos. a, Dasypus hybridus; b, Chaetophractus vellerosus; c, Tolypeutes matacus; d, Euphractus sexcinctus. Scale bar: 1 mm. Inset histological images of the rete mirabile. HE staining. Scale bar: 200 µm. RM: rete mirabile of the medial coccygeal artery, MAR: main artery of the rete mirabile, LCV: lateral caudal veins, cv: coccygeal vertebrae, sp: spinous process, tp: transverse process, bdp: bony dermal plate, mvs: medial ventral sacrocaudal muscle, lvs: lateral ventral sacrocaudal muscle, cvi: caudal ventral intertransverse muscle, cdi: caudal dorsal intertransverse muscle, lds: lateral dorsal sacrocaudal muscle, mds: medial dorsal sacrocaudal muscle.
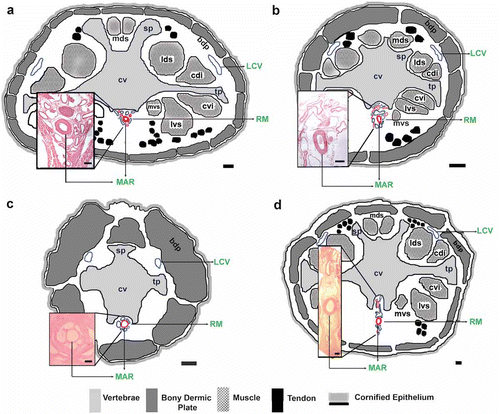
Table II. Diameter of the main artery of the rete mirabile (MAR) and lateral caudal vein (LCV) and MAR depth in five species of armadillos
In accordance with the histology of the rete mirabile, blood can be obtained from either arteries or veins. In the former, the syringe is charged without needing to pull the piston. In the latter, slow piston retraction is needed. Disinfection of the zone was important to prevent contamination with the skin bacterial flora. Because of the gradual increase in resistance and aggressiveness, the faster the procedure, the more successful the blood collection in medium-sized and large animals like C. villosus, Dasypus hybridus and Euphractus sexcinctus.
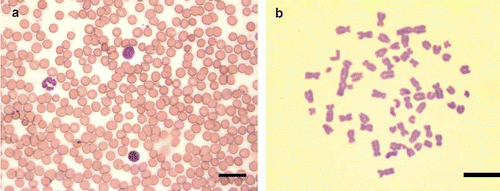
Cells obtained following this protocol presented normal morphology in blood smears () and viability under Trypan Blue stain. Lymphocytes were suitable in 89% (197/222) of the cultures and metaphases were obtained ().
Discussion
Some anatomic features of armadillos, such as thick skin, the presence of bony dermal plates and adipose tissue make the access to blood vessels difficult. Although the use of anesthetics is a useful option due to the exceptional anatomy and physical resistance of armadillos, it may reduce the quality of the sample and affect the animal's health. In addition, anesthetic interference over lymphocyte development in culture has been described (Siebert et al. Citation2007). In our experience, culture blood samples obtained from animals under ketamine chloride anesthesia showed low performance in all cases.
Due to the complex thermal regulation of armadillos (Roig Citation1969, Citation1970) and the fact that there are no data available about the effect of anesthetics in these species, mortality risk during sedation is an important feature. The use of anesthesia implies at least 1 h of animal manipulation. With the method described here we showed that manipulation can be minimized and blood collection in fully conscious animals requires less than 1 min. Because of that, when collecting blood samples in armadillos in field studies, if the pain is minimal and quick, anesthesia is not recommended because the animal can be released immediately (Gannon & Sikes Citation2009).
Excessive stress during manipulation leads to physiological changes, such as reduced number of viable leukocytes, blood pressure variations and an increase in blood glucose, which can also invalidate experimental results (Gannon & Sikes Citation2009). Because a correct restraint can shorten the length of manipulation, thus preventing a high exposure to stress, a fast procedure is crucial for blood collection success.
Through anatomic dissections and histological sections between the first and the second rings of the tail, we proved that the rete mirabile is an efficient site for easy blood collection in armadillos in field studies. We confirmed the presence of the peculiar rete mirabile, first described by Arruda and Arruda (Citation1999) in the nine-banded armadillo (D. novemcinctus), in six species of five genera of dasypodids.
Although Herbst and Webb (Citation1988) proposed the tail as a site for blood collection in armadillos, their results did not present enough information for the beginner operator, did not show precise evidence such as anatomo-histological studies, and did not evaluate cell viability in the blood samples obtained. In our work we confirmed, through anatomo-histological studies, the venipuncture site proposed by Herbst and Webb (Citation1988) for D. novemcinctus in six other species of five genera of armadillos. The presence of the rete mirabile demonstrates that the methodology, successfully applied in the laboratory by these authors, is attractive for the beginner operator and can also be used in field studies.
The use of the immobilization device in small armadillos proved to be very useful and allowed sampling by a single operator.
In conclusion, the present methodology for blood collection in armadillos is an effective technique for its application in field studies. Anatomo-histological analysis of the tail of all the armadillos studied showed that the rete mirabile makes blood collection easier, avoiding animal mortality, high-risk venipuncture sites, anesthetics and excessive stress.
Acknowledgements
This work was supported by ANPCyT PICT 00074, PICT 38013 and CONICET PIP 5185. We thank Dra. Maria Ines Pigozzi for valuable comments and advice, which have greatly improved the manuscript; D.V.M. Jorge Ibáñez and Roque Mira; Dr. Gabriel Angeleri for developing the immobilization device; Mario de Elias for advice on the restraint of armadillos.
References
- Affanni , JM , Cervino , CO and Aldana Marcos , HJ . 2001 . Absence of penile erections during paradoxical sleep. Peculiar penile events during wakefulness and slow wave sleep in the armadillo . Journal of Sleep Research , 10 : 219 – 228 .
- Arruda , OS and Arruda , MSP . 1999 . Study on the median sacral artery ramification and the body temperature of the armadillo (Dasypus novemcinctus) . Revista chilena de anatomía , 17 : 147 – 151 .
- Belcher , EH and Harriss , EB . 1957 . Studies of plasma volume, red cell volume and total blood volume in young growing rats . Journal of Physiology , 139 : 64 – 78 .
- Casanave , EB and Affanni , JM . 1994 . Body temperature of the armadillo Chaetophractus villosus (Mammalia, Dasypodidae) . Archives of Physiology and Biochemistry , 102 : 243 – 246 .
- Casanave , E , Bermúdez , P and Polini , N . 2006 . Principal coagulation factors and natural anticoagulants in the armadillo Chaetophractus villosus (Mammalia, Xenarthra, Dasypodidae) . Comparative Clinical Pathology , 14 : 210 – 216 .
- Cetica , PD , Aldana Marcos , HJ and Merani , MS . 2005 . Morphology of female genital tracts in Dasypodidae (Xenarthra, Mammalia): A comparative survey . Zoomorphology , 124 : 57 – 65 .
- Cetica , PD , Rahn , IM , Merani , MS and Solari , AJ . 1997 . Comparative spermatology in Dasypodidae II (Chaetophractus vellerosus, Zaedyus pichiy, Euphractus sexcinctus, Tolypeutes matacus, Dasypus septemcinctus and Dasypus novemcinctus) . Biocell , 21 : 195 – 204 .
- Cetica , PD , Sassaroli , J , Merani , MS and Solari , AJ . 1993 . Comparative spermatology in Dasypodidae (Priodontes maximus, Chaetophractus villosus and Dasypus hybridus) . Biocell , 18 : 89 – 103 .
- Deem , SL and Fiorello , CV . 2002 . “ Capture and immobilization of free-ranging edentates ” . In Zoological restraint and anesthesia , Edited by: Heard , D . Ithaca, NY : International Veterinary Information Service .
- Galbreath , GJ . 1985 . “ The evolution of monozygotic polyembryony in Dasypus ” . In The evolution and ecology of armadillos, sloths and vermilinguas , Edited by: Montgomery , GG . 243 – 246 . Washington and London : Smithsonian Institution Press .
- Galliari , FC , Carlini , AA and Sánchez-Villagra , MR . 2009 . “ Evolution of the axial skeleton in armadillos (Mammalia, Dasypodidae) ” . In Mammalian Biology – Zeitschrift fur Saugetierkunde
- Gannon , WL and Sikes , RS . 2009 . Guidelines of the American Society of Mammalogists for the use of wild mammals in research . Journal of Mammalogy , 88 : 809 – 823 .
- Herbst , LH and Webb , AI . 1988 . A simple technique for sampling blood from fully conscious nine-banded armadillos . Laboratory Animal Science , 38 : 335 – 336 .
- IUCN . 2006 . “ IUCN. 2004. List of Threatened Species ” . In A global species assessment , Gland : IUCN .
- Luaces , JP . 2007 . Estudio Citogenético de dos poblaciones geograficamente aisladas de Chaetophractus vellerosus , Buenos Aires : Universidad de Buenos Aires .
- Maeda , K , Hata , R and Hossmann , KA . 1999 . Regional metabolic disturbances and cerebrovascular anatomy after permanent middle cerebral artery occlusion in C57black/6 and SV129 mice . Neurobiology of Disease , 6 : 101 – 108 .
- Moore , DM . 1983 . Venipuncture sites in armadillos (Dasypus novemcinctus) . Laboratory Animal Science , 33 : 384 – 385 .
- Moorhead , PS , Nowell , PC , Mellman , WJ , Battips , DM and Hungerford , DA . 1960 . Chromosome preparations of leukocytes cultured from human peripheral blood . Experimental Cell Research , 20 : 613 – 616 .
- Nowak , R . 1999 . Walker's mammals of the world , Baltimore, MD : Johns Hopkins University Press .
- Roig , VG . 1969 . Termorregulación en Euphractus sexcinctus (Mammalia, Dasypodidae) . Physis (Argentina) , 26 : 27 – 32 .
- Roig , VG . 1970 . Observaciones sobre la termorregulación en Zaedyus pichiy . Acta Zoológica Lilloana (Argentina) , 28 : 13 – 18 .
- Siebert , JN , Posfay-Barbe , KM , Habre , W and Siegrist , CA . 2007 . Influence of anesthesia on immune responses and its effect on vaccination in children: Review of evidence . Pediatric Anesthesia , 17 : 410 – 420 .
- Storrs , EE and Burchfield , HP . 1985 . “ Leprosy in wild common long-nosed armadillos Dasypus novemcinctus ” . In The evolution and ecology of armadillos, sloths and vermilinguas , Edited by: Montgomery , GG . 265 – 268 . Washington and London : Smithsonian Institution Press .
- Strumia , M . 1963 . A rapid universal blood stain, May–Gruenwald–Giemsa in one solution . Journal of Laboratory Clinical Medicine , 21 : 930
- Tennant , JR . 1964 . Evaluation of the Trypan blue technique for determination of cell viability . Transplantation , 2 : 685 – 694 .
- Vizcaino , SF and Loughry , WJ . 2008 . “ Xenarthrans biology: Past, present and future ” . In The biology of the Xenarthra , Edited by: Vizcaino , SF and Loughry , WJ . 1 – 10 . Gainesville, FL : University Press of Florida .
- Wetzel , RM . 1985a . “ The identification and distribution of recent Xenarthra (= Edentata) ” . In The evolution and ecology of armadillos, sloths and vermilinguas , Edited by: Montgomery , GG . 5 – 21 . Washington and London : Smithsonian Institution Press .
- Wetzel , RM . 1985b . “ Taxonomy and distribution of armadillos, Dasypodidae ” . In The evolution and ecology of armadillos, sloths and vermilinguas , Edited by: Montgomery , GG . 23 – 46 . Washington and London : Smithsonian Institution Press .