Abstract
Sperm ultrastructure and some steps of spermatogenesis of the serpulids Hydroides dianthus, Serpula vermicularis and Vermiliopsis infundibulum are reported. In all the species examined the germinal cells originated from a germinative epithelium associated to blood vessels in the intersegmental septa. The spermatocytes were irregular in shape with a large nucleus and a thin cytoplasmic layer. In early spermatids in which the nucleus gradually condensed, a developed endoplasmic reticulum and some electron-dense bodies were observed. The nucleus was dehydrated in the late spermatids and assumed a cylindrical shape. In all the examined species, the morphology of the ripe spermatozoa can be ascribed to the ect-aquasperm type. The acrosome had a simple cup shape in V. infundibulum, whilst it was more developed extending laterally to the nucleus in H. dianthus, and cup-shaped with a swelling towards the nucleus in S. vermicularis. When the morphology of the serpulid spermatozoa was superimposed on a phylogenetic scheme, some trends could be highlighted. Although the paucity of data on serpulid spermatozoa ultrastructure at present prevents any phylogenetic inference, the comparison of acrosome ultrastructure within a group having similar reproductive strategies showed an increase in the internal complexity of the acrosome.
Introduction
Serpulimorph polychaetes constitute a discrete group of sedentary worms which secrete calcareous tubes. Traditionally they constitute the family Serpulidae that are currently placed in the clade Sabellida (Rouse & Pleijel Citation2001). However, support values for this clade were low (Kupriyanova et al. Citation2006); notably, the clade was not recovered in a recent morphological and molecular study based on a broad sampling of annelids (Rousset et al. Citation2004). Nevertheless, according to Kupriyanova and Rouse (Citation2008), the striking morphological similarity between serpulids and sabellids suggests that they are very closely related. As a result of the recent molecular analysis conducted on Serpulidae and Sabellidae, these authors revised the family Sabellidae, erecting the new taxon Fabricidae to which Serpulidae are more closely related. Traditionally Serpulidae were divided into three subfamilies: Serpulinae, Filograninae and Spirorbinae. However, a combined analysis of molecular and morphological data showed that Serpulinae and Filograninae are not monophyletic and Spirorbinae are a sister group to the monophyletic clade comprising both ‘filogranin’ and ‘serpulin’ taxa (Kupriyanova et al. Citation2006).
Information on the reproductive biology of the Serpulidae mostly refer to the larval phases and their settlement (Kupriyanova et al. Citation2001; Cotter et al. Citation2003; Denitto & Licciano Citation2006). Phylogenetic analysis including reproductive characters (Kupriyanova Citation2003) suggested that planktotrophy is an apomorphic feature in serpulids, with larval feeding probably evolved once in the clade of ‘Serpulinae’ sensu Pillai (Citation1970). Despite Serpulinae traditionally being considered gonochoric, there is a growing perception that sequential hermaphroditism in this taxon may be the rule rather than an exception (Kupriyanova et al. Citation2001).
Although gametogenesis was analysed in several species and ultrastructural studies are still scarce (Jamieson & Rouse Citation1989; West Citation1991; Selim et al. Citation2005), the morphology of Serpulidae spermatozoa correlates with their fertilisation strategies (Kupriyanova et al. Citation2001; Rouse Citation2005), as already reported for other polychaetes (Patti et al. Citation2003; Rouse Citation2005). Spermatozoa with an elongated head and mid piece (ent-aquasperm sensu Jamieson & Rouse Citation1989) are present in spirorbins, and in the ‘filogranin’ species investigated up to now (Kupriyanova et al. Citation2001), whilst sperms with a round head (ect-aquasperm sensu Jamieson & Rouse Citation1989) are present in species characterised by external fertilisation. The phylogenetic inferences could be misleading if based exclusively on the morphology of the spermatozoa being closely linked to functional and adaptative features.
However, ultrastructural information can be useful in solving taxonomic problems within species having the same reproductive strategy and a similar sperm type. In particular, as observed in other polychaetes such as Sabellidae and Syllidae (Patti et al. Citation2003; Musco et al. Citation2008, Citation2010), the structure of the acrosome can be useful to phylogenetic analysis within the clade.
This article describes the spermatogenesis of the species Hydroides dianthus (Verrill, 1873), Serpula vermicularis L., 1767, and Vermiliopsis infundibulum (Philippi, 1844), the species commonly recorded from shallow-water hard-bottom in the Mediterranean, in order to increase knowledge on the sperm ultrastructure of serpulids.
Material and methods
In total, 95 specimens of serpulids (38 of H. dianthus, 23 of S. vermicularis, 34 of V. infundibulum) were collected in the Mar Piccolo of Taranto (Ionian Sea). Hydroides dianthus is widespread in harbours and lagoons where it can tolerate wide fluctuations of salinity, temperature, and oxygen; some aspects of its population dynamics have already been investigated in the same biotope (Mercurio et al. Citation2008). The mature specimens collected measured 1–1.2 cm in total length. Serpula vermicularis is a species present on different substrata, from the subtidal to bathyal zones, particularly common in infralittoral and circalittoral bioconcretions. The mature specimens collected measured 1–1.2 cm in total length. Vermiliopsis infundibulum is a species abundant on littoral detritic bottoms, shells and small hard substrata. It is also common on coralligenous substrates and in submarine caves. Specimens reached a maximum of 80 mm in total length.
Samplings were carried out by scraping off a vertical dock characterised by the presence of a rich benthic community, at a depth of about 1 m. The specimens were sampled monthly for one year. After collection, samples were transferred to the laboratory where males were selected for spermatozoa analysis.
For transmission electron microscopy, specimens were fixed in a mixture of 2.5% glutaraldehyde, cacodylate buffer (0.4 M) and filtered seawater (pH 7.4). Subsequently they were post-fixed for 1 h in a mixture of 1.0% OsO4 and seawater at 4°C, rinsed in seawater, dehydrated with a graded series of acetone, and embedded in Araldite (Taab, Aldermaston, UK) for sectioning. The ultra-thin sections were cut with an LKB ultratome, contrasted with 5% uranyl acetate in 50% ethanol and lead citrate in distilled water, and examined with a Philips EM 208 compound microscope. Semi-thin sections (1 μm thick) were heat-stained with toluidine blue borate (Millonig Citation1976).
Results
In all the studied species mature specimens were only observed in the summer months from June to August. The germinal cells of the three examined species originate from a germinative epithelium associated to blood vessels in the intersegmental septa. The maturation of the gametes occurs in the coelomic cavity, which appeared to be filled by germinal cells. The region of the body containing gametes appeared widened, occupying most of the abdomen with only the last chaetiger devoid of gametes. The region filled with gametes was white in males, and red–orange in females.
Hydroides dianthus
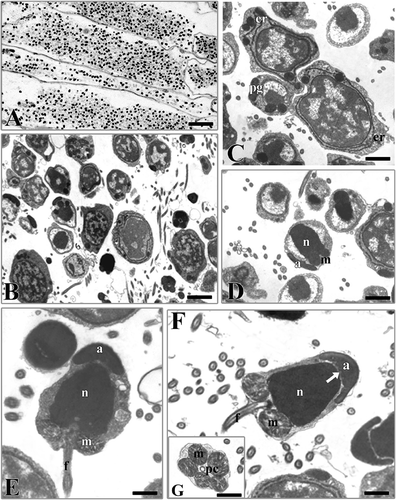
Male germinal cells in various phases of maturation were present in the same individual (). The spermatocytes were irregular cells with a large nucleus and a thin cytoplasmic layer; the chromatin was dispersed with small strongly electrondense zones (). In the early spermatids the nucleus gradually condensed with the irregular accumulation of chromatin in several areas within the nuclear membrane. A well-developed endoplasmic reticulum was observed in the thin layer of cytoplasm. Some electron-dense bodies were present from which probably the acrosomal vesicle originates (). In the late spermatids the nucleus was condensed and its content was compact, homogeneous, and assumed an almost cylindrical shape (). Subsequently, the acrosomal bodies fused giving rise to a small electrondense vesicle towards the front region of the nucleus opposite the mitochondria (). In the last phase of spermiogenesis (), the spermatozoon showed a large cellular body (approx. 4.5 μm × 2.5 μm) with a large nucleus, a thin cytoplasmic layer and a thin tail. A cap-shaped acrosome, devoid of subacrosomal space, enveloped the apical part of the nucleus. Some indentations could be detected in its basal portion facing the nucleus. Two centrioles surrounded by five mitochondria were located at the base of the nucleus (). The flagellum, thin and devoid of cytoplasm, was formed by a typical axoneme with 9x2+2 tubular organisation.
Figure 1. Hydroides dianthus. A, section showing segments full of male germinal cells. B, male germinal cells in various phases of maturation. C, early spermatids. er, endoplasmic reticulum; pg, proacrosomal granules. D, late spermatid. n, nucleus; a, acrosome; m, mitochondria. E, spermatozoon in the last phases of the spermioistogenesis. n, nucleus; a, acrosome; m, mitochondria; f, flagellum. F, spermatozoon. Note the indentations (arrow) in the lower part of the acrosome (a). n, nucleus; m, mitochondria; f, flagellum. G, section at the level of the proximal centriole (pc). m, mitochondria. Scale bars: A, 25 μm. B, 3 μm. C,D, 1 μm. E,F, 0.9 μm. G, 1.5 μm.
Serpula vermicularis
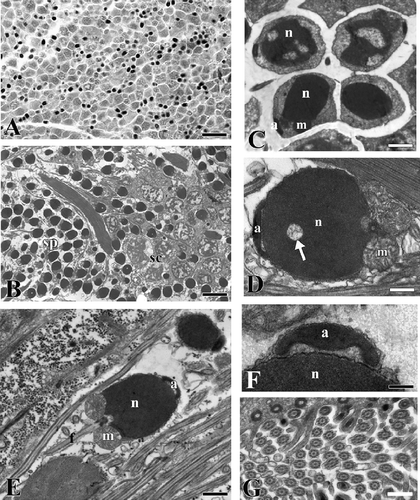
Male germinal cells at various phases of maturation were found in the same specimen, with numerous spermatozoa and groups of spermatocytes with small clods of heterochromatin (,B). In the late spermatids the chromatin was highly condensed and a dish-shaped acrosome not yet located in the definitive position was observed (). In an early phase of maturation the spermatozoon showed a spherical head showing areas of uncondensed chromatin (). At the end of maturation its head became ellipsoidal (4 μm × 2.5 μm) and the base of the nucleus showed a central fossa where the proximal centriole was located (). The acrosome lying on the top of the nucleus was cup-shaped with a swelling towards the nucleus (). Spherical mitochondria with numerous crests were located at the base of the nucleus. The flagellum had a typical 9x2+2 organisation ().
Figure 2. Serpula vermicularis. A, section showing a portion of the coelomic cavity full of male germinal cells in various phases of maturation. B, male germinal cells in various phases of maturation. sc, spermatocytes; sp, spermatozoa. C, spermatids. n, nucleus; a, acrosome; m, mitochondria. D, spermatozoon in which the condensation of the nucleus (n) is not yet complete (arrow). a, acrosome; m, mitochondria. E, spermatozoon. n, nucleus; a, acrosome; m, mitochondria, f, flagellum. F, picture at high magnification of the acrosome (a). n, nucleus. G, section at level of the flagella. Scale bars: A, 20 μm. B, 5 μm. C, 1.5 μm. D, 0.6 μm. E, 1 μm. F, 170 nm. G, 0.5 μm.
Vermiliopsis infundibulum
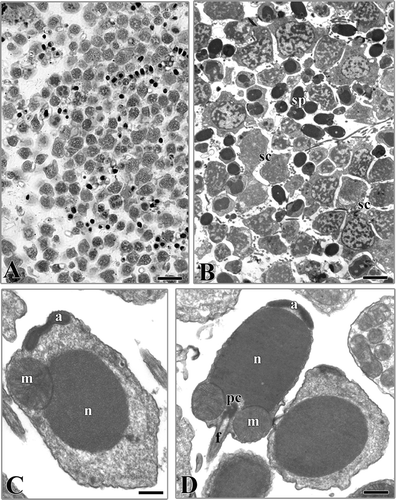
Male germinal cells at various phases of maturation were found in the same specimen (,B). The spermatogenesis is comparable to that of the previously examined species. In the late spermatids, abundant cytoplasm, large mitochondria and an acrosome, which had not yet acquired its final shape and position, were observed (). The spermatozoon (4 μm × 2 μm about) showed a large head with a cylindrical nucleus capped by a simple acrosome (). The base of the nucleus showed a central fossa where the proximal centriole was located as well as lateral fossae less marked for the location of the mitochondria surrounding the anchoring apparatus (). The flagellum consisted of a conventional axoneme surrounded by a plasma membrane.
Figure 3. Vermiliopsis infundibulum. A, section showing a portion of the coelomic cavity full of male germinal cells in various phases of maturation. B, male germinal cells in various phases of maturation. sc, spermatocytes; sp, spermatozoa. C, late spermatid. n, nucleus; a, acrosome; m, mitochondria. D, spermatozoon. n, nucleus; a, acrosome; m, mitochondria; f, flagellum; pc, proximal centriole. Scale bars: A, 15 μm. B, 5 μm. C, 0.4 μm. D, 0.7 μm.
Discussion
All the species examined showed a typical ect-aquasperm sensu Jamieson and Rouse (Citation1989), reported to be associated with external fertilisation (Rouse Citation2005). The sperm consists of a fairly spherical nucleus, a mid piece containing a small number of rounded mitochondria, and a free flagellum. All the species have a quite simple cup-like acrosome. Literature data concerning the type of fertilisation and larval growth of the three examined species are summarised in .
Table I. Some spermatozoa and developmental features of the up to now investigated serpulids. NF, non-feeding larva; F, feeding larva; ?, unknown
In more detail, the acrosome of S. vermicularis is very similar to that observed in the congeneric species Serpula sp. (Jamieson & Rouse Citation1989), although S. vermicularis shows a smaller subacrosomal space and a central swelling towards the nucleus. Hydroides dianthus shows a more developed acrosome compared to that of Serpula, laterally enlarged to cover the nucleus. The external morphology of H. dianthus spermatozoa differs from that of the congeneric species H. elegans previously investigated only by SEM analysis (Nishi Citation1992), in having a more pointed and thin acrosome. The acrosome of H. dianthus also differs from that of an already investigated species reported as H. exagonus (Bosc, 1802) (Colwin & Colwin Citation1961). However, it is not clear which taxon was involved in the experimental literature. Hydroides exagonus is today an unaccepted taxon apparently forgotten for more than a century and afterwards synonymised with the better-known name of H. dianthus not following the ICZN (World Register of Marine species) (Appeltans et al. Citation2010).
In our opinion the significant difference in the sperm morphology between the presently investigated taxon and that investigated by Colwin and Colwin (Citation1961) suggests they are different taxa.
The acrosome of V. infundibulum shows a flattened shape similar to Pomatoleios kraussi (Jamieson & Rouse Citation1989); however, in this latter species it is laterally more elongated over a more rounded nucleus, with a vesiculated subacrosomal space not present in V. infundibulum.
According to Rouse (Citation1999), even if the term ect-aquasperm places no phylogenetic significance on the kind of fertilisation, sperm ultrastructural characters have proved to be informative in clades with uniform reproductive mechanisms. Within the Sabellidae, for instance, the acrosome morphology in broadcasting species appeared constant within each single clade, with a trend of increasing of elaboration in internal structure of the acrosome, going from the more plesiomorphic to the more apomorphic clades (Patti et al. Citation2003).
In , the sperm morphology and acrosomal structure were superimposed to the molecular serpulid phylogeny by Lehrke et al. (Citation2007), who obtained very similar results to a previous analysis by Kupriyanova et al. (Citation2006). In both analyses two main clades were observed: one (clade A) including typical serpulin taxa divided into two subclades. The other (clade B), also divided into two subclades, one containing the Spirorbinae, and the other containing taxa traditionally attributed to Filograninae together with some taxa usually attributed to Serpulinae. In this latter clade, the species Chitinopoma serrula, for which information on spermatozoa structure is available, is present in the analysis by Kupriyanova et al. (Citation2006), but not in that by Lehrke et al. (Citation2007).
Figure 4. The spermatozoa morphology of the up to now investigated serpulid species is superimposed on the most parsimonious tree obtained, with permission, from Lehrke et al. (Citation2007). a, Vermiliopsis infundibulum (present paper); b, Salmacina sp. (redrawn, with permission, from Rouse Citation1996); c, Serpula vermicularis (present paper); d, Hydroides dianthus (present paper); e, Spirobranchus tetraceros (redrawn, with permission, from Selim et al. Citation2005); f, Galeolaria caespitosa (redrawn, with permission, from Jamieson & Rouse Citation1989).
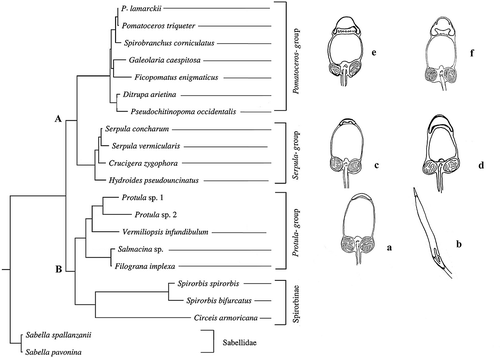
A trend from ect- to ent-aquasperm going from Serpulinae to Spirorbinae can be highlighted. The Serpulinae included in clade A are free-spawners; by contrast, taxa included in clade B show high variability in reproductive strategies, with a prevalence of brooding. All the Spirorbinae are brooders with internal fertilisation, and modified spermatozoa. The filogranins Chitinopoma serrula and Salmacina sp. are brooder forms, as confirmed by the shape of their spermatozoa and the occurrence of sperm storage (Rouse Citation1996). Protula, Salmacina sp. and Chitinopoma serrula have non-feeding larvae (). Finally, Vermiliopsis infundibulum is a free-spawner (Nishi Citation1993) in accordance with the shape of its spermatozoa, but is unclear whether its larvae are feeders.
As generally occurs in marine invertebrates, the reproductive strategy in Sabellidae is linked to size (Rouse & Fitzhugh Citation1994), with brooding and internal fertilisation linked to small-sized species. All the Spirorbinae and Filogranins fit well with this rule (Kupriyanova et al. Citation2001), although the reproductive strategy of C. serrula could also be linked to the colonised environment, since the genus is distributed at high latitudes. This species broods in a special chamber of the tube-like Pseudochitinopoma and not within the operculum as occurs in the Spirorbinae (Zibrowius Citation1969; Kupriyanova et al. Citation2001).
On the basis of the presently available data, the comparison of acrosome ultrastructure within groups having similar reproductive strategy (sperm released into water and fertilisation without sperm storage by females) shows an increase in internal complexity of the acrosome proceeding from Vermiliopsis (Protula group) to Spirobranchus (Pomatoceros group) (). Although the paucity of data on serpulid spermatozoa ultrastructure at present prevents any phylogenetic inference, it seems that the similarity of acrosome shape and ultrastructure could be indicative of closeness. This is the case, for instance, of Galeolaria caespitosa and S. tetraceros, which are closely related taxa, and show a very similar acrosome morphology. By contrast, the acrosome morphology seems to be highly variable within the clade including the closely related taxa Serpula and Hydroides, which differ in the shape and development of the acrosome. Moreover, the acrosome morphology appears conservative within the genus Serpula, but extremely variable within the genus Hydroides. Even more unclear is the external acrosomal similarity of Pomatoleios kraussi and Vermiliopsis infundibulum, two taxa not closely related phylogenetically. Indeed, according to ten Hove and Kupriyanova (Citation2009), it is very likely that the genus Pomatoleios is a synonym of Pomatoceros and Spirobranchus, genera located in the more apomorphic area. By contrast, Vermiliopsis is the most phylogenetically distant genus among the investigated ‘serpulin’ species.
Possibly the acrosomal shape within Serpulidae underwent a more complex evolutionary trend than in Sabellidae, also reflecting the more complex and not yet solved phylogeny of this group compared to the Sabellidae. Further studies are needed to confirm the above-described trend.
Acknowledgements
The work of the authors AG and ML was financed by CoNISMa and Italian MURST, the research of the other authors was supported by grants from the Italian MURST (Scientific Research Ateneo of Bari University).
References
- Appeltans W, Bouchet P, Boxshall GA, Fauchald K, Gordon DP, Hoeksema BW, Poore GCB, van Soest RWM, Stöhr S, Walter TC, Costello MJ, editors. 2010. World Register of Marine Species. http://www.marinespecies.org (http://www.marinespecies.org) (Accessed: 30 September 2010 ).
- Colwin , AL and Colwin , LH. 1961 . Fine structure of the spermatozoon of Hydroides hexagonus (Annelida), with special reference to the acrosomal region . The Journal of Biophysical and Biochemical Cytology , 10 : 211 – 230 .
- Cotter , E , O'Riordan , RM and Myers , AA. 2003 . Recruitment patterns of serpulids (Annelida: Polychaeta) in Bantry Bay, Ireland . Journal of the Marine Biological Association of the United Kingdom , 83 : 41 – 48 .
- Denitto , F and Licciano , M. 2006 . Recruitment of Serpuloidea (Annelida: Polychaeta) in a marine cave of the Ionian Sea (Italy, Central Mediterranean) . Journal of the Marine Biological Association of the United Kingdom , 86 : 1373 – 1380 .
- Franzen , Å. 1982 . Ultra-structure of spermatids and spermatozoa in three polychaetes with modified biology of reproduction: Autolytus sp, Chitinopoma serrula and Capitella capitata . International Journal of Invertebrate Reproduction , 5 : 185 – 200 .
- Giangrande , A. 1997 . Polychaete reproductive patterns, life cycles and life histories: An overview . Oceanography and Marine Biology: An Annual Review , 35 : 323 – 386 .
- Hove , HA ten and Kupriyanova , EK. 2009 . Taxonomy of Serpulidae (Annelida, Polychaeta): The state of affairs . Zootaxa , 2036 : 1 – 26 .
- Jamieson , BG and Rouse , GW. 1989 . The spermatozoa of the Polychaeta (Annelida): An ultrastructural review . Biological Reviews , 64 : 93 – 157 .
- Kupriyanova , EK. 2003 . Live history evolution in Serpulimorph polychaetes: A phylogenetic analysis . Hydrobiologia , 496 : 105 – 114 .
- Kupriyanova , EK , Macdonald , TA and Rouse , GW. 2006 . Phylogenetic relationships within Serpulidae (Sabellida, Annelida) inferred from molecular and morphological data . Zoologica Scripta , 35 : 421 – 439 .
- Kupriyanova , EK , Nishi , E , ten Hove , H and Rzhavsky , AV. 2001 . Life-history patterns in serpulimorph polychaetes: Ecological and evolutionary perspectives . Oceanography and Marine Biology: An Annual Review , 39 : 1 – 101 .
- Kupriyanova , EK and Rouse , GW. 2008 . Yet another example of paraphyly in Annelida: Molecular evidence that Sabellidae contains Serpulidae . Molecular Phylogenetics and Evolution , 46 : 1174 – 1181 .
- Lehrke , J , ten Hove , HA , Macdonald , TA , Bartolomaeus , T and Bleidorn , C. 2007 . Phylogenetic relationships of Serpulidae (Annelida: Polychaeta) based on 18S rDNA sequence data, and implications for opercular evolution . Organisms, Diversity and Evolution , 7 : 195 – 206 .
- Mercurio , M , Sciscioli , M , Lepore , E and Gherardi , M. 2008 . Note sulla popolazione di Hydroides dianthus (Verrill, 1873) , Polychaeta : Serpulidae . del Mar Piccolo di Taranto (Mar Ionio). 39° Congresso della Società Italiana di Biologia Marina, 9–13 Giugno 2008, Cesenatico (FC)
- Millonig , G. 1976 . Laboratory manual of biological electron microscopy , Vercelli : Edizioni Saviolo .
- Musco , L , Giangrande , A , Gherardi , M , Lepore , E , Mercurio , M and Sciscioli , M. 2008 . Sperm ultra-structure of Odontosyllis ctenostoma (Polychaeta: Syllidae) with inferences on syllid phylogeny and reproductive biology . Scientia Marina , 72 : 421 – 427 .
- Musco , L , Lepore , E , Gherardi , M , Sciscioli , M , Mercurio , M and Giangrande , A. 2010 . Sperm ultra-structure of three Syllinae (Annelida, Phyllodocida) species with considerations on syllid phylogeny and Syllis vittata reproductive biology . Zoomorphology , 129 : 133 – 139 .
- Nishi , E. 1992 . Sperm morphology of serpulid polychaetes Pomatoleios kraussi (Baird), Spirobranchus giganteus corniculatus Pallas, Hydroides elegans Haswell and Salmacina dysteri (Huxley) . Galaxea , 11 : 9 – 14 .
- Nishi , E. 1993 . Notes on reproductive biology of some serpulid polychaetes at Sesoko Island, Okinawa, with brief accounts of setal morphology of three species of Salmacina and Filograna implexa . Marine Fouling , 10 : 11 – 16 .
- Patti , P , Gambi , MC and Giangrande , A. 2003 . Preliminary study on the systematic relationships of Sabellinae (Polychaeta: Sabellidae), based on the C1 domain of the 28S rDNA, with discussion of reproductive features . Italian Journal of Zoology , 70 : 269 – 278 .
- Pillai , TG. 1970 . Studies on a collection of spirorbids from Ceylon, together with a critical review and revision of spirorbid systematics and an account of their phylogeny and zoogeography . Ceylon Journal of Science (Biological Sciences) , 8 : 100 – 172 .
- Rouse , GW. 1996 . Variability of sperm storage by females in the Sabellidae and Serpulidae (Polychaeta, Sabellida) . Zoomorphology , 116 : 179 – 193 .
- Rouse , GW. 1999 . Polychaete sperm: Phylogenetic and functional considerations . Hydrobiologia , 402 : 215 – 224 .
- Rouse , GW. 2005 . Annelid sperm and fertilization biology . Hydrobiologia , 535/536 : 167 – 178 .
- Rouse , GW and Fitzhugh , K. 1994 . Broadcasting fables: Is external fertilization really primitive? Sex, size, and larvae in sabellid polychaetes . Zoologica Scripta , 23 : 271 – 314 .
- Rouse , GW and Pleijel , F. 2001 . Polychaetes , 354 Oxford : Oxford University Press .
- Rousset , V , Rouse , GW , Siddall , ME , Tillier , A and Pleijel , F. 2004 . The phylogenetic position of Siboglinidae (Annelida), inferred from 18S rRNA, 28S rRNA, and morphological data . Cladistics , 20 : 518 – 533 .
- Selim , SA , Abdel Naby , F , Gab-Alla , AA-FA and Ghobashy , A. 2005 . Gametogenesis and spawning of Spirobranchus tetraceros (Polychaeta, Serpulidae) in Abu Kir Bay, Egypt . Mediterranean Marine Science , 6 : 89 – 97 .
- West , SA. 1991 . An ultrastructural study of gametogenesis in Hydroides ezoensis (Okuda) (Polychaeta: Serpulidae) . Ophelia, Supplement , 5 : 702 – 703 .
- Zibrowius , H. 1969 . Review of some little known genera of Serpulidae (Annelida, Polychaeta) . Smithsonian Contributions to Zoology , 42 : 1 – 22 .