Abstract
In females of Isopoda Oniscidea, the genital system displays a remarkable variability of its morphological and functional organization as possible adaptation to different strategies of sperm storage. In all the oniscidean species, the sperm received from the male during mating are temporarily stored in the bursa copulatrix, a chitinous pouch of the oviduct. In 27 of the 32 species we studied, the sperm that remain in the bursa copulatrix after the fertilization of eggs of the first oviposition are transferred into the seminal receptacle, where they can be stored for a variable time. In these species, the seminal receptacle is a small kidney-shaped cup, localized at the insertion of the oviduct into the ovary. Only in two species of the genus Trichoniscus, in which the oviduct is very short, is the bursa copulatrix modified to form a large lateral diverticle.
Instead, in three species of the family Halophilosciidae, Halophiloscia couchii, Halophiloscia hirsuta and Stenophiloscia glarearum, the ovary is consistently shorter, while the seminal receptacle is greater; after mating, both seminal receptacle and ovary appear completely filled with sperm. Finally, in two species of the family Tylidae, Tylos europaeus and Helleria brevicornis, the female genital system lacks specialized structures for sperm storage, and every oviposition requires a mating for the eggs fertilization.
The authors present some hypotheses to explain the variability of the female genital system morphology and the sperm storage strategies.
Introduction
The oniscidean isopods are the crustacean taxon that have had the widest success in the colonization of emerged land (Edney Citation1968). This success is testified by over 520 genera, assembled in 39 families and by approximately 3600 valid species (Schmalfuss Citation2003).
Probably, the evolutionary success of oniscideans is derived from the considerable genetic, biochemical, physiological and morphological ‘flexibility’ shown by these organisms, which is translated into an exceptional adaptive radiation which has allowed them to inhabit an enormous variety of habitats except higher summits and poles (Vandel Citation1964); in particular, the oniscideans have been able to combine the most efficacious adaptive peculiarities of amphipods (for example, direct development inside a cavity of the maternal body) and of decapods (the presence of locomotor versatile appendages, suitable for a large variety of substrata).
The high flexibility of the oniscideans can also be seen in their reproductive biology, as pointed out by Dangerfield and Telford (Citation1995): ‘such flexibility, particularly in reproductive tactics, may be the key to the success of woodlice as terrestrial animals’.
Some species of oniscideans are known to be semelparous, e.g. Tylos punctatus, (Hamner et al. Citation1969), Hemilepistus reamuri (Warburg Citation1987) and Schizidium tiberianum (Warburg & Cohen Citation1991; Warburg et al. Citation1993), whose females breed only once in their reproductive lifetime.
Most species of oniscideans are iteroparous; i.e. they breed more than once in their lifetime or, even, several times within a single breeding season (Warburg Citation1993).
Also the relationships among mating, vitellogenesis and oviposition can be highly variable. In some species, such as Tylos latreillei and Helleria brevicornis (Mead Citation1973), Ligia oceanica (Besse et al. Citation1969), Ligidium hypnorum and Philoscia muscorum (Vandel Citation1928) mating occurs only when the female shows functional oostegites and it accompanies each parturial moult. In these species the vitellogenesis does not seem to depend on mating and each parturial moult requires a mating to become fertile (Besse Citation1976).
In other species, the first mating occurs during the intermoult phase before the first parturial moult and happens before the female has functional oostegites. In this case, the establishment of a stockpile of sperm within the female genital tract always occurs, and eggs can be fertilized without further matings.
The need to realize an effective conservation of sperm in the female genital tract is certainly linked to the variability of the sex ratio in the different species of oniscideans. Whereas in some species of oniscideans, e.g. Trichoniscus pygmaeus or Armadillidium nasatum (Vandel Citation1925), the male/female ratio does not vary greatly from 1:1; in other species, such as Philoscia muscorum, it is about 1:11 and in Trichoniscus pusillus (Frankel et al. Citation1981) and Ocelloscia floridana (Johnson Citation1986) it is less than 1:200. In other species, such as Platyarthrus aiasensis, the sex ratio of the population differs in relation to the geographic area of its provenance ranging from a male/female ratio of 1:1 to total lack of males, with obvious recourse to parthenogenesis (Caruso Citation1968; Montesanto et al. Citation2008). The female-biased sex ratio is often due to Wolbachia, an endocellular α-proteobacteria that causes feminization of genetic males in many species of Oniscidea (Martin et al. Citation1973; Bouchon et al. Citation1998; Rigaud et al. Citation1999), while in Cylisticus convexus (Moret at al. Citation2001) and in Porcellio dilatatus (Legrand et al. Citation1978; Rousset et al. Citation1992) it induces cytoplasmic incompatibility, an effect commonly found in insects. In Armadillidium vulgare, a species studied intensively, the presence of Wolbachia in the females ranges from 0 to 64%, but most populations are uninfected (Juchault et al. Citation1993).
The variability of the reproductive strategies in Oniscidea is related to the variability of the morphological and functional organization of the female genital system and, in particular, of the structures and sites used for the sperm storage. In the few species studied until now under this aspect, sperm are stored in the seminal receptacle, which represents a specialized region of the female genital tract, localized at the confluence of the oviduct with the ovary (Besse Citation1976; De Luca et al. Citation1987; Longo et al. Citation1998).
Instead, in Trichoniscus pusillus provisorius, the sperm are stored, according to Vandel (Citation1937), in a diverticle of the oviduct wall, described by the author as a spermatheca. Vandel has also suggested a possible sperm storage in the ovary.
In spite of the stimulating information arising from the researches of Vandel (Citation1925, 1928, 1937), who has pointed out how diversified strategies of sperm storage in the female genital system were implemented by the different oniscidean species, only few studies have improved the knowledge of these aspects.
On these premises and having personally observed in some species of the genus Halophiloscia a great variability in the morphology of their female genital system in relation to sperm storage, we have started a more punctual study with the aim of investigating the morphological variability of the genital system in Oniscidea, extending the observations to numerous species belonging to different families of this suborder of isopods.
Materials and methods
The observations were carried out on sexually mature females of 32 species belonging to 14 families of Oniscidea (after Schmalfuss Citation2003).
| • | Tylidae: Helleria brevicornis Ebner, 1968; Tylos europaeus Arcangeli, 1938; | ||||
| • | Ligiidae: Ligia italica Fabricius, 1798; | ||||
| • | Trichoniscidae: Haplophthalmus siculus Dollfus, 1896, Siciloniscus tulliae Caruso, 1982, Trichoniscus alexandrae Caruso, 1978, Trichoniscus provisorius Racovitza, 1908; | ||||
| • | Stenoniscidae: Stenoniscus carinatus Silvestri, 1897; | ||||
| • | Platyarthridae: Platyarthrus aiasensis Legrand, 1954, Trichorhina sicula Vandel, 1969; | ||||
| • | Detonidae: Armadilloniscus ellipticus (Harger, 1878); | ||||
| • | Bathytropidae: Bathytropa schembrii Caruso & Lombardo, 1982; | ||||
| • | Halophilosciidae: Halophiloscia couchii (Kinahan, 1858), Halophiloscia hirsuta Verhoeff, 1928, Stenophiloscia glarearum Verhoeff, 1908; | ||||
| • | Philosciidae: Anaphiloscia sicula Arcangeli, 1934, Chaetophiloscia elongata (Dollfus, 1884), Ctenoscia dorsalis (Verhoeff, 1928), Philoscia affinis Verhoeff, 1908; | ||||
| • | Oniscidae: Oniscus asellus Linnaeus, 1758; | ||||
| • | Porcellionidae: Acaeroplastes melanurus (Budde-Lund, 1885), Leptotrichus naupliensis (Verhoeff, 1901), Porcellio albicornis (Dollfus, 1896), Porcellio lamellatus Budde-Lund, 1985, Porcellionides pruinosus (Brandt, 1833), Proporcellio quadriseriatus Verhoeff, 1917; | ||||
| • | Trachelipodidae: Trachelipus arcuatus (Budde-Lund, 1885); | ||||
| • | Armadillidiidae: Armadillidium aelleni Caruso & Ferrara, 1982, Armadillidium granulatum Brandt, 1833, Armadillidium nasatum Budde-Lund, 1885, Armadillidium vulgare (Latreille, 1804); and | ||||
| • | Armadillidae: Armadillo officinalis Duméril, 1816. | ||||
Most species were collected in different places in Sicily (Italy); the specimens were determined by Professor Domenico Caruso who, moreover, provided us with Armadillidium aelleni females collected in the island of Malta. Females of Helleria brevicornis were collected in Sardinia (Italy) by Dr Rosario Grasso; females of Oniscus asellus were collected near Tübingen (Germany) and kindly furnished by Dr Reinhard Gerecke.
At least 20 specimens were studied for each species. The female genital system was dissected out in Ringer's saline solution modified for land isopods (Besse Citation1976). The female genital system was studied and photographed with a stereomicroscope, both fresh and after a brief fixation in 2.5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.2. Some specimens were in toto stained with 0.1% toluidine blue in 0.1 M phosphate buffer, pH 7.2 or with Sudan black IV B (saturated solution in isopropyl alcohol).
Results
The observations carried out on 32 species of oniscideans highlighted a variability of the morphological organization of the female genital system and in particular of the structures used for sperm storage. We have identified four patterns of morphological organization of the female genital system.
‘Common’ pattern
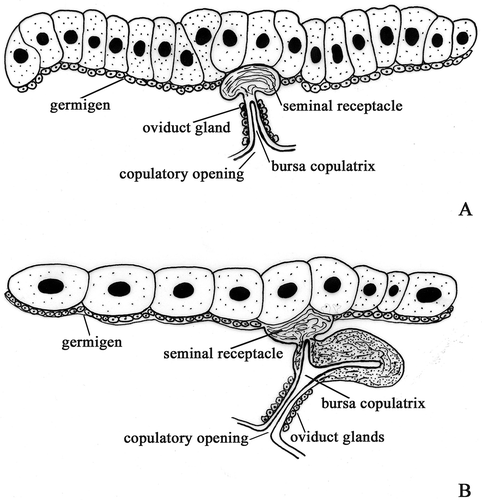
It is the pattern found in 25 of the 32 species observed (Ligia italica, Haplophtalmus siculus, Siciloniscus tulliae, Stenoniscus carinatus, Platyarthrus aiasensis, Trichorhina sicula, Armadilloniscus ellipticus, Bathytropa schembrii, Anaphiloscia sicula, Chaetophiloscia elongata, Ctenoscia dorsalis, Philoscia affinis, Oniscus asellus, Acaeroplastes melanurus, Leptotrichus naupliensis, Porcellio albicornis, Porcellio lamellatus, Porcellionides pruinosus, Proporcellio quadriseriatus, Trachelipus arcuatus, Armadillidium aelleni, Armadillidium granulatum, Armadillidium nasatum, Armadillium vulgare, Armadillo officinalis) and it presents the basic characteristics reported for other oniscideans in literature. The female genital system consists of two separate ovaries located dorsolaterally to the intestine and extended from the second to the seventh segment of the pereion (). An oviduct is connected to each ovary and opens into the copulatory opening (), situated on the lateral margin of the sternite in the fifth pereionite, near the base of the fifth pereiopod. Each ovary is connected to the lateral wall of the body by suspension ligaments.
Figure 1. Schematic drawing of the female genital system of an oniscidean isopod (ventral vision). Ov, ovaries; od oviduct; sr, seminal receptacle; g, germigen.
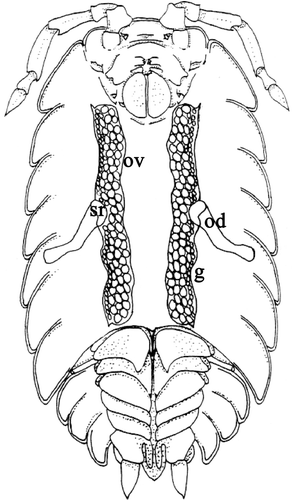
Figure 2. Schematic drawing of female genital system. A, ‘Common’ pattern. B, ‘Trichoniscus’ pattern.
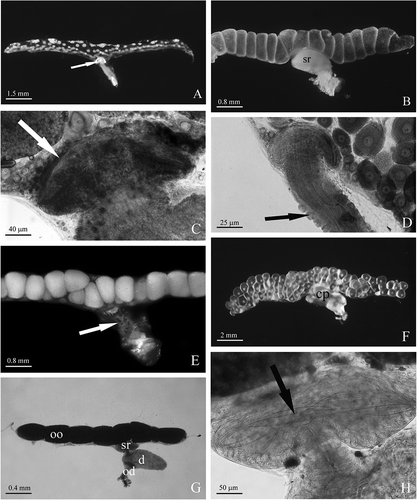
The aspect of the ovary changes depending on the growth stages of oocytes; when it contains small oocytes, it looks like a dorsoventrally flattened sac (); when it contains mature oocytes, its diameter increases markedly and its wall appears irregularly swollen ().
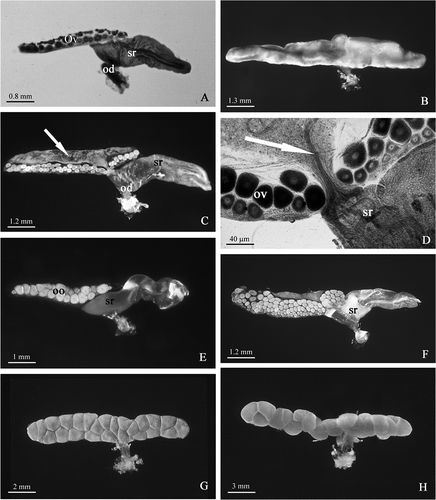
Figure 3. A, Armadillidium granulatum. Female at intermoult stage: the flattened ovary contains small oocytes. The seminal receptacle contains bundles of sperm (arrow). B, Oniscus asellus. In mated females, the seminal receptacle (sr) is large and clearly visible as a kidney dilatation of the ovary's wall. C, Armadillidium aelleni. Mated female: a bundle of sperm (arrow) is present in the lumen of the seminal receptacle. D, Armadillidium nasatum. The oviduct is inserted on the wall of the ovary forming an acute angle; two rows of small glands (arrow) protrude from the oviduct wall. E, Ligia italica. Numerous chromatophores (arrow) are scattered on the surface of the oviduct. F, Armadillidium vulgare. Female just after mating: the chitinous pouch (cp) of the oviduct (bursa copulatrix) is filled of sperm and considerably enlarged. G, Trichoniscus alexandrae. Female with brood pouch: the ovary contains few large oocytes (oo); sr, seminal receptacle; d, diverticle of the oviduct emptied of sperm; od, oviduct. H, Trichoniscus alexandrae. Female with brood pouch: some sperm (arrow) are visible into the seminal receptacle.
A germinal zone, or germigen, which forms a band containing primary oogonia and follicular cells, is present along the lateral margin of each ovary; it is interrupted in correspondence of the insertion of the oviduct into the ovary, where the seminal receptacle is present ().
In some species, e.g. Haplophtalmus siculus, Stenoniscus carinatus, Trichorhina sicula, Bathytropa schembrii, the seminal receptacle is small and almost entirely localized in the wall of the ovary; in the other species it is larger and clearly visible as a kidney-shaped dilatation of the ovary wall (). In mated females, it is easy to see the presence of bundles of sperm in the lumen of the seminal receptacle ().
In most species, the oviduct is connected to the wall of the ovary forming a straight angle; in some species, e.g. Armadillidium nasatum and Chaetophiloscia elongate, it forms, instead, an acute angle (). One or two rows of small, pear-shaped glands protrude from the oviduct wall (); in Ligia italica, numerous star-shaped chromatophores are scattered on the outer surface of the oviduct (). In virgin females, the cuticle lining the lumen thickens considerably at the cranial end of the oviduct forming a chitinous cap that occludes the lumen completely; after mating the sperm enter the lumen of the oviduct, which dilates considerably, assuming the role of bursa copulatrix (). Before the release of the mature eggs into the brood pouch, the chitinous cap undergoes lysis and a large quantity of sperm are transferred into the lumen of the seminal receptacle.
‘Trichoniscus’ pattern
In Trichoniscus provisorius and Trichoniscus alexandrae the ovaries extend from the second to the seventh segment of the pereion. Each ovary contains a small number of oocytes arranged in a single row (, ). A seminal receptacle is present in the form of a small cup-like protrusion of the ovary wall (, ). A short oviduct arises from the seminal receptacle and, immediately below; it dilates forming a large diverticle that extends in the sixth segment (). This diverticle presents a large lumen lined, like the oviduct, by a thick cuticle. Immediately after mating, the lumen of this diverticle is filled with sperm embedded in a dense secretion. In females with a brood pouch, the diverticle is emptied of sperm while some are still present in the lumen of the seminal receptacle ().
Distal to the diverticle the oviduct continues as a short tube, which opens into the copulatory opening at the base of the fifth pereiopod ().
‘Halophiloscia’ pattern
The female genital system of Halophiloscia couchii, Halophiloscia hirsuta and Stenophiloscia glarearum exhibits peculiar characteristics that differentiate it greatly from the known pattern for the other species of oniscideans (). Moreover, its morphological aspect and organization change considerably according to the phase of the reproductive cycle.
Figure 4. Schematic drawing of female genital system. A, ‘Halophiloscia’ pattern. B, ‘Tylos’ pattern.
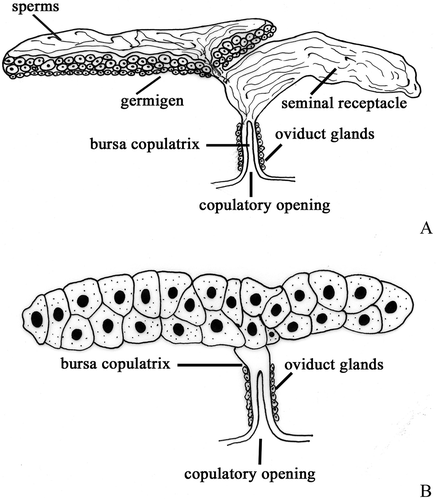
In virgin, sexually mature females the genital system consists, as usual, of two separated ovaries that are situated dorsolaterally to the intestine. In these species, however, the ovaries are shorter: in Halophiloscia couchii and Halophiloscia hirsuta they extend between the second and the fifth segment of the pereion while in Stenophiloscia glarearum they extend only to the fourth segment. Each ovary contains growing oocytes (10–20) generally arranged in one or two rows. The germinal zone is located, as usually, along the lateral margin of the ovary. The seminal receptacle arises from the caudal end of the ovary (, ); it is like a large bag that extends to the seventh segment of the pereion. The oviduct originates from the lateral surface of the seminal receptacle; it is short and emerges into the copulatory opening at the base of the fifth pereiopod ().
Figure 5. A, Halophiloscia couchii. Virgin female: Ov, ovary; sr, seminal receptacle; od, oviduct. B, Halophiloscia hirsuta. Female just after mating: the genital system is entirely filled with sperm and its different regions are not more recognizable each from the other. C, Halophiloscia hirsuta. Female with brood pouch: the seminal receptacle (sr) is still partially filled with sperm such as a small pouch located along the medial margin of the ovary (arrow). od, oviduct. D, Halophiloscia hirsuta. Female with brood pouch: a bundle of sperm (arrow) is present in the thin isthmus connecting the ovary (ov) with the seminal receptacle (sr). E, Halophiloscia hirsuta. Female at intermoult stage: the seminal receptacle (sr) is partially emptied of sperm; large oocytes (oo) are present in the ovary while the region before occupied by the sperm is disappeared. F, Halophiloscia couchii. Female 2 years old at intermoult stage: the ovary contains numerous growing oocytes arranged in three to four rows; the seminal receptacle (sr) is filled with sperm. G, Tylos europaeus. Female just before parturial moult with ovaries containing mature oocytes. The genital system lacks of seminal receptacle. H, Helleria brevicornis. Female just before parturial moult. Also in this semelparous species the seminal receptacle is absent.
In the females of these species, after mating, sperm completely fill not only the seminal receptacle but also the lumen of the ovary; consequently, the female genital system frequently appears as a single bag filled with sperm and its different structures are no more recognizable from each other ().
In females who have undergone the reproductive moult and have released fertilized eggs into the brood pouch, the seminal receptacle still contains a large quantity of sperm (); in the ovary of these females it is possible to distinguish again the germinal zone and the growing oocytes and, moreover, a small pouch filled with sperm is visible along the medial margin of the ovary (). This region of the ovary is connected with the seminal receptacle through a thin isthmus inside which a thin bundle of sperm is visible ().
If the female does not mate again later in the reproductive season, the seminal receptacle is progressively emptied of sperm while in the ovary, where large oocytes are now present, the region previously occupied by the sperm is progressively reduced until it disappears (). In 2-year-old and large-sized females, 40–60 growing oocytes, arranged in three to four rows, are present in the ovary ().
‘Tylos’ pattern
In Tylos europaeus and in Helleria brevicornis, the ovaries extend from the first to the seventh thoracic segment and contain 15–25 oocytes generally arranged in two rows. In these semelparous species, both the germinal zone and the seminal receptacle are absent (, , ); therefore, sperm, after mating, are stored in the chitinous bursa copulatrix until the release of the eggs into the brood pouch.
Discussion
In many species of vertebrates and invertebrates, the females may store sperm for many months or even years after mating in specialized districts of the reproductive system (Selmi Citation1992).
A prolonged storage of sperm can be aimed at different goals such as:
| a. | to allow the female gonads to complete the maturation and release of eggs, events often sequentially related to mating; | ||||
| b. | to fulfill the specific physiological requirements of the reproductive process, that are not always well known; this is the case of many species of chiropterans in which mating takes place in autumn and the fertilization takes place the following spring; and | ||||
| c. | to represent a kind of ‘insurance policy’ in the case of a missed further mating. | ||||
This last aspect particularly concerns all those species in which the meeting between males and females is aleatory, as in most oniscidean species in which the sex ratio is more or less biased toward females. In the oniscideans, this sex distortion is frequently due to the presence of Wolbachia, an α-proteobacteria intracellular symbiont, responsible for the feminization of the genetic males (Martin et al. Citation1973; Bouchon et al. Citation1998; Stouthamer et al. Citation1999).
Prolonged sperm storage in the female genital system of oniscideans has been known for a long time (Schöbl Citation1880); the sperm reserve allows females to fertilize their eggs for a variable time after mating, e.g. for 3–4 months in Trichoniscus provisorius (Vandel Citation1937) or up to 17 months in Armadillidium vulgare (Vandel Citation1941).
A long-term sperm storage in the female genital system does not, however, seem to be realized in all species of oniscideans; according to the data present in literature, this seems be due to a great variability in the relationships between mating, vitellogenesis and oviposition present in the various species so far investigated (Vandel Citation1928; Besse et al. Citation1969; Mead Citation1973; Besse Citation1976; Warburg Citation1993).
Our investigation has identified four different patterns of morphological organization of the female genital system, the diversity of which seems to be clearly related to different strategies of sperm storage.
In all the species belonging to the families Porcellionidae, Trachelipodidae, Armadillidiidae and Armadillidae, which include the most evolved species of Oniscidea (Schmalfuss Citation1989), the organization pattern of the female genital system is always similar to that known from literature (Besse Citation1976; Longo et al. Citation1998; Suzuki & Ziegler Citation2005; Longo & Trovato Citation2008) and denominated in the present paper as ‘common pattern’. A similar pattern has been found in many species belonging to other families, even phylogenetically distant, such as Ligia italica (Ligiidae), Siciloniscus tulliae (Trichoniscidae), Stenoniscus carinatus (Stenoniscidae), Platyarthrus aiasensis (Platyarthridae), Armadilloniscus ellipticus (Detonidae), Chaetophiloscia elongata and Philoscia affinis (Philosciidae), and Oniscus asellus (Oniscidae).
In females characterized by this pattern of genital system, during mating males transfer an abundant number of sperm into the bursa copulatrix – a chitinous pouch present inside the oviduct – which dilates notably (Besse Citation1976; De Luca et al. Citation1987). In the course of the following parturial moult or immediately after it, the bursa copulatrix undergoes lysis of its cranial end, probably consequent to the chitinolitic activity performed by the glands of the oviduct (Nemec Citation1896; De Luca et al. Citation1987).
After release of the fertilized eggs into the brood pouch, a portion of the sperm present in the bursa copulatrix is transferred into the seminal receptacle, where it is stored for a time which is long enough to assure – in the absence of further mating – the fertilization of the eggs.
A second pattern of female genital system organization was found in two species of the Trichoniscus genus; as it has previously been remembered, Vandel (Citation1937) described in Trichoniscus pusillus provisorius (syn. of Trichoniscus provisorius acc. Schmalfuss Citation2003) the existence of a spermatheca, a lateral pouch of the oviduct that extends toward the caudal region of the body. Our observations have shown that the sperm storage in this structure is limited to the time between the mating and the following release of fertilized eggs into the brood pouch; after this event the spermatheca appears emptied of sperm, a part of which, in the opinion of Vandel (Citation1937), would go up again into the ovary to constitute a reserve of sperm useful to fertilize further eggs.
In our opinion, the assessments of Vandel concerning sperm storage in Trichoniscus pusillus provisorius should be revised; the structure identified by Vandel as a spermatheca is nothing other than a bursa copulatrix equivalent to the chitinous pouch of the oviduct of other species of oniscideans. This variation, observed only in the species of the genus Trichoniscus, would be made necessary by the reduced size of the oviduct that makes the storage of all the sperm transferred during the mating impossible; otherwise, in Haplophtalmus siculus and in Siciloniscus tulliae, species belonging to the same family, where the oviduct has a greater size, there is no lateral diverticle (the so-called ‘spermatheca’ by Vandel) and the sperm are stored into the chitinous pouch of the oviduct.
A more consistent variation of the female genital system organization has been found in some species of the family Halophilosciidae, namely Halophiloscia couchii, Halophiloscia hirsuta and Stenophiloscia glarearum, in which the ovaries are shorter than in other oniscideans and the seminal receptacle arises from the caudal end of the ovary in the form of a large bag.
In the mated females of these species, both the seminal receptacle and the ovary appear filled with sperm. After release of the mature eggs into the brood pouch, a residual number of sperm are still present into the seminal receptacle and also in a small region present along the medial surface of the ovary. This region is in continuity with the lumen of the seminal receptacle through a thin isthmus that runs transversally into the ovary.
Finally, the genital system of the females of Tylos europaeus and Helleria brevicornis lacks specialized sites for sperm storage. This finding confirms what has been previously sustained by Vandel (Citation1928), Mead (Citation1973), and Besse et al. (Citation1969) for these species, in which the mating takes place only when the female presents functional oostegites and, therefore, every further egg laying needs a new mating for fertilization. This makes sperm storage useless.
What may be the reasons of the great variability showed by the female genital system and by the strategies of sperm storage in the Oniscidea? A hypothesis based on phylogenetic constraints is not supported by any data in our possession. A plausible hypothesis seems to arise from variable relationships between mating, vitellogenesis and oviposition observed in the oniscidean species so far investigated.
In some species, e.g. Tylos latreillei and Helleria brevicornis (Mead Citation1973), Ligia oceanica (Besse et al. Citation1969), Ligidium hypnorum and Philoscia muscorum (Vandel Citation1928), as mentioned above, mating occurs only when the female shows functional oostegites and it accompanies each parturial moult. The vitellogenesis does not seem to depend on the mating and each parturial moult needs a further mating in order to be fertile (Besse Citation1976).
In other species, instead, the first mating occurs during the intermoult preceding the first parturial moult and, consequently, when the female does not yet show functional oostegites; in this case, the establishment of a reserve of sperm in the female genital system – useful to guarantee the eggs fertilization in absence of a further mating – is always puts into effect.
Another hypothesis could be founded on the variability of the paternal investment of trophic resources finalized to support ovogenesis and eggs maturation; some our preliminary ultrastructural investigations carried out on the seminal receptacle of Halophiloscia couchii, Halophiloscia hirsuta and Stenophiloscia glarearum (Longo and Trovato, unpublished results) have shown that a portion of the sperm stored in the lumen is captured by the epithelial cells of the seminal receptacle and then drawn into tubular cavities, as has been observed in the seminal receptacle of Porcellio laevis (Longo et al. Citation1998), Armadillidium vulgare (Suzuki & Ziegler Citation2005) and Armadillidium granulatum (Longo & Trovato Citation2008).
A spermiophagic activity regarding the sperm stored into the female genital system does not represent something new; in fact, it has already been described in the spermatheca of some species of Oligochaeta (Richards & Fleming Citation1982), in the seminal receptacle of an annelid polychaete (Westheide Citation1988) and in the spermathecal duct of some species of Orthoptera Tettigoniidae (Viscuso et al. Citation1996; Brundo et al. in press).
In the oniscideans, however, the capture involves almost exclusively the long sperm tails that successively undergo progressive digestion.
The role of the sperm tail of the oniscideans – which is essentially made up of proteins – could represent a transfer of nutrients from the male to the female, useful to support its reproductive effort. This hypothesis agrees with our findings that in Halophiloscia and Stenophiloscia species a large quantity of sperm – certainly excessive in comparison to the need of the eggs fertilization – is transferred into the female genital system during the mating.
Finally, a further motivation could take into consideration the effects of Wolbachia, which could have acted as agent responsible of a selective pressure turned to modify the female genital system organization to favor a more efficient strategy of sperm storage, useful to assuring repeated fertilization even in the absence of further mating. No experimental evidence exists to support such a hypothesis; recent research carried out on Platyarthrus aiasensis (Montesanto et al. Citation2008; Montesanto et al., personal communication) have pointed out that, in the different populations of this species, the presence of Wolbachia results to be extremely variable and there are parthenogenetic populations where the endosymbiont is totally absent. In the populations of Platyarthrus aiasensis studied in this research (one of which was parthenogenetic), no variation regarding the organization of the female genital system has been found. Still, Wolbachia represents a powerful manipulator of the reproductive biology of its hosts, and it would be very interesting to further the research on its possible influence on the aspects concerning the evolution of the structures used for sperm storage.
Acknowledgements
The authors wish to thank Professor Domenico Caruso for his valuable help during sampling of oniscideans, for his determination and for critically reading the manuscript. We also thank Dr Rosario Grasso of our Department who provided us with specimens of Helleria brevicornis and Dr Reinhard Gerecke (University of Tübingen, Germany) who provided us with specimens of Oniscus asellus.
References
- Besse , G. 1976 . Contribution a l’étude expérimentale de la physiologie sexuelle femelle chez les crustacés Isopodes terrestres [Thèse Doctorat d'Etat] , Université de Poitiers, CNRS France, n° AO 13 017 .
- Besse , G , Juchault , P , Legrand , JJ and Mocquard , JP. 1969 . Contribution à l’étude de la physiologie sexuelle femelle chez Ligia oceanica L. (Crustacé Oniscoĭde) . Différenciation des oostégites et contrôle neurohumoral de la maturation ovarienne. Comptes rendus hebdomadaire des Séances de l'Académie des Sciences (Paris), Série D , 269 : 733 – 736 .
- Bouchon , D , Rigaud , T and Juchault , P. 1998 . Evidence for widespread Wolbachia infection in isopod crustaceans: Molecular identification and host feminisation , Vol. B 265 , 1081 – 1090 . London : Proceedings of the Royal Society .
- Brundo , MV , Longo , G , Sottile , L , Trovato , M , Vitale , D and Viscuso , R . in press . Morphological and ultrastructural organization of the spermatheca of some Tettigoniidae (Insecta, Orthoptera) . Italian Journal of Zoology ,
- Caruso , D. 1968 . Partenogenesi e spanandria in Platyarthrus aiasensis Legrand (Crustacea Isopoda) . Bollettino dell'Accademia Gioenia di Scienze Naturali di Catania, Serie IV , 9 : 451 – 457 .
- Dangerfield , JM and Telford , SR. 1995 . “ Reproduction in woodlice: Flexibility to maximise individual fitness ” . In Terrestrial isopod biology , Edited by: Alikhan , MA . Vol. 9 , 69 – 82 . Rotterdam : A.A. Balkema. Crustacean Issues .
- De Luca , V , Longo , G , Sottile , L , La Spina , G and Viscuso , R. 1987 . Scanning electron microscopy and histochemistry of the reproductive female system and sperm storage in Porcellio laevis Latreille (Isopoda Oniscoidea) . Acta embryologiae et morphologiae experimentalis , 8 : 243 – 255 .
- Edney , EB. 1968 . Transition from water to land in isopod crustaceans . American Zoologist , 8 : 309 – 326 .
- Frankel , B , Sutton , SL and Fussey , GD. 1981 . The sex ratios of Trichoniscus pusillus Brandt (Crustacea: Oniscoidea) . Journal of Natural History , 15 : 301 – 307 .
- Hamner , W , Smith , M and Mulford , E. 1969 . The behaviour and life history of a sand-beach isopod, Tylos punctatus . Ecology , 50 : 442 – 453 .
- Johnson , C. 1986 . Parthenogenetic reproduction in the philosciid isopod, Ocelloscia floridana (Van Name, 1940) . Crustaceana , 51 : 123 – 132 .
- Juchault , P , Rigaud , T and Mocquard , JP. 1993 . Evolution of sex determination and sex ratio variability in wild populations of Armadillidium vulgare (Latr.) (Crustacea, Isopoda): A case study in conflict resolution . Acta Oecologia , 14 : 547 – 562 .
- Legrand , JJ , Martin , G and Artault , JC. 1978 . Corrélation entre la présence d'un symbiote bactérien dans les ovocytes de Porcellio dilatatus petiti, et la stérilité du croisement P. d. petiti mâle × P. d. dilatatus femelle . Archives de l'Institut Pasteur de Tunis , 55 : 507 – 514 .
- Longo , G , Musmeci , R , Privitera , R and Sottile , L. 1998 . Ultrastructural organization of seminal receptacle and sperm storage in Porcellio laevis Latreille (Crustacea: Isopoda Oniscidae) . Tissue & Cell , 30 : 464 – 474 .
- Longo , G and Trovato , M. 2008 . Ultrastructure of seminal receptacle and sperm storage in Armadillidium granulatum Brandt (Isopoda, Oniscidea) . Italian Journal of Zoology , 75 : 113 – 123 .
- Martin , G , Juchault , R and Legrand , JJ. 1973 . Mise en évidence d'un micro-organisme intracytoplasmique symbiote de l'Oniscoïde Armadillidium vulgare L., dont la présence accompagne l'intersexualité ou la féminisation totale des mâles génétiques de la lignée thélygène . Comptes rendus hebdomadaire des Séances de l'Académie des Sciences (Paris), Série D , 276 : 2313 – 2316 .
- Mead , F. 1973 . Recherches sur la reproduction et le comportement sexuel des Isopodes terrestres [Thèse Doctorat d'Etat] , Université de Provence, CNRS France n° AO 8177 .
- Montesanto , G , Caruso , D and Lombardo , BM. 2008 . “ Genetic variability in parthenogenetic and amphigonic populations of Platyarthrus aiasensis Legrand from Sicily (Crustacea, Isopoda, Oniscidea) ” . In Proceedings of the International Symposium of Terrestrial Isopod Biology – ISTIB -07. M Edited by: Zimmer , M , Charfi-Cheikhrouha , F and Taiti , S . 53 – 62 .
- Moret , Y , Juchault , P and Rigaud , T. 2001 . Wolbachia endosymbiont responsible for cytoplasmic incompatibility in a terrestrial crustacean: Effects in natural and foreign hosts . Heredity , 86 : 325 – 332 .
- Nemec , B. 1896 . Studie o isopodech II . Mémoires de la Société royale des Lettres et des Science de Bohème , 25 : 1 – 55 .
- Richards , KS and Fleming , TP. 1982 . Spermatozoal phagocytosis by the spermathecae of Dendrobaena subrubicunda and other lumbricids (Oligochaeta, Annelida) . International Journal of Invertebrate Reproduction , 5 : 233 – 241 .
- Rigaud , T , Moreau , J and Juchault , P. 1999 . Wolbachia infection in the isopod Oniscus asellus: Sex ratio distortion and effect on fecundity . Heredity , 83 : 469 – 475 .
- Rousset , F , Bouchon , D , Pintureau , B , Juchault , P and Solignac , M. 1992 . Wolbachia endosymbionts responsible for various alterations of sexuality in arthropods . Proceedings of the Royal Society, London, B , 250 : 91 – 98 .
- Schmalfuss , H. 1989 . Phylogenetics in Oniscidea . Monitore Zoologico Italiano, , 4 : 3 – 27 .
- Schmalfuss , H. 2003 . World catalog of terrestrial isopods. (Isopoda: Oniscidea) . Stuttgarter Beitrăge zur Naturkunde, Serie A, Nr , 654 : 341
- Schöbl , J. 1880 . Ueber die Fortpflanzung Isopoder Crustaceen . Archiven Mikroskopie Anatomie Entwick Mechanik , 17 : 125 – 140 .
- Selmi , MG. 1992 . “ Sperm storage and capacitation ” . In Sex origin and evolution. Selected Symposia and Monographs U.Z.I. vol 6. Modena: Mucchi Edited by: Dallai , R . 251 – 265 .
- Stouthamer , R , Breeuwer , JA and Hurst , G D. 1999 . Wolbachia pipientis: Microbial manipulator of arthropod reproduction . Annual Review of Microbiology , 53 : 71 – 102 .
- Suzuki , S and Ziegler , A. 2005 . Structural investigations of the female genitalia and sperm-storage sites in the terrestrial isopod Armadillidium vulgare (Crustacea, Isopoda) . Arthropod Structure & Development , 34 : 441 – 454 .
- Vandel , A. 1925 . Recherches sur la sexualité des Isopodes. I. Les conditions naturelles de la reproduction chez les Isopodes terrestres . Bulletin Biologique de la France et de la Belgique , 59 : 317 – 371 .
- Vandel , A. 1928 . La Parthènogenèse gèographique. Contribution à l’ètude biologique et cytologique de la parthènogenèse naturelle . Bulletin Biologique de la France et de la Belgique , 62 : 164 – 281 .
- Vandel , A. 1937 . Recherches sur la sexualité des Isopodes. II. Les conditions de la fécondation chez Trichoniscus (Spiloniscus) provisorius Racovitza . Bulletin Biologique de la France et de la Belgique , 71 : 206 – 219 .
- Vandel , A. 1941 . Recherches sur la génétique et la sexualité des Isopodes terrestres. Sur la longévité des spermatozoïdes à l'intérieur de l'ovaire d’Armadillidium vulgare . Bulletin Biologique de la France et de la Belgique , 75 : 364 – 368 .
- Vandel , A. 1964 . De l'emploi des appareils respiratoires pour l’établissement d'une classification rationnelle des isopodes terrestres (Oniscoidea) . Bulletin de la Société Zoologique de France , 89 : 730 – 736 .
- Viscuso , R , Barone , N , Sottile , L and Narcisi , L. 1996 . Spermiolytic activity of the epithelium of the spermathecal duct of Rhacocleis annulata Fieber (Orthoptera: Tettigoniidae) . International Journal of Insect Morphology and Embryology , 25 : 135 – 144 .
- Warburg , MR. 1987 . Isopods and their terrestrial environment . Advances in Ecological Research , 17 : 187 – 242 .
- Warburg , MR. 1993 . Evolutionary biology of lands isopods , Berlin : Springer-Verlag. 159 .
- Warburg , MR and Cohen , N. 1991 . Reproductive pattern, allocation, and potential in a semelparous isopod from the Mediterranean region of Israel . Journal of Crustacean Biology , 11 : 368 – 374 .
- Warburg , MR , Cohen , N , Weinstein , D and Rosenberg , M. 1993 . Life history of a semelparous oniscid isopod, Schizidium tiberianum Verhoeff, inhabiting the Mediterranean region of Northern Israel . Israel Journal of Zoology , 39 : 79 – 93 .
- Westheide , W. 1988 . The ultrastructure of the spermatozoon in Pisione remota (Annelida: Polychaeta) and its transformation in the receptaculum seminis . Journal of Submicroscopic Cytology and Pathology , 20 : 169 – 178 .