Abstract
Encystment is a reversible cell differentiation process that also requires an antagonistic process, i.e. excystment. Both processes are genetically encoded. Encystment is of common occurrence among free-living ciliates and may serve several different purposes. In some ciliates, this process is part of the regular life cycle (reproductive cyst), while many other ciliates undergo encystment when environmental conditions become adverse (resting cyst). In this review, the different phases and aspects of the encystment process in ciliates, such as the ecological role, the cyst formation, the morphological characteristics of the cysts and their dynamic state during the time and the excysting process are reviewed on the basis on our own results and literature data.
Introduction
In many free-living protozoa, during constant environmental conditions, the phenotype remains fairly constant, except for changes in cell size and, of course, except during cell division. In some species, however, the phenotypic appearance may change considerably as an obligatory part of the life cycle. For example, the peculiar ciliates Suctoria are sessile, have tentacles and lack cilia in the adult phase but, by budding, they produce young swarmers which lack both tentacles and stalks but have cilia, while many histophagous ciliates, during their life cycle, pass through distinct developmental stages very different in size and shape (Lynn Citation2007).
Other protozoa are able to change their phenotypic appearance as a response to environmental variations. The ability to sense alterations in the environment and to respond to new situations rapidly and appropriately is vital for every organism and in particular for unicellular ones (Corliss & Esser Citation1974). The ability to form resting cysts, whose function in the life cycle of protozoa is in general considered a protection against ‘unfavourable environmental conditions’, plays an important role in their ecology and may have played an important role in the long evolutionary history and the enormous diffusion of these microorganisms.
The encystment is a reversible cell differentiation process that requires also an antagonistic process, i.e. excystment, which involves vegetative cell emergence from the cyst. As Gutierrez et al. (Citation2001) emphasise in a review on this topic, both processes are genetically encoded. This process falls within the microbial strategies called cryptobiosis (Keilin Citation1959), i.e. processes involving the formation of a resting stage, and consists of a reversible cytodifferentiation.
Mention of encysted state occurrence among ciliates can be found in such taxonomic monographs as that by Kahl (Citation1930). Thereafter, many data have accumulated over the decades and encystment appears now of common occurrence among free-living ciliates.
Ciliates are one of the most important groups of protozoa. In spite of their diversity, they are easy to recognise; certain unique characters (nuclear dualism, the presence of cilia and of a specialised region of the body for food uptake, i.e. a cytostomial area) are almost constantly present and set them apart from other protozoa. Some of the mostly highly differentiated and specialised eukaryotic cells described to date are found among ciliates. Not only is their morphological structure complex, but also their physiological, biochemical, ecological and behavioural traits may be highly specialised and complex. Moreover, ciliates are ubiquitous: they have colonised virtually every aquatic and humid environment. Basically all ciliates are heterotrophs. Using various feeding strategies they consume all sizes of autotrophic cells from bacteria to the larger dinoflagellates or diatoms (Verni & Gualtieri Citation1997; Hausmann Citation2002; Hausmann et al. Citation2003). In addition, they consume other heterotrophic protists. So ciliates are found at various trophic levels of the microbial loop and through them every component of prokaryotic and eukaryotic nanoproducer biomass can be transferred to higher trophic levels (Rosati et al. Citation2008). Furthermore, some large ciliates are predatory species that do not belong to the microbial loop but feed on rotifers, nematodes and micro-Crustacea (Foissner et al. Citation1999).
Due to all these characteristics the study of ciliate adaptive strategies such as the ability to form cysts, appears of interest from an ecological point of view.
This review is mainly based on our observations on the ciliate Oxytricha bifaria. The structure, the significance and the ecological value of O. bifaria cysts are also discussed based on literature data on other ciliates.
This review includes the following sections:
| 1. | Ecological significance of encystment. | ||||
| 2. | The cyst formation. | ||||
| 3. | The cyst wall. | ||||
| 4. | Cytoplasm and nucleus in resting cysts. What is retained and why? | ||||
| 5. | Resting cysts are dynamic structures. | ||||
| 6. | Benefits and costs of encystment and excystment. | ||||
| 7. | Concluding remarks and perspective. | ||||
Ecological significance of encystment
Cyst formation in free living ciliates may serve several different purposes. In some ciliates, the process is part of the regular life cycle. For example in Colpodida, under favourable conditions, the vegetative form feeds, grows and then, after a time, encysts to form reproductive cysts (Lynn Citation2008). The encysted cells divide twice or three times, then excyst and begin feeding again (Mueller & Mueller Citation1970). In this case, the excystment is spontaneous according to a determined genetic program and does not require a specific environmental stimulus. Similarly, in most histophagous ciliates asexual reproduction takes place in division cysts only. For example, in the presence of food, Prorodon aklitolophon feeds for 3–6 h, then forms reproductive cysts and two to four cells exit the cyst after 2–3 days (Hiller & Bardele Citation1988).
Many ciliates undergo encystment when environmental conditions become adverse. When the environmental stimulus appears, it is recognised by membrane receptors and opens a specific genetic programme that leads to resting cyst formation. When the stimulus disappears an inverse programme is activated: every cryptobiotic state is a direct result of the opening and closing of specific genes (Gutierrez et al. Citation2001).
What are the factors controlling encystment and excystment in Ciliates? Many authors consider starvation as the most universal exogenous inducer of ciliate encystment (Gutierrez et al. Citation2001). Water is one of the universal requirements for protozoan activity. In fact, species living in terrestrial or semi-terrestrial environments form cysts in response to desiccation. However, a large proportion of the population may encyst under optimal environmental conditions. So the factors governing the dynamics of vegetative and encysted cells in the soil are not well understood. Ekelund et al. (Citation2002) reported that encystment is likely to be controlled by a density-dependent mechanism. In other words, the rate of encystment depends on the ciliate density in a population and could perhaps be triggered by a signalling substance excreted by the ciliates themselves. This internally governed encystment may be an essential adaptation to an unpredictable environment in which only those organisms that have encysted in time will survive desiccation. Other factors such as temperature, day length, pH and predator avoidance are also candidate stimuli especially for planktonic species, but there is an experimental evidence only in a few cases (Weisse Citation2006). In Euplotes rariseta, the cyst formation was induced by exposing cells to environmental salinities >0.5%. The authors demonstrated experimentally that this species actually encysts in response to excess salinity, and not to excess osmosity (Dallai et al. Citation1987). Recent observations with the rare oligotrich Meseres corlissi revealed intraspecific differences between tropical and temperate strains in the proximate factors triggering encystment. In the tropical strain, encystment was induced when temperature dropped below 20°C (Weisse Citation2004); encystment, excystment and cyst survival of two temperate strains depended strongly on the presence or absence of soil extract in the culture medium (Muller et al. Citation2006).
Some ciliates form temporaneous cysts (rhythmic cysts) in response to rapid, periodic environmental changes. For example, on leaves, Colpoda cucullus is capable of completing its life cycle by excysting in dew drops during the night and encysting when the water evaporates during the day (Fenchel Citation1987). The tide pool oligotrich ciliate Strombidium oculatum alternates between an encysted and free-swimming stage with tidal rhythm. For ∼6 h at low tide, the ciliate is free-swimming in pools, and ∼20–60 min before flushing of the pools it encysts on a substrate. Encystment last for ∼19 h: two high tides and one intervening low tide. Excystment occurs the next day, only 30–40 min after the pools are isolated. This behaviour greatly increases the probability of remaining in a tide pool during flooding by the high tide. This excystment–encystment pattern is similar in S. oculatum populations from the north coast of France and from the Isle of Man (Fauré-Fremiet Citation1948; Jonsson Citation1994; Montagnes et al. Citation2002).
The cysts generally referred to as resting cysts represent a strategy by which ciliates escape from unfavourable conditions for extended periods via cryptobiosis or dormancy. Their formation requires a definite stimulus and some time, during which there is a period of reorganisation. Their longevity is very likely correlated with the environment in which they are produced (Foissner et al. Citation2007), so in desert regions cysts remain viable in air-dried conditions for several or many years (Foissner et al. Citation2002) while those produced in rainforest soil lose viability within a few months (Foissner Citation1997, 2006).
Detailed morphological and physiological data on resting cysts in ciliates are available from less than 40 species. These studied resting cysts differ for resistance and have a great morphological and ontogenetic diversity. The most remarkable changes in their formation are, in general, a drastic decrease in cellular volume through autophagy and cytoplasmic dehydration, the metabolic inactivation and the building of a resistant but permeable barrier (the cyst wall). Formation of reproductive and rhythmic cysts involves ‘work’ and less extreme changes. There are ciliates able to form different types of cysts such as Colpodidae, which have reproductive and resting cysts (Muller & Muller 1970), and istophagous ciliates (Hiller & Bardele Citation1988) in which, in the absence of food, reproductive cysts transform into resting cysts.
Cyst formation
A number of current descriptions of cyst formation in ciliates are available in the literature. A good description for the process is represented by the detailed, well-illustrated, account reported for the raptorial ciliate Spathidium spathula (Foissner & Xu Citation2007).
Here we report our observations in vivo on the filter-feeding ciliate Oxytricha bifaria (Ricci et al. Citation1985).
In this ciliate, cyst formation takes about 36–48 h, comprising two main steps (1) cytoplasmic reorganisation along with a sharp decrease in cell volume; and (2) formation of the cyst wall. These steps are of common occurrence in cyst formation of aquatic ciliates. As differences in their progress have been reported among the few species in which the process has been described so far, some significant data from the literature are also reported.
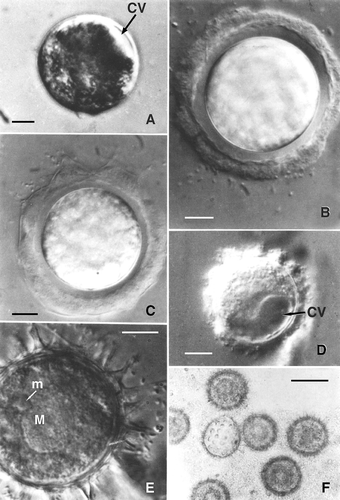
A vegetative cell of Oxytricha can be simulated as one-half of an ellipsoid, with its semi-axes a = 65.3 ± 10.2, b = 43.5 ± 5.8, and c = 40.1 ±4.4 μm. Accordingly, the cell volume (4/3π abc) is approximately 234,000 μm3; when the rounded cell is ready to encyst, the volume is only 47,000 μm3, about 20% of the initial volume. After this decline in volume, the cell has a large contractile vacuole (CV), which becomes active and is easily observed until encystment is completed. The frequency of vacuolar contraction ranges from 30 to 120 s depending in general upon the progress of the encystment process achieved by the cell: the further encystment has progressed, the more frequent the rate of discharge of the CV. Soon after reaching the spherical stage, the cell produces an external, strongly refractile jelly coat that, at first, adheres to the cell membrane. In this phase, the activity of the CV appears to play a major role in the process. After each discharge the cell rotates alternately clockwise and counter-clockwise, apparently facilitating the separation of cell membrane from jelly coat. As a result, a somewhat regular space of about 4 μm is formed around the cell. This space becomes filled with products of the cell; these products in turn are organised and arranged into a regular, homogeneous layer. During the latter phase, the cytoplasm is densely vacuolised, the two macronuclear components become rounded and approach one another in the central area of the cell. The final step of differentiation involves the progressive formation of protrusions of the cyst wall (–F), referred to as finger-like protrusions for their shape.
Figure 1. Light micrographs of encystment and resting cyst of Oxytricha bifaria. A, starved cell with decreased volume. The contractile vacuole can be observed easily. B, the first external layer of the cyst has been produced and the space between it and rotating cytoplasm formed. C, the formation of finger-like protrusion begins at one pole of the forming cyst. D, cyst formation is almost completed and large contractile vacuole is still visible. E, a resting cyst; Nomarsky interference phase-contrast; the finger-like protrusions appear hollow. F, relationship of the finger-like protrusions and mucilaginous substance enveloping resting cysts. CV: contractile vacuoles; M: macronucleus; m: micronucleus. Scale bars: C, E, 10 μm. F, 45 μm.
Formation of the finger-like protrusions begins at one pole and terminates when the protrusions are formed over the entire surface of the cyst. The cyst wall is about 5 μm thick. The finger-like protrusions appear as empty structures. The cytoplasm is homogeneous and the nuclear apparatus consists of only one macronucleus and one micronucleus (instead of two macronuclei and two micronuclei as in the vegetative form). The cysts are always ‘aggregated’ and embedded in a mucilaginous matrix. Perhaps the substances making up the matrix are released continually by the cyst, because they are still present after many washing both by centrifugation and sonication.
A well-formed cyst of Oxytricha bifaria is shown in .
The cyst wall structure and composition
Numerous ultrastructural studies of resting cysts have demonstrated that the cyst walls are composed of several layers, in general four (Rosati et al. Citation1984; Calvo et al. Citation1986; Gutierrez et al. Citation2003).
The presence of different precursors that are synthesised ‘de novo’ giving rise to the different cyst wall layers during the encystment of various ciliate protozoa has been reported. These precursors originate from endoplasmic reticulum in Colpoda steinii (Ruthmann & Kuck Citation1985), or from Golgi vesicles in Histriculus similis (Calvo et al. Citation1986). Recently, Foissner and Pichler (Citation2006) studied the genesis of the cyst wall precursors and the assemblage of the cyst wall in Meseres corlissi. This ciliate has five types of cyst wall precursor. None of the precursors is similar to those reported from other ciliates, suggesting the oligotrich as a very distinct group of ciliates.
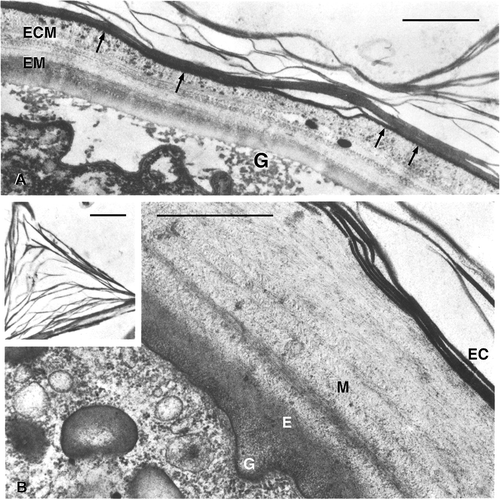
The ultrastructural investigation revealed that in 1-day-old cysts of O. bifaria, the wall consists of four distinct layers: the ectocyst, namely the external lamellar layer now well-organised into an internal subspherical part and into an external one which forms the finger-like protrusions; the fibrous mesocyst, about 2 μm thick, structured into many concentric layers; the endocyst, very homogeneous and regular both in shape and thickness; and the granular layer, the most internal one regularly discontinuous and uniting the spherical cyst wall to the wrinkled cell surface (). A similar four-layer configuration of the wall has been described for several other species (Walker et al. Citation1975; Walker & Maugel Citation1980; Rosati et al. Citation1983).
Figure 2. Section of the cyst wall at TEM of Oxytricha bifaria. A, 1-day-old cyst. G: granular layer; E: endocyst; M: mesocyst; EC: ectocyst. The insert shows the internal structure of a finger-like structure. B, 14-day-old cyst. The granular layer (G) is very rarefied. The mesocyst is differentiated in an ecto (ECM) and an endomesocyst (EM). Many electron-dense granules are present in ECM and are regularly arranged forming an electron-dense layer underneath the ectocyst (arrows). Scale bars: A, 1 μm. B, 10 μm.
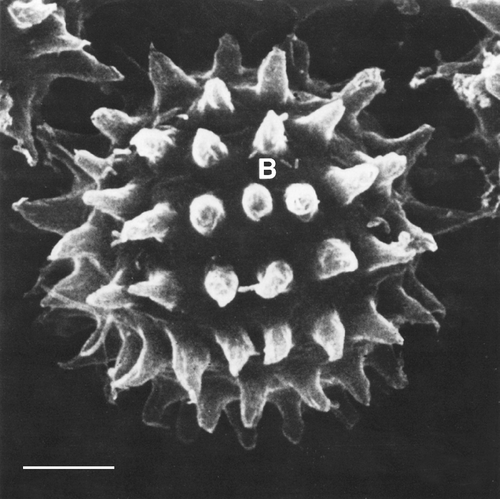
An interesting feature of the cysts wall of O. bifaria is the presence of finger-like protrusions ( inset). These conical structures, measured on SEM preparation, are about 7.5 μm high and occur at a frequency of 80–100 per cyst. Their external surface is irregular.
In a comparative study on the fine structure of resting cysts in some representative ciliate species, Foissner et al. (Citation2007) evidenced three different, very likely non-homologous cyst surface ornamentations: those generated by the ectocyst, such as the finger-like protrusions of O. bifaria and the papilla-like structures described in Colpoda inflata (Chessa et al. Citation1994); those generated by the mesocyst; and those produced intracellularly like lepidosomes. According to these authors, cyst morphology, cyst wall composition and the cell restructuring should contain considerable phylogenetic and ecological information.
At least three hypotheses of the role of the specialisation of the cyst wall in O. bifaria have been offered: (1) the finger-like protrusions may anchor the cyst to the substrate, thus stopping it from being washed away by flowing water; (2) they may ensure the simultaneous dispersion of several cell lines by causing the cysts to adhere to one another (thus, two or more mating types could be transferred together to a new habitat by animals or the wind); (3) they can ensure food availability on the basis of the observation that many bacteria adhere to or partially emerge from the outer layer of the cyst even after being vigorously sonicated.
Cytoplasm and nucleus in resting cysts. What is retained and why?
As reported in detail for Oxytricha bifaria in the previous sections, besides the building of the wall, cyst formation includes drastic changes in shape and volume, at least in most ciliates. In general, cysts are spherical and the volume sharply decreases. Only a few exceptions have been reported: for example, Pelagostrombidium fallax forms flask-shaped cysts while the cyst volume of Strombidium oculatum is larger than that of vegetative cells () (Foissner Citation2006; Foissner et al. Citation2007). By comparing the vegetative cell volume and the surface with the cyst's cell volume and surface, respectively, Ricci et al. (Citation1985) calculated that even if the cyst cell had the same volume as a vegetative cell, its spherical shape would ensure a saving of about 75% of the cell surface. The maintenance of an as-large-as-possible outer surface, together with a decreasing volume, provides the cyst the best power to have exchanges with the outside. On the other hand, the greater the radius of the sphere, the thicker its wall must be to ensure mechanical protection. Thus, the reduction of the cell volume is also useful to reduce the volume of cyst wall to be secreted. The size of resting cysts probably represents an optimum combination between the need for the cell volume to be as small as possible and the need for enough biological material to undergo excystment. The dimensions of vegetative and encysted cells from 14 different species of ciliates are compared in . It appears that the cyst diameters are closer to each other than the lengths of vegetative forms. Perhaps there is a certain cytoplasmic ‘quantum’ not further reducible without severely affecting the survival potentiality of the cell (Ricci et al. Citation1985).
Table I. Resting cyst volume as percentage of the vegetative cell volume
Table II. Comparative synopsis of cell vs. cyst dimensions in 14 species of ciliates
The volume reduction is mainly achieved by elimination of water. As a consequence cytoplasmic viscosity increases, as can also be manifested in the different sedimentation rates of resting cysts and vegetative cells on a discontinuous gradient of Percoll (Gutierrez et al. Citation1998). In the dense cytoplasm organelles are reduced in number and crowded. The reduction of cellular organelles is mainly due to authophagic activity that provides energy and endogenous material useful for synthesising the new macromolecules directly or indirectly involved in cyst formation. A more or less pronounced dedifferentiation of cortical structures takes place. With respect to the kinetosomes there are three types of resting cysts (Walker & Maugel Citation1980; Gutierrez et al. Citation2003): kinetosome-resorbing (KR) cysts, partial kinetosome-resorbing (PKR) cysts and non-kinetosome-resorbing (NKR) cysts. The most common are KR and PKR. Major differences are reported in the cytoplasmic and nuclear organisation between these three kinds of cysts. The classic description of Diophrys scutum cysts by Walker and Maugel (Citation1980) can be used as an example of NKR cysts: cilia, kinetosomes and most microtubules are maintained intact; membrane food vacuole precursors (pharingeal discs) are maintained intact; mitochondria remain scattered; macronuclei do not fuse. These characteristics suggest that less energy would be required for the excystment. Indeed, D. scutum shows a rapid excisting ability.
The cysts of O. bifaria belong to the KR type (for other KR ciliate species see Gutierrez et al. Citation2003). In well-formed resting cysts of this ciliate the major traits evidenced by ultrastructural analysis are: (1) the ciliary organelles are completely resorbed; (2) the subcortical microtubules are still present; (3) the cytoplasm is very condensed and rich in ribosomes; (4) the mitochondria are grouped in clusters; (5) the paraglycogen granules, i.e. the polysaccharidic reserve substances, are numerous and mainly localized at one pole; lipid droplets are also present; (6) authophagic vacuoles are rare; and (7) the macronuclei fuse and their chromatin appears to be arranged in small, spherical, dense bodies, regularly scattered throughout the nuclear matrix (Verni et al. Citation1984). Apparently the cell retains only the structures to maintain viability and useful to resume the vegetative form.
Polysaccharidic and lipidic reserve substances, which are readily utilised by the ciliate cells during short-lasting starvation, are maintained in resting cysts (Rosati et al. Citation1981). They probably represent the ‘fuel’ to start and carry on the energetically expensive excystment process. The accumulation of mRNAs in the resting cysts of some ciliates has been reported (Gutierrez et al. Citation2001). They may represent the remaining molecules required during the encystment process or transcripts, biosynthesised during encystment but only required during the excystment process of the cell. The maintenance of subcortical microtubules observed in O. bifaria recall for a structural characteristic, revealed by SEM observation during long-term resting encystment in Colpoda inflata, a ciliate forming PKR cysts (Chessa et al. Citation1994). Remarkably, despite the lack of any polarising structure on the cyst wall outer surface, Colpoda cells long retain spatial arrangement of the completely reabsorbed somatic and oral cortical structures. Indeed, the complete removal of the cyst wall layers, reveals, on the outer surface of encysted cells, traces of the interkinetal argirome and the longitudinal furrows of the somatic kineties. This, according to the authors (Chessa et al. Citation1994), accounts for long retaining of both spatial arrangement of cortical structures and cell polarity during resting encystment.
Resting cysts are dynamic structures
Much evidence has been reported that cysts in ciliates are a sort of dynamic state, and far from being fixed soon after their formation, undergo significant morphological and chemical changes during ageing.
The following experimental studies on O. bifaria and two species of Colpoda genus, respectively, well illustrate ciliate cyst ‘dynamism’. When 1-day-old cysts and 14-day-old cysts of O. bifaria were compared by ultrastructural and cytochemical analysis it appeared that not only the morphology but also the distribution within the wall layers and/or the arrangement of the cyst chemical components vary during time (Rosati et al. Citation1984; Verni et al. Citation1984). Indeed, in 14-day-old cysts the mesocyst consists of two layers, the ecto- and endomesocyst, which can be either separated by a less condensed and rather discontinuous layer or not. Heavily electron-dense granules form in the ectomesocyst while the granular layer appears rarefied (). The reactivity to the tests specific for polysaccharides of both granular layer and endocyst clearly increases from 1- to 14-day-old cysts. Moreover, while protease treatment is completely ineffective on the cyst wall of young cysts, it partially affects the granular layer in 14-day-old cysts. Finally, the presence of chitin in the ectomesocyst of the older cysts has been demonstrated. The presence of chitin in the cyst wall of ciliates is not unusual. (Foissner et al. Citation2006).
Analogous differences in cytochemical reactivity have been observed for other cellular components such as macronuclear chromatin and paraglycogen granules. Macronuclear chromatin remains unmodified after a prolonged protease treatment in young cysts while it is easily removed from sections of 14-day-old cysts. In the same sections, paraglycogen granules (which in these old cysts are grouped forming ‘clouds’) are not selectively stained by methods specific for polysaccharides. All the cytochemical tests have been performed on sections of O. bifaria cells processed for TEM observation. Fixation and embedding procedures may certainly influence the reactivity toward the cytochemical treatments. However, as young and old cysts have been processed in the same way and contemporaneously only intrinsic differences can account for the different results. Thus, although the presence of polysaccharides in the cyst wall of 1-day-old cysts cannot be excluded, the strong positive reaction observed in the wall layers of 14-day-old cysts is indicative of a quantitative variations of these molecules or of their different arrangement or different links with other components. It is worth noting that even the reactivity of paraglycogen granules varies with cyst ageing.
Quantitative variations of macronuclear DNA and total protein content have been reported in increasingly old cysts in Colpoda inflata. In this ciliate macronuclear and protein syntheses has been observed in cysts as old as 27 days. Protein synthesis is probably relevant to the formation of new material for the cyst wall, allowing the cell to be even more protected and, therefore, to increase its powers of survival. DNA synthesis is preceded by a high percentage (77%) of 20–25–day-old cysts showing macronuclear extrusions. Thus, this phenomenon indicative of a regulation of total DNA content, typical of the trophic state, occurs in resting cysts of C. inflata at a distance from the encystment onset (Chessa & Delmonte Corrado Citation1994). Indeed, chromatin extrusion activity takes place only after the second day of encystment (Corrado et al. Citation1996).
The presence of glycoconjugates, whose function as receptors of signals from extracellular environment is known, has been demonstrated in the cyst wall of a number of ciliates. Chessa et al. (Citation2002) investigated the glycopretein composition and lectin-binding site localisation of the cyst wall of Colpoda cucullus, at increasing cyst ages. During an 18-day resting encystment, the cyst wall undergoes structural modifications resulting in the compaction of its previously formed layers and the production of new material that is placed on the inner side of the cyst wall. Changes in the distribution of wheat germ agglutinin (WGA) and Concanavalin A (ConA) binding sites were also found: this suggests that the cyst wall of C. cucullus undergoes cytochemical rearrangements of its glycoprotein components in the time. Moreover, differences in the electrophoretic and ConA/WGA-positive patterns have been found when 18-day-old cysts are compared to 1-day-old ones.
So, as it appears from these studies mentioned by way of examples, the encysted cells, although resting, therefore having a strikingly reduced metabolism, are not ‘sleeping’ and are somehow ready to excyst, as soon as environmental conditions allow it.
Excystment
Detailed descriptions of excystment in ciliates are somehow less frequent in the literature than those of encystment. Basically, two different excystment modes are recognised: (1) escape through an emergence pore with removable plug, and (2) no preformed emergence pore. Direct observations in ciliates escaping from cysts that possess a preformed emergence pore have been performed on Blepharisma stoltei (Repak & Pfister Citation1967), Bursaria truncatella (Beers Citation1948), Strombidium oculatum (Montagnes et al. Citation2002), and Strombidium conicum (Kim & Taniguchi Citation1995). The ciliates, prior to excystment, actively rotate within the cyst, the plug material disappears and the ciliate emerges by the protoplasm squeezing through the excystment pore not enclosed in a membrane. Pressure from within, intensive ciliary beating and enzymatic activity may be involved. As concern cysts without preformed emergence pore, a recent description by live observation is offered by the spirotrich Meseres corlissi (Muller Citation2007). Excysting cells were first recognised by the appearance of a non-pulsating vacuole. With increasing size of this vacuole, the outer cyst wall ruptured, creating a narrow slit. The emerging protoplast was enclosed by an inner flexible membrane. Oral membranelles were faintly visible, but inactive at this stage. Within the next hour, the oral membranelles and a pulsating vacuole became active, and the ciliate started to rotate. During a phase of rapid movement, a flexible membrane became wider and thinner. Finally it burst open and released the now fully differentiated ciliate. The emergence of M. corlissi from the cyst is similar to descriptions in the literature in several stichotrich ciliates (for related literature see Muller Citation2007). The cyst wall ruptures are due to inner pressure. No doubt the growth of the vacuole contributes to the internal pressure. Whether enzymatic activity is also involved is an open question.
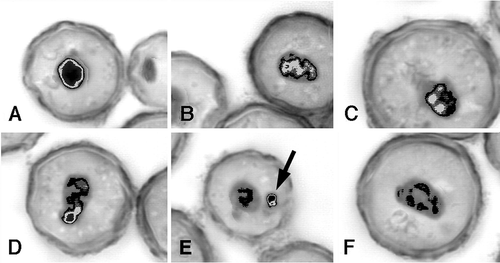
In both excystment modes, structures absorbed during encystment develop again. This development implies that the macronucleus is transcriptionally active during early excystment. In line with this assumption, Chessa et al. (Citation2001) studied, by image analysis, the macronucleus of Colpoda inflata to determine variations that occur in chromatin structure during excystment from resting cysts. A comparative morphometric and densiometric analysis was performed on macronuclei of differently aged cysts: from 1-day-old cysts to 1-year-old cysts. Marked variations in densiometric values and chromatin texture were found only in 1-day-old excysting cells, in which a dramatic decrease in DNA content occurs 2 h after induction of excystment. This suggests that the chromatin extrusion process mentioned above, not yet accomplished in these very young cysts, is triggered by excystment (Chessa & Delmonte Corrado Citation1994). The results obtained by densiometric analysis of excysting cells derived from older cysts, revealed that chromatin decondensation, indicative of a more intense transcriptional activity, increases during time. Moreover, the percentage of macronuclei with decondensed chromatin that also show an extrusion body of condensed chromatin increases as excystment progresses, thus suggesting that the macronucleus is somehow renewed by extruding chromatin that is unable to decondense (). This event appears as a possible mechanism at the basis of the ‘rejuvenescence’ of senescent cell lines (Chessa & Delmonte Corrado Citation1994).
Figure 4. Pattern of the macronuclear chromatin structure dynamics, shown by optical microscopic images of excysting cells of Colpoda inflata. Samples derived from 1-year-old cysts of a senescent culture, examined 24 h after the excystment induction. During chromatin decondensation, the highly condensed and compact mass (A) becomes first irregular in shape (B); then undergoes fragmentation (C,D); finally, part of the chromatin unable to decondense (arrow) is extruded into the cytoplasm (E), where it is digested (F). Black refers to very condensed chromatin. Chromatin gradual decondensation is represented by the decreasing intensity of the shade of grey (modified from European Journal of Protistology, Vol. 37, Issue 3, Maria Giovanna Chessa, Lorenzo Gallus, Luca Tiano, Francesca Trielli, Maria Umberta Delmonte Corrado, Variations in macronuclear chromatin structure and chromatin extrusion in excystment from resting cysts of Colpoda inflata, pp. 281–290, Copyright (2001), with permission from Elsevier).
It is known that in most cases the addition of fresh medium is sufficient to recover the vegetative form from the cyst. Anyway, as reported above for O. bifaria, in general not all encysted individuals succeed in the excysting process. The process of excystment involves several phenomena and steps, so it is difficult to understand why some fully formed individual are able to break through the cyst wall while others cannot escape. Since reversal morphogenetic processes may be repeated several times by the active protoplasm within the cyst, it scarcely seems plausible to attribute the failure of excystment to any fundamental defect in the cell itself which might have been caused by the internal changes accompanying encystment. It appears more probable that the difficulty lays in the nature of the cyst wall, which proved impervious to rupture from within. To verify this hypothesis, Foissner (Citation2006) performed several experiments on Spathidium. The author reports that in most cases, following the addition of fresh medium, only few individuals on a slide were able to penetrate the cyst wall, while the majority could not. On the contrary, when the cyst wall was artificially broken, a greater number of normal individuals was obtained.
Benefits and costs of encystment/excystment process
Like most biological adaptive strategies, the encystment/excystment process has not only benefits for the species but also costs. Certainly, the most general advantage of encystment is to enhance species survival. The observations that the cyst wall is not only a mechanical shelter but also a defence against possible extreme values of environmental parameters, as suggested by the different, contiguous and sophisticated layers of the wall itself reported in most cases, concur with the idea that the meaning of this process is actually that of allowing the species to survive during extremely unfavourable conditions. Thus, the protective cysts of soil ciliates of genus Colpoda remain viable for many years in a desiccated state, and for short periods they can survive dry heat up to 120°C. These cysts also survive passage through the gut of animals (Fernandez-Galiano et al. Citation1986). However, in spite of its complexity and resistance, the cyst wall should be partially permeable, in intimate contact with the cell surface, in order to allow molecular information coming from the extracellular medium to reach specific receptors to induce excystment. Interestingly, cysts that are able to excyst rapidly, like Diophrys scutum cysts mentioned above, have a thinner, three-layered cyst wall.
A dispersive role of cysts has been also supposed. It is often assumed that the ability to disperse is an advantageous trait for microorganisms as dispersal would facilitate the possibility to encounter favourable conditions. Certainly desiccation-resistant cysts, smaller and lighter than vegetative forms, may be dispersed widely by wind, animals, etc. (Lynn Citation2008). However, it is very hard to evaluate the actual role of encystment in ciliate dispersal. A short-distance dispersal of cysts, however, seems to play a role in the peculiar ciliate Sorogena.
Sorogena stoianovitchae is the only ciliate that undergoes fruiting body development. When S. stoianovitchae is mildly starved, several hundreds of cells aggregate beneath the water surface, and the aggregate develops into an aerial fruiting body. The developmental process is divided into five distinct stages: aggregation, compact aggregation, secretion of a mucous matrix, stalk elongation, and completion of the fruiting body. Essential requirements for fruiting body development are high cell density, a light–dark cycle, and a dark period of more than 8 consecutive hours. Recently the genes involved in stalk formation have been investigated (Sugimoto & Endoh Citation2008). The cells forming the fruiting body are then encysted. After desiccation, a slight mechanical disturbance will lead to dispersal of the cysts. As reported above, a dispersive role has been also supposed for O. bifaria cysts.
The cost of forming cysts was calculated in the case of O. bifaria as the percentage of cells that had died because of encystment. In five replicate experiments, 30–35% of about 3000 cells died while encysting, 10–15% died during the resting cyst phase, and 20–25% died during and soon after excystment. Thus the proportion of living excysted individual cells ranges between about 25 and 40% of the encysting population: probably enough to ensure the survival of the species, despite the sacrifice of many individuals.
Concluding remarks and perspectives
The literature on cysts in ciliates is still highly scattered. Most studies deal with single species or genera and do not consider the process on the whole, i.e. from the cyst formation to its modification in time and to excystment. In their review article, Corliss and Esser (Citation1974) discussed the data accumulated over the former decades. The aim of the present article is to pull together, step by step in a single account, more recent findings on the encysting–excysting cycles in ciliates. Not pretending to present an exhaustive review of the existing literature (which is really too abundant), we used, as a thread, our observation on O. bifaria and provided a survey of the different steps of the process in ciliates of the most-studied species.
The process appears as an extremely complex and varied trait in which a number of biological mechanisms are involved (Gutierrez et al. Citation1990). The recognition of specific stimuli by membrane receptors and the consequent opening of a specific genetic programme come into play, while an inverse programme is activated when the stimulus disappears. Some ciliates are even able to develop different kinds of cysts (resting, reproductive, etc.) in response to different stimuli. Furthermore the process involves an extensive ‘protoplasmic and nuclear reorganisation’ and the activation of reversible genetic and biochemical mechanisms. Further studies are required to increase the present incomplete knowledge on these mechanisms. Anyway, as ciliates are ubiquitous, easy to be collected and a number of them cheaply cultivable in the lab, they could represent a suitable tool in the study of reversible cell differentiation in eukaryotes.
In the end, as there are problems in cyst taxonomic identification and even in recognising cysts as such, to determine the dynamics of active and encysted populations of ciliates in nature could be very important in ecological studies. However, it must be considered that species react differently to the apparently same environmental conditions. Thus quantitative data, which are now cumulating cannot be generalised and must be evaluated one by one. The ‘in situ’ significance of encystment–excystment in ciliates is still an open question.
Notes
*This paper is dedicated to Maria Umberta Delmonte Corrado for her contribution to the development of Italian Protistology.
References
- Beers , CD . 1948 . Excystment in the ciliate . Bursaria truncatella. The Biological Bulletin , 94 : 86 – 98 .
- Calvo , P , Torres , A and Perez-Silva , J. 1986 . Ultrastructural and cytochemical study of the encystment in the hypotricous ciliate . Histriculus similis. Archiv fur Protistenkunde , 132 : 201 – 211 .
- Chessa , MG and Delmonte Corrado MU . 1994 . Encystment and excystment in Colpoda inflata . Macronuclear DNA and total protein content quantitative variations Archiv fur Protistenkunde. , 144 : 207 – 211 .
- Chessa , MG , Delmonte Corrado MU , Carcupino , M , Pelli , P. and Grassi Milano , E . 1994 . “ Ultrastructural cortical aspects of cell differentiation during long-term resting encystment in Colpoda inflata ” . In Contribution to animal biology , Edited by: Cirrotto , C and Maastrolia , L . Palermo : Halocyntia Association .
- Chessa , MG , Gallus , L , Tiano , L , Trielli , F and Delmonte Corrado MU . 2001 . Variation in macronuclear chromatin structure and chromatin extrusion in excystment from resting cysts of Colpoda inflata . European Journal of Protistology , 37 : 281 – 290 .
- Chessa , MG , Largana , I , Trielli , F , Rosati , G , Politi , H , Angelini , C and Corrado Delmonte MU . 2002 . Changes in the ultrastructure and glycoproteins of the cyst wall of Colpoda cucullus during resting encystment . European Journal of Protistology , 38 : 373 – 381 .
- Corliss , JO and Esser , SC. 1974 . Comments on the role of the cyst in the life cycle and survival of free-living protozoa . Transactions of the American Microscopy Society , 93 : 578 – 593 .
- Corrado , MU , Pelli , P , Chessa , G , Margallo , E , Lattes , A and Nicolò , G. 1996 . A study on macronuclear chromatin structure in Colpoda inflata (Protozoa, Ciliophora) by means of image analysis . European Journal of Histochemestry , 40 : 181 – 188 .
- Dallai , R , Miceli , C and Luporini , P. 1987 . Euplotes rariseta Curds et al. (Ciliophora Hypotrichida) from the Somalian coast: Description and preliminary observations on cyst induction and ultrastructure . Monitore Zoologico Italiano (N.S.) , XXII : 263 – 280 .
- Ekelund , F , Frederiksen , HB and Rønn , R. 2002 . Population dynamics of active and total ciliate populations in arable soil amended with wheat . Applied and Environmental Microbiology , 68 : 1096 – 1101 .
- Fauré-Fremiet , E. 1948 . Le rythme de mare du Strombidium aculeatum Gruber . Bulletin Biologique de la France et de la Belgique , 82 : 1 – 20 .
- Fenchel , T. 1987 . Ecology of Protozoa: The biology of free living phagotrophic protists , Madison, WI : Science Tech. Pub .
- Fernandez-Galiano , MT , Fernandez-Galiano , D and Madrigal-Sesma , MJ. 1986 . Presence of viable cysts of Colpoda (Ciliophora Colpodida) in digestive tube of reptiles and amphibians . Revista Ibérica de Parasitología , 46 : 223 – 228 .
- Foissner , W. 1997 . Soil ciliates (Protozoa Ciliophora) from evergreen rain forests of Australia, South America and Costa Rica: Diversity and description of new species . Biology and Fertility of Soil , 25 : 317 – 339 .
- Foissner , W. 2006 . Biogeography and dispersal of the micro-organisms: A review emphasizing protists . Acta Protozoologica , 45 : 111 – 136 .
- Foissner , W , Agatha , S and Berger , H. 2002 . Soil ciliates (Protozoa Ciliophora) from Namibia (Southwest Africa), with emphasis on two contrasting environments, the Etosha Region and Namib desert . Denisia , 5 : 1 – 1459 .
- Foissner , W , Berger , H and Schaumburg , J. 1999 . Identification and ecology of limnetic plankton ciliates. Informationsberichte des Bayer . Landesamtes für Wasserwirtschaft, Heft , 3/99 : 793
- Foissner , W , Müller , H and Agatha , S. 2007 . A comparative fine structural and phylogenetic analysis of resting cysts in oligotrich and hypotrich Spirotrichea (Ciliophora) . European Journal of Protistology , 43 : 295 – 314 .
- Foissner , W and Pichler , M. 2006 . The unusual, lepidosome-coated resting cyst of Meseres corlissi (Ciliophora: Oligotrichea): Genesis of four complex types of wall precursors and assemblage of the cyst wall . Acta Protozoologica , 45 : 339 – 366 .
- Foissner , W , Pichler , M , Al-Rashedid , K and Weisse , T. 2006 . The unusual, lepidosome-coated resting cyst of Meseres corlissi (Ciliophora: Oligotrichea): Encystment and genesis and release of lepidosomes . Acta Protozoologica , 45 : 323 – 338 .
- Foissner , W and Xu , K. 2007 . Monograph of the Spathidiida (Ciliophora, Haptoria). Volume I: Protospathidiidae, Arcuospathidiidae, Apertospathulidae . Monographiae Biologicae , 81 : 1 – 485 .
- Gutierrez , JC , Callejas , S , Borniquel , S , Bénitez , L and Martίn-Gonzáles , A. 2001 . Ciliate cryptobiosis: A microbial strategy against environmental starvation . International Microbiology , 4 : 151 – 157 .
- Gutierrez , JC , Diaz , S , Ortega , R and Martίn-Gonzales , A. 2003 . Ciliate resting cysts walls: A comparative review. Recent Research Developments in Microbiology , 7 : 361 – 379 .
- Gutierrez , JC , Izquierdo , A , Martίn-Gonzáles , A and Callejas , S. 1998 . “ Cryptobiosis of colpodid ciliates: A microbial eukaryotic cell differentiation model ” . In Recent research and development in microbiology , Edited by: Pandalai , SG . 1 – 15 . India : Research Signpost .
- Gutierrez , JC , Martίn-Gonzáles , A and Matsusaka , T. 1990 . Towards a generalized model of encystment (cryptobiosis) in ciliates: A review and hypothesis . BioSystems , 24 : 17 – 24 .
- Hausmann , K. 2002 . Food acquisition, food ingestion and food digestion by protists . Japanese Journal of Protozoology , 35 : 85 – 95 .
- Hausmann , K , Hülsmann , N and Radek , R. 2003 . Protozoology , 3rd , Edited by: Thieme , G . New York, NY : Verlag Stuttgart .
- Hiller , S and Bardele , CF. 1988 . Prorodon aklitolophon n. spec and the dorsal brush as a character to identify certain subgroups in the genus Prorodon . Archiv fur Protistenkunde , 136 : 213 – 236 .
- Jonsson , PR. 1994 . Tidal rhythm of cyst formation in the rock pool ciliate Strombidium oculatum Gruber (Ciliophora, Oligotrichea): A description of the functional biology and analysis of the tidal synchronization of encystment . Journal of Experimental Marine Biology and Ecology , 175 : 77 – 103 .
- Kahl , A. 1930-1935 . “ Urtiere, oder Protozoa. I ” . In Wimpertiere oder Ciliata (Infusoria) , Edited by: Dahl , F . Jena : Gustav Fischer .
- Keilin , D. 1959 . The Leeuwenhoek lecture: The problem of anabiosis or latent life . History and current concept. Proceedings of the Royal Society Biological Science , 150 : 149 – 191 .
- Kim , YO and Taniguchi , A. 1995 . Excystment of the oligothric ciliate Strombidium conicum . Aquatic Microbial Ecology , 9 : 149 – 156 .
- Lynn , DH. 2007 . The life cycle of the histophagous ciliate Tetrahymena corlissi Thompson, 1955 . Journal Eukaryotic Microbiology , 22 : 188 – 195 .
- Lynn , DH . 2008 . The ciliated Protozoa , 605 Springer .
- Montagnes , DJS , Lowe , CD , Poulton , A and Jonsson , P. 2002 . Redescription of Strombidium oculatum Gruber 1884 (Ciliophora, Olitrichia) . Journal of Eukaryotic Microbiology , 49 : 329 – 337 .
- Muller , H . 2007 . Live observation of excystment in the spirotrich ciliate Meseres corlissi European Journal of Protistology , 43 : 95 – 100 .
- Muller , H , Foissner , W and Weisse , T. 2006 . Role of soil in the life cycle of Meseres corlissi (Ciliophora Oligotrichea): Experiment with two clonal strains from the type locality, an astatic meadows pond . Aquatic Microbial Ecology , 42 : 199 – 208 .
- Mueller , JA and Mueller , WP. 1970 . Colpoda cucullus: A terrestrial aquatic . American Midland Naturalist , 84 : 1 – 12 .
- Repak , AJ and Pfister , RM. 1967 . Electron microscopical observations on the extracellular structures of the resting cyst of Blepharisma stoltei . Transactions of the American Microscopical Society , 86 : 417 – 421 .
- Ricci , N , Verni , F and Rosati , G. 1985 . The cyst of Oxytricha bifaria (Ciliata: Hypotrichida) . I. Morphology and significance. Transactions of the American Microscopical Society , 104 : 70 – 78 .
- Rosati , G , Modeo , L and Verni , F. 2008 . “ Micro-game hunting: Predatory behavior and defensive strategies in ciliates ” . In Microbial ecology research trend , Edited by: Van Dijk , T . 65 – 86 . New York, NY : Nova Science Publishers .
- Rosati , G , Verni , F and Nieri , L. 1983 . Investigation on the cyst wall of the hypotrich ciliate Gastrostyla steini Engelmann . Monitore Zoologico Italiano , 17 : 19 – 26 .
- Rosati , G , Verni , F , Nobili , R and Angeli , M. 1981 . Starvation in Euplotes crassus (Ciliata, Hypotrichida): Ultrastructure modifications and effects on production . Acta Protozoologica , 20 : 225 – 231 .
- Rosati , G , Verni , F and Ricci , N. 1984 . The cyst of Oxytricha bifaria (Ciliata: Hypotrichida) . III. Cytochemical investigation. Protistologica , 20 : 197 – 204 .
- Ruthmann , A and Kuck , A . 1985 . Formation of the cystwall of the ciliate Colpoda steinii Journal of Protozoology , 32 : 677 – 682 .
- Sugimoto , H and Endoh , H. 2008 . Differentially expressed genes during fruiting body development in the aggregative ciliate Sorogene stoianovitchae (Ciliophora: Colpodea) . Journal of Eukaryotic Microbiology , 55 : 110 – 116 .
- Verni , F and Gualtieri , P. 1997 . Feeding behaviour in ciliated Protists . Micron , 28 : 487 – 504 .
- Verni , F , Rosati , G and Ricci , N. 1984 . The cyst of Oxytricha bifaria (Ciliata: Hypotrichida) . II. The ultrastructure. Protistologica , 20 : 87 – 95 .
- Walker , GK and Maugel , TK. 1980 . Encystment and excystement in hypotrich ciliates. II Diophrys scutum and remarks on comparative features . Protistologica , 16 : 525 – 531 .
- Walker , GK , Maugel , TK and Goode , D. 1975 . Some ultrastructural observations on encystment in Stylonychia mytilus (Ciliophora: Hypotrichida) . Transactions of the American Microscopy Society , 94 : 147 – 154 .
- Weisse , T. 2004 . Meseres corlissi: Rare oligotrich ciliate adapted to warm water and temporary habitats . Aquatic Microbial Ecology , 37 : 75 – 83 .
- Weisse , T. 2006 . Freshwater ciliates as ecophysiological model organism – Lesson from Daphnia, major achievements and future perspectives . Archiv für Hydrobiologie , 167 : 371 – 402 .