Abstract
Active masking behaviours have been widely reported for Majoid spider crabs, although it is known that species living on sandy and muddy bottoms are frequently less masked than those living on rocky substrates. On the other hand, crabs living on soft bottoms may represent rare hard substrates available for larval settlement of other organisms. Inachus communissimus Rizza, 1839 is a spider crab belonging to the Inachidae family, common in the Mediterranean Sea. Individuals collected in two different locations on the sandy–muddy bottoms of the Northern Adriatic Sea (Senigallia and Pedaso), showed exoskeletons mainly covered by the colonial ascidian Diplosoma listerianum (Milne-Edwards, 1841). The large size of the ascidians, encompassing all the portions of the crabs' exoskeleton, and the lack of differences in the abundance of the covering, between adult males and females, support the idea that D. listerianum is an epibiont on the carapace of the crabs. The low level of carapace covering in the juveniles may be attributed to the regular moulting that the crabs undergo until puberty. On the other hand, juveniles actively mask themselves with small fragments of filamentous green algae, affixing them to the hooked setae. This active behaviour is lost when the crabs reach their final size after the pubertal moult.
Introduction
The evidence that many spider crabs belonging to the superfamily Majoidea (Crustacea, Brachyura) use the characteristic hooked setae, spread all over their exoskeletons, to keep masking materials in place is widely reported in literature (Wicksten Citation1993; Berke & Woodin Citation2008; Hultgren & Stachowicz Citation2009). This behaviour offers a series of advantages to the crabs and, in particular, allows them to hide in the environment, becoming invisible to prey and predators (Woods & McLay Citation1994; Sallam et al. Citation2007). The composition of the crabs' covering normally reflects the abundance of the materials present in the surrounding habitat (Wicksten Citation1993; Parapar et al. Citation1997). As a consequence, species living on rocky substrates mask themselves more abundantly than those living on muddy or sandy bottoms, which are more frequently covered by sediment (Dudgeon Citation1980).
Not all the organisms recorded on their exoskeleton are actively collected by spider crabs. Hartnoll (Citation1993) distinguished four categories of materials: (i) pieces of organic or inorganic detritus affixed to the hooked setae; (ii) parts of sessile living organisms attached by the crab that continue to grow; (iii) organisms directly settled while in their larval stage; and (iv) vagile organisms that can migrate to and from the environment. Indeed, the crabs represent a suitable substrate for many epibionts that may reap advantages in terms of food supply and larval dispersion (Maldonado & Uriz Citation1992), and the crabs tolerate their presence as they increase their masking level (Dudgeon Citation1980).
Several kinds of organisms, such as algae, sponges, hydroids, bryozoans, amphipods, cirripeds, polychaetes, etc., have been recorded on the crabs (Sato & Wada Citation2000), and their amount seems to differ in relation to the chemical/mechanical properties of the organism and to the sex and maturity stages of the host (Dudgeon Citation1980; Maldonado & Uriz Citation1992; Woods & McLay Citation1994; Woods & Page Citation1999; Sato & Wada Citation2000; Cruz-Rivera Citation2001; Hultgren et al. Citation2006; Martinelli et al. Citation2006; Berke & Woodin Citation2008; Hultgren & Stachowicz Citation2008). It is also known that juvenile crabs undergo frequent moults, each time losing their covering materials (Wirtz & Diesel Citation1983). On the other hand, there are crabs (e.g. Maja squinado) that are extensive carapace decorators as juveniles but reduce this activity with maturity, when their larger size offers a suitable substrate to many epibionts settling on them (Fernández et al. Citation1998).
On sandy and muddy bottoms, epibiosis on decapod crustaceans may represent a highly valuable process for the survival of sessile organisms; the size of the crustacean host, together with its carapace renewal, due to moulting, are some important factors regulating the pattern of colonisation of the epibionts (Abelló et al. Citation1990; Gili et al. Citation1993; Di Camillo et al. Citation2008).
The aim of this work was to investigate the presence of epibionts or masking materials on the exoskeleton of the spider crab Inachus communissimus Rizza, 1839 from the Northern Adriatic Sea. This species is Atlanto-Mediterranean and is mainly recorded on sandy and muddy bottoms between 5 and 200 m depth (Mori Citation1991; d'Udekem d'Acoz Citation1999). Mori (Citation1991) reported a short life cycle for this species with only one mating season and a life span not exceeding 2 years; ovigerous females can probably be found all around the year, but their number increases in late spring and summer. Inachus communissimus is a detritivorous species that may sometimes act as a predator on small vagile organisms (Mori Citation1991).
Materials and methods
Sampling was carried out in May 2007 by means of a ‘rapido’ trawl, on the sandy–muddy bottoms of the Northern Adriatic Sea. The ‘rapido’ trawl is a bottom gear characterised by a rigid frame that assures the vertical and the horizontal opening of a net and that has iron teeth along the lower part. This is typically used in the Northern Adriatic Sea to exploit benthic species such as flatfishes (Cosimi et al. Citation2001; Rossetti et al. Citation2006) and spider crabs of the genus Inachus are common by-catch in this kind of fishing activity (Hall-Spencer et al. Citation1999). Samples of I. communissimus were collected during two cruises on board commercial fishing vessels using ‘rapido’ trawl between 2 and 8 miles offshore in two different sites (Senigallia and Pedaso; ).
Both sampling areas are characterised by the biocoenosis of Fine Well Sorted Sand (SFBC, Pérès & Picard Citation1964) widely diffused in the inshore bottoms of the Northern and Central Adriatic Sea.
A total of 74 specimens of I. communissimus were collected off Senigallia (27 individuals: 9 adult females, 11 adult males and 7 juveniles) and Pedaso (47 individuals: 16 adult females, 28 adult males and 3 juveniles) and preserved in formalin 4%. Observations of the ventral region and the claws were carried out in order to determine sex and maturity stage of each sampled crab. All the crabs were photographed and their carapace surfaces were measured using the ImageJ software.
Statistical analyses were performed on the collected data using Statistica 7 (StatSoft®) software. Log-transformed data concerning the size of the carapace of the three considered categories of crabs, from both the sampled populations, were analysed by a two-way ANOVA test and a Tukey Unequal N HSD post-hoc test. The surface of the carapace occupied by epibionts and masking materials was measured, and the arcsine transformed percentage covering was also analysed by a two-way ANOVA test and a Tukey Unequal N HSD post-hoc test. Furthermore, the exoskeleton of each collected crab was observed to investigate the presence of epibionts and masking materials on it, considering the following regions: rostrum (A), left side of carapace (B), right side of carapace (C), central region of carapace (D), back region of carapace (E), claws (first pair of pereopods) (1), second pair of pereopods (2), third pair of pereopods (3), fourth pair of pereopods (4), fifth pair of pereopods (5) (Dudgeon Citation1980). Arcsine-transformed data, referring to the percentage of crabs covered in each region, were analysed by the Nested design ANOVA test and Tukey post-hoc test.
Observations on the morphology of the hooked setae found on the exoskeleton of one adult male specimen were also carried out by means of scanning electron microscopy (SEM). With this intent, the sample was washed with distilled water, dehydrated in a graded ethanol series, then coated with gold–palladium in a Balzer Union evaporator and finally examined with a Philips® XL20 SEM.
Results
The size of the collected adult specimens () showed significant differences between the two sexes (ANOVA F(3,60) = 36.55, p < 0.001). Adult males revealed a carapace surface significantly larger than females (p < 0.001). Furthermore, the crabs were significantly larger in Pedaso than in Senigallia (p < 0.05). All collected adult females were ovigerous.
Table I. Carapace average surface (± SD) (mm2) of adult males and females and range of carapace surface (mm2) for juveniles of Inachus communissimus collected off Senigallia and Pedaso
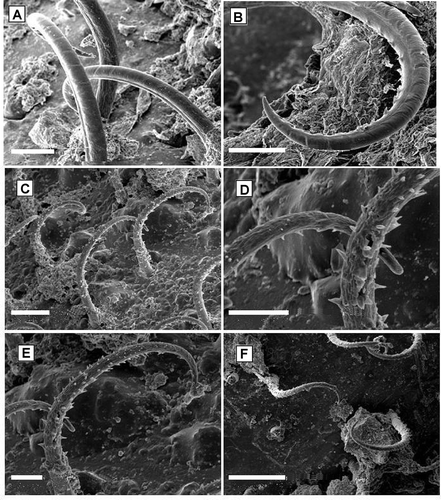
SEM analysis of the crab exoskeleton allowed, for the first time, the morphological description of the hooked setae of the carapace of Inachus communissimus. Three kinds of hooked setae, similar to those described by Szebeni and Hartnoll (Citation2005) for other species belonging to the genus Inachus, were recorded. The three kinds of setae were as follows: (i) On the rostral and central region of the carapace, the setae had a bent shaft and a single line of denticules on the distal portion of the inner side of the curve ( and B); (ii) On the lateral, central and back regions, the setae had the shaft curved and completely covered by scattered denticules (–E). (iii) On the lateral and back regions, similar denticulate hooked setae, deeply curved or twisted, were present ().
Figure 2. Hooked setae found on the rostral and central region of the carapace of Inachus communissimus sampled in the Northern Adriatic Sea: shaft curved, denticules on the inside of the curve that appear to form distally a single row (A,B); hooked setae found on the lateral, central and back regions of the carapace: shaft curved with denticules all around and all along (C–E); hooked setae found on the lateral and back region of the carapace (F). Scale bars: A–C, 50 μm. D and E, 20 μm. F, 100 μm.
The organism mainly recorded on the exoskeleton of the observed crabs was the didemnid tunicate Diplosoma listerianum (Milne-Edwards, 1841). More rarely, a filamentous green alga was found. The ascidian was recorded on all the adult specimens from both populations, on all the juveniles from Pedaso and on 72% of the juveniles from Senigallia. The alga was observed in 30% of the adults and 100% of the juveniles in Senigallia, and 23% of the adults and 75% of the juveniles in Pedaso.
Moreover, unidentified amphipods, the sabellid polychaetes Amphiglena mediterranea (Leydig, 1851) and Chone sp., and the hydroid Clytia gracilis (Sars, 1850) were occasionally found on the crabs collected off Pedaso.
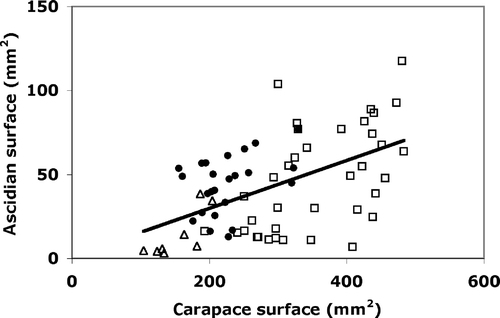
The algae found on the carapace were present as fragments affixed on the hooked setae, while the ascidian colonies grew on the crabs encompassing the setae in their tunics. The size of the ascidian colonies was correlated to the sizes of the exoskeleton (n = 74, r = 0.55, p < 0.001; ).
Figure 3. Relationships between ascidian cover and carapace area of each studied crab (y = 0.15x − 1.54, n = 74, r = 0.55, p < 0.001). Female, empty squares; males, black spots; juveniles, empty triangles.
The samples collected off Pedaso showed an average ascidian covering corresponding to 20.55 ± 8.94% of the carapace surface in adult females, 11.4 ± 7.96% in adult males and 5.79 ± 2.44% in juveniles. Similar results were obtained for the samples collected off Senigallia: 18.4 ± 4.7% in adult females, 16.6 ± 5.5% in adult males and 6.70 ± 8.40% in juveniles. No significant differences were found in the percentage of ascidian cover between the two sexes and the two populations, while juveniles appeared significantly less covered than adults (p < 0.05; ANOVA: F(5,68) = 4.46, p < 0.001).
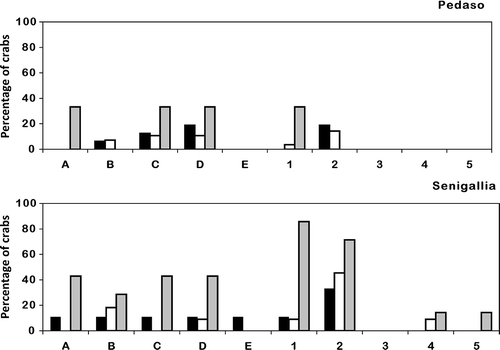
ANOVA showed significant differences in the covering of the various exoskeleton regions (F(9,54) = 14.98, p < 0.001). In adult crabs, the ascidian covering involved all the considered regions of the body ( and B) without significant differences between males and females. On the contrary, algae were mainly recorded on the carapace and the first two pairs of pereopods (p < 0.05; and B). In juveniles, the portions that were most covered by ascidians were the lateral sides, the central part of the carapace and the first and second pair of pereopods (p < 0.05; and B), while the first pair of pereopods was the region that was the most covered with algae (p < 0.05; and B). The back region of carapace and the third pair of pereopods were always free from algal covering.
Figure 4. Percentage of crabs covered by ascidians on the various regions of the exoskeleton: rostrum (A), left side of carapace (B), right side of carapace (C), central region of carapace (D), back region of carapace (E), claws (first pair of pereopods) (1), second pair of pereopods (2), third pair of pereopods (3), fourth pair of pereopods (4), fifth pair of pereopods (5). Females, black bars; males, white bars; juveniles, grey bars.
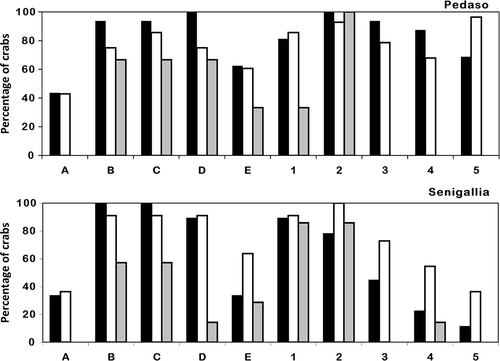
Figure 5. Percentage of crabs covered with algae on the various regions of the exoskeleton: rostrum (A), left side of carapace (B), right side of carapace (C), central region of carapace (D), back region of carapace (E), claws (first pair of pereopods) (1), second pair of pereopods (2), third pair of pereopods (3), fourth pair of pereopods (4), fifth pair of pereopods (5). Females, black bars; males, white bars; juveniles, grey bars.
Discussion
The colonial ascidian Diplosoma listerianum was the main component of the covering of the exoskeleton of the spider crab Inachus communissimus living on the sandy–muddy bottoms of the Northern Adriatic Sea. The ecology and biology of D. listerianum were deeply described by Brunetti et al. (Citation1988), and the ability of this didemnid to settle on decapod exoskeletons was already reported by Lafargue (Citation1975). On each examined specimen of I. communissimus, D. listerianum always appeared as a unique colony growing on the crab exoskeleton, encompassing the setae and occupying the entire available surface. These evidences suggested that this ascidian is a real epibiont that can settle on the exoskeleton of the crabs while in its larval stages using its adhesive papillae (Lane Citation1973). Diplosoma listerianum has previously been recorded as a pioneer species on bare surfaces (Schmidt & Warner Citation1986). Therefore, the exoskeleton of newly moulted I. communissimus represents a suitable hard substrate in a sandy–muddy environment. In addition, the denticulate hooked setae may offer a useful support to the larvae during settlement.
As observed for I. phalangium from the Adriatic Sea and its sponge cover (Martinelli et al. Citation2006), a positive correlation between the carapace size and the ascidian cover was found for I. communissimus. On the other hand, the absence of significant differences in the ascidian covering between adult males and females confirms the idea that the didemnid settles on the crab exoskeleton at the larval stage. In fact, in other Inachus species that have been reported to actively decorate their exoskeletons, the intensity of masking behaviour is sexually driven due to the more sedentary lifestyle of females (Martinelli et al. Citation2006).
The lower level of ascidian covering in juveniles may be attributed to the regular moulting that the crabs undergo until the typical Majoidea puberty moult, after which growth ceases (Hartnoll Citation1963; Gonzalez-Gurriaran et al. Citation1995). Thus, the frequent moults do not allow the growth of big ascidian colonies on juveniles as observed for other epibionts on other majoids (Winter & Masunari Citation2006).
On the crab's exoskeletons, algae were always recorded as fragments, indicating that their presence is due to active decoration. Contrary to that observed for adult crabs, the higher number of juveniles with algae attached to the exoskeleton allows us to conclude that their masking behaviour declines or is substantially lost at maturity, as occurs in other spider crab species (Dudgeon Citation1980; Hultgren & Stachowicz Citation2009). This idea is supported by the presence of a recurrent distribution pattern of ascidians and algal pieces on the exoskeleton of the juveniles. In fact, the lateral and central part of the carapace, together with the first and second pair of pereopods, are the most exposed regions of the crab's body for the ascidian larvae to settle. The ascidian colonisation probably starts in these areas and then develops on all the available portions of the exoskeleton in adult crabs. The back region and the last two pairs of pereopods are generally covered by sediment that prevents ascidian settling. The high rate of algal fragments recorded on the first pair of pereopods of juveniles may be related to a need for protection of this region. It is known that the urge for protection of the various body regions can drive the decoration patterns in different species or in the same species according to sex and life stage (Dudgeon Citation1980; Martinelli et al. Citation2006).
Berke and Woodin (Citation2008) suggested that the decoration pattern arises from the interaction between metabolism, body/claw size, and predation risk. Together with the increment in body size of adults, epibiosis definitely contributes to the reduction of predation risk by reducing the visibility of the crabs and protecting them with powerful anti-feeding compounds (Hughes & Cava Citation1998; Vervoort et al. Citation1998). In the present study, this turned out to be particularly evident in adult specimens of I. communissimus on which the ascidian epibiont found a stable substrate. According to Fernández et al. (Citation1998), epibiosis is more susceptible to environmental and biological constraints than self-decoration, and this phenomenon is quantitatively more important on those parts of the body where camouflage is not essential and on those individuals that lose their decorative behaviour during a life stage.
Acknowledgements
The authors are indebted to Dr Alessandro Lucchetti, ISMAR CNR Ancona, for the sample collection.
References
- Abelló , P , Villanueva , R and Gili , JM. 1990 . Epibiosis in deep-sea crab populations as indicator of biological and behavioural characteristic of the host . Journal of Marine Biological Association of the United Kingdom , 70 : 687 – 695 .
- Berke , SK and Woodin , SA. 2008 . Energetic costs, ontogenetic shifts and sexual dimorphism in spider crab decoration . Functional Ecology , 2 : 1118 – 1124 .
- Brunetti , R , Bressan , M , Marin , M and Libralato , M. 1988 . On the ecology and biology of Diplosoma listerianum (Milne Edwards. 1841) (Ascidiacea, Didemnidae) . Vie et Milieu , 38 : 123 – 131 .
- Cosimi , G , Leonori , I , Sala , A and Palumbo , V. 2001 . “ Principali attrezzi per la pesca professionale marittima – sistematica e normativa. (Cap. XI) ” . In La gestione della pesca marittima in Italia. Fondamenti tecnico-biologici e normative vigenti , Edited by: Gramitto , ME . 241 – 265 . CNR, Monografie scientifiche, serie scienze e tecnologie dell'ambiente .
- Cruz-Rivera , E. 2001 . Generality and specificity in the feeding and decoration preferences of three Mediterranean crabs . Journal of Experimental Marine Biology and Ecology , 266 : 17 – 31 .
- Di Camillo , CG , Bo , M , Puce , S , Tazioli , S , Froglia , C and Bavestrello , G. 2008 . The epibiontic assemblage of Geryon longipes (Crustacea: Decapoda: Geryonidae) from the Southern Adriatic Sea . Italian Journal of Zoology , 75 : 29 – 35 .
- d'Udekem and d'Acoz , C . 1999 . Inventaire et distribution des crustacés décapodes de l'Atlantique nord-oriental, de la Méditerranée et des eaux continentales adjacentes au nord de 25°N . Patrimoines naturels (M.N.H.N./S.P.N.) , 40 : 1 – 383 .
- Dudgeon , R. 1980 . “ Some inter- and intraspecific differences in the decorating patterns of majid crabs (Crustacea: Decapoda) from the coastal waters of Hong Kong ” . In Proceedings of First International Marine Biological Workshop: The Marine Flora and Fauna of Hong Kong and Southern China , Edited by: Morton , BS and Tseng , CK . 825 – 835 . Hong Kong : Hong Kong University Press .
- Fernández , L , Parapar , J , González-Gurrián , E and Muiño , R. 1998 . Epibiosis and ornamental cover patterns of the spider crab Maja squinado on the glacian coast, Northwestern Spain: Influence of behavioral and ecological characteristics of the host . Journal of Crustacean Biology , 18 : 728 – 737 .
- Gili , JM , Abelló , P and Villanueva , R. 1993 . Epibionts and intermoult duration in the crab Bathynectes piperitus . Marine Ecology Progress Series , 98 : 107 – 113 .
- Gonzalez-Gurriaran , E , Freire , J , Parapar , J , Sampedro , MP and Urcera , M. 1995 . Growth at moult and moulting seasonality of the spider crab, Maja squinado (Herbst) (Decapoda: Majidae) in experimental conditions: Implications for juvenile life history . Journal of Experimental Marine Biology and Ecology , 189 : 183 – 203 .
- Hall-Spencer , JM , Froglia , C , Atkinson , RJA and Moore , PG. 1999 . The impact of Rapido trawling for scallops, Pecten jacobaeus (L.), on the benthos of the Gulf of Venice . ICES Journal of Marine Science , 56 : 111 – 124 .
- Hartnoll , RG. 1963 . The biology of Manx spider crabs . Proceedings of the Zoological Society of London , 141 : 423 – 496 .
- Hartnoll , RG. 1993 . The epibiota of spider crabs . Scientific Annals of the School of Biology , 1 : 163 – 176 .
- Hughes , TV and Cava , MP. 1998 . Total synthesis of didemnimide A and B . Tetrahedron Letters , 39 : 9629 – 9630 .
- Hultgren , KM and Stachowicz , JJ. 2008 . Alternative camouflage strategies mediate predation risk among closely related co-occurring kelp crabs . Oecologia , 155 : 519 – 528 .
- Hultgren , KM and Stachowicz , JJ. 2009 . Evolution of decoration in Majoid crabs: A comparative phylogenetic analysis of the role of body size and alternative defensive strategies . The American Naturalist , 173 : 566 – 578 .
- Hultgren , KM , Thanh , PD and Sato , M. 2006 . Geographic variation in decoration selectivity of Micippa platipes and Tiarinia cornigera in Japan . Marine Ecology Progress Series , 326 : 235 – 244 .
- Lafargue , F. 1975 . Révision taxonomique des Didemnidae des còtes de France (Ascidies composées). Description des espèces de Banyuls-sur-Mer. Généralités. Genre Polysyncraton . Vie et Milieu , 25 : 133 – 164 .
- Lane , DJW. 1973 . Attachment of the larva of the Ascidian Diplosoma listerianum . Marine Biology , 21 : 47 – 58 .
- Maldonado , M and Uriz , MJ. 1992 . Relationships between sponges and crabs: Patterns of epibiosis on Inachus aguiarii (Decapoda: Majidae) . Marine Biology , 113 : 281 – 286 .
- Martinelli , M , Calcinai , B and Bavestrello , G. 2006 . Use of sponges in the decoration of Inachus phalangium (Decapoda, Maijdae) from the Adriatic Sea . Italian Journal of Zoology , 73 : 34 – 353 .
- Mori , M. 1991 . Notizie biologiche su Inachus communissimus Rizza (Crustacea, Decapoda, Majidae) del Golfo di Genova . Bollettino del Museo dell'Istituto di Biologia dell'Università di Genova , 54/55 : 59 – 69 .
- Parapar , J , Fernández , L , Gonzáles-Gurriarán , E and Muíño , R. 1997 . Epibiosis and masking material in the spider crab Maja squinado (Decapoda: Majidae) in Ría de Arousa (Galicia, NW Spain) . Cahiers de Biologie Marine , 38 : 221 – 234 .
- Pérès , JM and Picard , J. 1964 . Nouveau manuel de bionomie benthique de la mer Méditerranée . Recueil des Travaux de la Station Marine d'Endoume , 31 : 1 – 137 .
- Rossetti , I , Sartor , P , Francesconi , B , Mori , M and Belcari , P. 2006 . Biological aspects of Medorippe lanata (Linnaeus, 1767) (Brachyura: Dorippidae) from the eastern Ligurian Sea (western Mediterranean) . Hydrobiologia , 557 : 21 – 29 .
- Sallam , WS , Madkour , FF and Wicksten , MK. 2007 . Masking behaviour of the spider crab, Hyastenus hilgendorfi (De Man, 1887) (Brachyura, Majidae) from the Suez Canal, Egypt . Crustaceana , 80 : 235 – 245 .
- Sato , M and Wada , K. 2000 . Resource utilization for decorating in three intertidal majid crabs (Brachyura: Majidae) . Marine Biology , 137 : 705 – 714 .
- Schmidt , GH and Warner , GF. 1986 . Spatial competition between colonial ascidians: The importance of stand-off . Marine Ecology Progress Series , 31 : 101 – 104 .
- Szebeni , T and Hartnoll , RG. 2005 . Structure and distribution of carapace setae in British spider crabs . Journal of Natural History , 39 : 3795 – 3809 .
- Vervoort , HC , Pawlik , JR and Fenical , W. 1998 . Chemical defense of the Caribbean ascidian Didemnum conchyliatum . Marine Ecology Progress Series , 164 : 221 – 228 .
- Wicksten , MK. 1993 . A review and a model of decorating behaviour in spider crabs (Decapoda, Brachyura, Majidae) . Crustaceana , 64 : 314 – 325 .
- Winter , VC and Masunari , S. 2006 . Macroepizoísmo em Libinia ferreirae (Crustacea, Brachyura, Majidae) . Iheringia, série Zoologia , 96 : 135 – 140 .
- Wirtz , P and Diesel , R. 1983 . The social structure of Inachus phalangium, a spider crab associated with the sea anemone Anemonia sulcata . Zeitschrift für Tierpsychologie , 62 : 209 – 234 .
- Woods , CM and McLay , CL. 1994 . Use of camouflage materials as a food store by the spider crab Notomithrax ursus (Brachyura: Majidae) . New Zealand Journal of Marine and Freshwater Research , 28 : 97 – 104 .
- Woods , CM and Page , MJ. 1999 . Sponge masking and related preferences in the spider crab Thacanophyris filholi (Brachiura: Majidae) . New Zealand Journal of Marine and Freshwater Researches , 50 : 135 – 143 .