Abstract
The present study reports the morphology of leukocytes of 12 European lacertid lizards (Podarcis sicula, P. tiliguerta, P. melisellensis, P. bocagei, P. muralis, Algyroides nigropunctatus, Lacerta viridis, L. bilineata, L. trilineata, L. oxycephala, Timon lepidus, and Zootoca vivipara) stained using May–Grünwald/Giemsa method. The morphology of white blood cells was very similar among species, suggesting a relative morphological uniformity within the lacertid lizards. For six species (i.e. P. sicula, P. tiliguerta, P. melisellensis, P. bocagei, P. muralis, and A. nigropunctatus), we determined the leukocyte differential counts, which may be considered representative of the normal values of the corresponding populations. These results may be useful either in clinical investigation to detect pathologies in wild individuals, as in management and conservation projects to assess the general health conditions of natural wild lizard populations.
Introduction
In clinical investigation, blood samples are of great diagnostic value, and can be easily obtained (e.g. Frye Citation1991). Haematological data provide clues to the existence of conditions that affect the cellular component of peripheral blood, and can be used to detect such condition as anaemia, parasitaemia, chronic stress and disorder of haemostasis (Mader Citation2000). Moreover, the haemogram is also of particular interest in ecological immunology (Sheldon & Verhulst Citation1996; Ots et al. Citation1998; Norris & Evans Citation2000), since it can support field researchers to analyse, in wild-living reptiles, the effects of health condition of individuals on different life history traits. For example, the leukograms of tortoises have been useful to explain why individuals differ from each other with respect to their reproductive decisions (Galeotti et al. Citation2005). Finally, the knowledge of the haematologic parameters of free-living individuals is important for assessing and managing their populations (Christopher et al. Citation1999; Dickinson et al. Citation2002). This is because haemograms supply a useful tool to easily detect pathologic processes within populations, which is a crucial point in managing endangered species (Martinez-Silvestre et al. Citation2005).
Many authors have described the blood cells of different reptile species, particularly tortoises (Alleman et al. Citation1992; O'Connor et al. Citation1994; Christopher et al. Citation1999; Kolle et al. Citation2001; Dickinson et al. Citation2002; Knotek et al. Citation2002, Citation2003; Knotkova et al. Citation2002; Azevedo & Lunardi Citation2003; Lopez-Olivera et al. Citation2003; Ugurtas et al. Citation2003; Keller et al. Citation2004), snakes (Bounous et al. Citation1996; Lamirande et al. Citation1999; Salakij et al. Citation2002a,Citationb; Arikan et al. Citation2004, Citation2009a; Arikan & Çiçek Citation2010) and lizards (Pica et al. Citation1986; Divers & Redmayne Citation1996; Puerta et al. Citation1996; Eliman Citation1997; Tosunoglu et al. Citation2001; Sevinc & Ugurtas Citation2004; Pejrilova et al. Citation2004; Sacchi et al. Citation2007; Arikan et al. Citation2009b; Arikan & Çiçek Citation2010).
Although erythrocytes, thrombocytes, basophils, lymphocytes and monocytes are morphologically similar, remarkable differences occur in heterophils, eosinophils and monocytes among different reptile taxa, and frequently among species within a single taxon (Le Blanc et al. 2000; Mader Citation2000; Martinez-Silvestre et al. Citation2005). For example, heterophils vary in number and in nuclear lobules, whereas azurophils are often recognised as a distinct cell type from monocytes (Eliman Citation1997). Similarly, the eosinophils differ widely among reptiles for both shape and colouration of cytoplasmatic granules (Frye Citation1991; Mader Citation2000; Salakij et al. Citation2002a; Knotkova et al. Citation2002). Thus, some ambiguity still exists that can make the evaluation of the haemogram of reptile difficult to do (Cannon et al. Citation1996; Canfield Citation1998).
In addition, reptiles are the class among vertebrates in which immunology has been less investigated in respect, for example, to birds and mammals. Consequently, haematological studies are of great interest as anticipatory of immunological studies.
In this article we describe the morphology of circulating blood cells of 12 species of European lizards and we determine reference values for leukocyte differential counts for six of them.
Materials and methods
During summer 2007–2009, we collected 129 blood samples from both males and females of the following six lacertid species: Podarcis sicula (five males and five females, Latina, Italy), P. tiliguerta (four males and four females, Punta Sebera, Italy), P. melisellensis (seven males and five females, Kres, Croatia), P. bocagei (five males and six females, Oporto, Portugal), P. muralis (71 males and 10 females, Pavia, Italy), Algyroides nigropunctatus (five males and two females, Kres, Croatia). In addition, we collected blood samples from one to two individuals from Lacerta viridis (one male, Haj, Slovakia), L. bilineata (one male and one female, Pavia, Italy), L. trilineata (one male, Kres, Croatia), L. oxycephala (one female, Kres, Croatia), Timon lepidus (one juvenile, Oporto, Portugal), Zootoca vivipara (one female, Alpe Siusi, Italy). All individuals except T. lepidus were sexually mature, and appeared to be healthy and with no detectable abnormalities (such as injuries by predators).
Blood was collected immediately after capture from the orbital sinus using a heparinized glass capillary tube (see McLean et al. Citation1973). Air-dried smears were stained with May–Grünwald/Giemsa stain and scanned using a light microscope at 100× oil immersion following standard routines (Canfield Citation1998). In each microscope field, the red blood cells were counted and the leukocytes classified as lymphocytes, monocytes, eosinophils, heterophils, and basophils. In each smear, we counted 150–200 leukocytes and the corresponding red blood cells; when present, we also counted haemoparasites. The white blood cell count (WBC) and haemoparasite count were expressed as numbers per 10,000 erythrocytes.
The WBC, thrombocytes and haemoparasites counts were log-transformed to achieve normality, whereas the leukocyte percentages were arcsin-transformed. Linear models were used to check for differences in blood variables among species and between sexes, using sex, species and sex × species interaction as predictors. The initial models were subjected to a stepdown simplification procedure, where non-significant terms (P > 0.05) were removed sequentially, starting from the interaction terms, until the minimal adequate models, including only significant variables, were obtained (Crawley Citation1993). All statistical analyses were performed using the R 2.6.1 statistical software (R Development Core Team Citation2007), and unless otherwise stated, values are means ± SE.
Results
Blood cell morphology
Mature erythrocytes of lizards are nucleated ellipsoidal cells with rounded poles and uniform grey–blue cytoplasm, even though colour varied among smears probably as an effect of the heparin within capillary tubes or the time elapsed between collection and staining (, ). The nucleus is central and elongated with the long diameter parallel to that of the cell; it stains violet with a dense dark purple chromatin.
Figure 1. Heterophils, eosinophils and basophils of cells of the Laceta bilineata, L. viridis, L. trilineata, L. oxycephala, Timon lepidus and Zootoca vivipara (May–Grünwald/Giemsa stain). Scale bar: 10 μm.
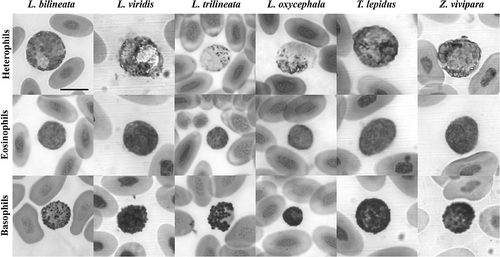
Figure 2. Heterophils, eosinophils and basophils of cells of the Podarcis tiliguerta, P. muralis, P. melisellensis, P. sicula, P. bocagei, and Algyroides nigropunctatus (May–Grünwald/Giemsa stain). Scale bar: 10 μm.
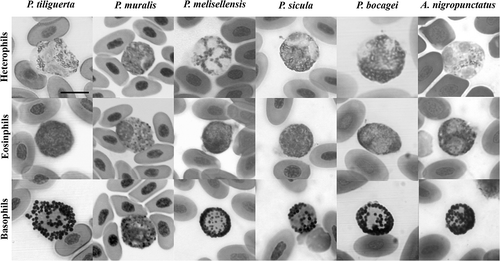
Heterophils (, ) are large, rounded cells with a light pink cytoplasm filled with small granules stained red to reddish-orange. In most cases, the granules are so numerous that they displace the nucleus against the side of the cell, while in others, the granules are less numerous and the nucleus is in the centre of the cell; the nucleus is light purple on staining and appears normally polylobed.
The eosinophils (, ) are as large as the heterophils and contain numerous small, weakly eosinophilic granules that often obscure the nucleus. The few intense eosinophilia in some cases could depend on the time elapsed between collection and staining. In some species, the granules are so thin and dense that their shape is not clearly defined, appearing as an undefined matrix filling the cytoplasm. The nucleus is variably positioned and appears dark purple on staining; sometimes it shows two or three lobes.
The basophils (, ) show the most conservative morphology among the 12 species analysed: they are as large as the other granulocytes and contain a round and a centrally positioned nucleus; the cytoplasm is densely filled by round granules that appear dark purple–black on staining.
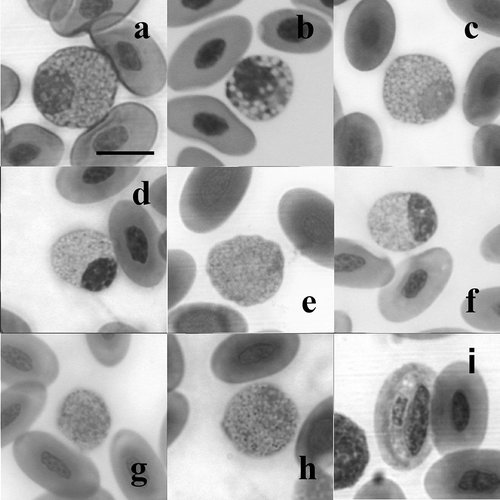
In some individuals, we detected a fourth type of granulocyte completely different from the other types (): it is a rounded cell with a non-segmented nucleus and an azurophilic cytoplasm densely filled by large, clear and rounded granules, which displace the nucleus off to the side of the cell.
Figure 3. The fourth granulocyte type (a–h), and an erythrocyte infected by haemogregarine spp. (i) (May–Grünwald/Giemsa stain); (a) Podarcis tiliguerta, (b) P. muralis, (c) P. melisellensis , (d) P. sicula, (e) Algyroides nigropunctatus, (f) Lacerta bilineata, (g) L. trilineata, and (h) L. oxycephala. Scale bar: 10 μm.
The monocytes in all species are rounded cells with the diameter not larger than the long diameter of the erythrocytes. The cytoplasm is moderately granular and appears light pink on staining. The nucleus may appear ‘C’ shaped, centrally positioned, and with a violet pigmentation and a granular, dark purple chromatin.
The lymphocytes in European lizards, as for all other vertebrates, are mononuclear with an azurophilic scant cytoplasm covering a narrow area around the nucleus.
Finally, the thrombocytes have a round to oval nucleus staining dark purple and a uniform grey–blue cytoplasm, without granules, and frequently form small groups up to a dozen cells within the smear. The thrombocytes are similar among all the species we investigated in this study.
Leukocyte differential count and haemoparasites
Total WBC (N/10,000 erythrocytes) ranged from 171 ± 22 in P. melisellensis to 280 ± 44 in P. tiliguerta, and we found a significant effect of the sex × species interaction (F 5,117 = 4.73, P < 0.001), which reflected the lower concentration of leucocytes in Common wall lizard females in respect to males () and in respect to both males and females of all other species (–). On the contrary, no significant differences were detected between the sexes in the other species, likely due to the lower sample of individuals analysed in respect to the P. muralis one. The number of heterophils differed significantly among species (F 5,123 = 3.54, P = 0.005), and were more numerous in A. nigropunctatus (15.7%) in respect to P. bocagei (7.9%) and P. tiliguerta (7.1%, see –). The eosinophils ranged from 7.7% (in A. nigropunctatus, Table II) to 16.8% (in P. tiliguerta, ), but this range did not differ among species or between sexes. The percentage of basophils varied depending both on sex (F 1,122 = 7.07, P = 0.008) and species (F 5,122 = 4.40, P = 0.001). In all species apart from A. nigropunctatus (), the basophils were detected more frequently in females than in males (11.1 and 9.2%, respectively). Irrespective of the sex, P. melisellensis had a significantly smaller percentage of basophils (5.1%) than all other species, whose basophils ranged from 7.4% in P. muralis to 13.6% in P. tiliguerta (–). Finally, the lymphocytes were the most abundant type of leukocytes in all the six species analysed, ranging from 60.3% in P. tiliguerta to 73.6% in P. melisellensis (–), but no significant differences were found among the species or between the sexes.
Table I. WBC, differential leukocyte count and thrombocyte count from the blood of P. muralis
Table II. WBC, differential leukocyte count and thrombocyte count from the blood of A. nigropunctatus
Table III. WBC, differential leukocyte count and thrombocyte count from the blood of P. bocagei
Table IV. WBC, differential leukocyte count and thrombocyte count from the blood of P. melisellensis
Table V. WBC, differential leukocyte count and thrombocyte count from the blood of P. sicula
Table VI. WBC, differential leukocyte count and thrombocyte count from the blood of P. tiliguerta
The number of thrombocytes differed significantly among the species (F 5,123 = 7.38, P < 0.001), being lower in P. melisellensis in respect to the other species (–); no differences were detected between males and females (mean thrombocyte proportion among species, N/10,000 erythrocytes, males: 84 ± 33, females: 75 ± 29).
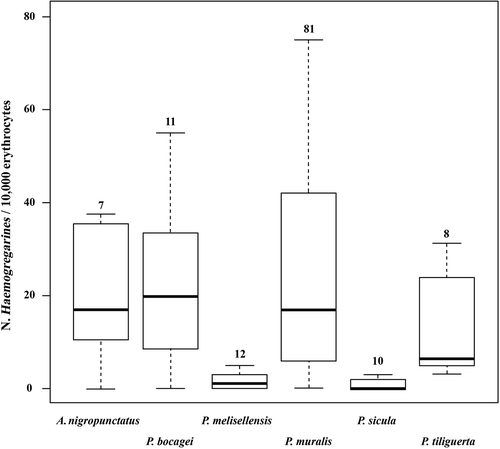
Haemoparasites (i.e. Haemogregarines, ) were detected in all species, and parasite loads varied significantly among the species (F 5,123 = 5.12, P < 0.001), but not between sexes. P. sicula and P. melisellensis had significant lower parasite concentrations than the other species ().
Discussion
The blood cells of the 12 species of lacertids we analysed in this study can be basically classified as erythrocytes, thrombocytes and leukocytes that can be furthermore assigned to five main classes: heterophils, eosinophils, basophils, monocytes and lymphocytes. Lymphocytes and heterophils were the most common leukocytes in the blood, while monocytes and basophils were the rarest, as reported in other reptile groups (Saint-Girons Citation1970; Pica et al. Citation1986; Frye Citation1991; Campbell Citation1996; Mader Citation2000). The classification of the leukocytes in reptiles might be more difficult than in other vertebrates due to the high levels of morphological variation among species (reviewed in Canfield Citation1998), particularly with regard to the acidophilic granulocytes (Saint-Girons Citation1970; Divers & Redmayne Citation1996; Eliman Citation1997; Christopher et al. Citation1999; Kolle et al. Citation2001; Dickinson et al. Citation2002; Knotek et al. Citation2002, 2003; Knotkova et al. Citation2002; Arikan et al. Citation2004). However, the morphology of the blood cells was very similar in all 12 species of lacertids with regard to lymphocytes, basophils and monocytes but also to granulocytes, suggesting that blood cells have a relative morphological uniformity within Lacertids. Despite this uniformity, further investigations are needed, particularly in order to exclude the possibility for the morphology of the Lacerta species, T. lepidus and Z. vivipara blood to be unusual, as only a few individuals were analysed. In addition, reptiles are the only class of vertebrates in which lymphocytes have not been characterised at the molecular level. Nevertheless, widespread evidences based on functional tests allow us to infer the existence of T and B lymphocytes (Cuchens & Clem Citation1979). In our 12 species, the morphology of lymphocytes is quite variable in size and nucleus/cytoplasm ratio, suggesting that different morphologies may actually correspond to different functional classes. Thus, our data need further improvements to clarify the morphology of live leucocytes by other approaches, such as flow cytometry.
As observed by Frye (Citation1991), and recently in Mediterranean Geckos (Sacchi et al. Citation2007), we found a fourth type of granulocyte in all the 12 species, whose morphology differed highly from that of the heterophils, eosinophils and basophils. Frye (Citation1991) names this cell type neutrophil, since granules do not stain selectively to May–Grünwald/Giemsa method. Alberio et al. (Citation2005), reported in the lizard Ameiva ameiva four different granulocyte types, referring them as types I, II, III and IV. However, the essential differences between type I and III cells were the bilobed nucleus, the amount of heterochromatin and the more heterogeneous granules present in type III. Consequently, both cells were likely to constitute the same cell lineage, type III being a more mature state of type I granulocyte (Alberio et al. Citation2005). Despite this, the morphology of the fourth type we found in European lizards does not resemble any granulocyte types reported for A. ameiva. Further electron microscope analyses and investigations on the specific enzymatic activities of this cell type are needed to definitively assess if it is a distinct granulocyte rather than an activated or toxic state of the other granulocyte types and monocytes (Sacchi et al. Citation2007; Strik et al. Citation2007).
Recently, Arikan et al. (Citation2009b) described the morphology of blood cells of 16 Lacertid lizards from Turkey, including L. bilineata, L. trilineata, P. muralis and P. sicula. Surprisingly, these authors did not find the heterophils in five species, including P. sicula, and reported a low occurrence of basophils in all species. Moreover, they provided no information about the sample sizes, or the sex of the lizards. Contrary to Arikan et al. (Citation2009b), in our blood survey the heterophils were detected in all 10 individuals of P. sicula analysed, with percentages similar to those observed in the other four Podarcis species and also to those reported by Pica et al. (Citation1986). In addition, in all species the basophils were not rare, as they were present with percentages between 5.1 and 12.3%. In two species (i.e. P. sicula and P. tiliguerta), basophils were as frequent as the heterophils and eosinophils. Finally, the percentages of granulocytes we find in our study were similar to those generally reported for the granulocytes of reptiles (Frye Citation1991; Mader Citation2000), and the leukocyte differential counts we obtained for P. muralis perfectly matched that previously published by Duguy (Citation1967). The heterophils and basophils are easily discernible from the other granulocytes because of their cytoplasmatic granules staining orange and dark purple. Thus the lack of heterophils as the rarity of the basophils reported by Arikan et al. (Citation2009b) might reflect the low sample of individuals they probably considered or, alternatively, the occurrence within the sample of some anomalous individual affected by undetected pathologies.
A second relevant result of this study was the definition of the leukocyte differential counts for six of the 12 species analysed. The percentages of the leukocytes were quite similar among species, particularly within the genus Podarcis. Indeed, the more evident significant differences regarded the heterophils, which were more frequent in A. nigropunctatus than in all the other species, and the basophils, which were more abundant in males than in females in all the species but A. nigropunctatus. Since all sampled lizards appeared in good condition and no evident pathologies were detected, we assume that the differential counts here provided can be considered as representative of the normal values of the sampled populations. However, these data are based on small samples (with the exception of P. muralis), collected from single populations, so caution should be exercised when extrapolating the results to other natural populations of the six species.
Even though preliminary, our data represent a first important step in order to obtain an accurate set of baseline reference intervals for clinically healthy free-ranging lizards in different sites and under a variety of environmental conditions (Christopher et al. Citation1999). Once obtained, haemograms will be useful tools in the diagnosis and monitoring of lizard health and diseases, both for conservation and scientific purposes.
Finally, haemogregarines were detected in all six species analysed in detail. Parasite loads did not differ between sex but only among species. Overall, a four species group (i.e. A. nigropunctatus, P. bocagei, P. tiliguerta and P. muralis) showed higher parasite loads then the other two (P. melisellensis and P. sicula). This difference may be due to the time difference among blood sampling of species (and consequently a difference in the cycle of the parasite), rather than a true difference in the susceptibility to haemogregarines among species. Therefore, further analyses basing on a repeated blood sampling of individuals in different period of the breeding season would be necessary to fully understand the causes of the difference in parasite loads among species we found in our survey. Finally, we did not find any parasite infecting other blood cells than erytrocytes, contrary to the recent confirmation that haemogregarines are able to infect monocytes (Bonadiman et al. Citation2010).
Acknowledgements
We thank Luca Cavigioli, Mario Pikalik, Alessandra Binada for the help during field work, and Corrado Guzzanti, and Dr Jeffrey Lebowski for his helpful suggestions on a previous version of the manuscript. We also thank four anonymous referees for their useful comments to a previous version of the manuscript.
References
- Alberio , SO , Diniz , JA , Silva , EO , De Souza , W and DaMatta , RA. 2005 . Cytochemical and functional characterization of blood and inflammatory cells from the lizard Ameiva ameiva . Tissue & Cell , 37 : 193 – 202 .
- Alleman , AR , Jacobson , ER and Raskin , RE. 1992 . Morphometric and cytochemical characteristics of blood cells from the desert tortoise (Gopherus agassizii) . American Journal of Veterinary Research , 53 : 1645 – 1651 .
- Arikan , H and Çiçek , K. 2010 . Morphology of peripheral blood cells from various species of Turkish herpetofauna . Acta Herpetologica , 5 : 179 – 198 .
- Arikan , H , Goçmen , B , Atatur , MK , Kumlutas , Y and Cicek , K. 2009a . Morphology of peripheral blood cells from various Turkish snakes . North-Western Journal of Zoology , 5 : 61 – 73 .
- Arikan , H , Goçmen , B , Yildiz , MZ , Ilgaz , C and Kumlutas , Y. 2009b . Morphology of peripheral blood cells from some lacertid lizards from Turkey . Russian Journal of Herpetology , 16 : 101 – 106 .
- Arikan , H , Kumlutas , Y , Turkozan , O , Baran , I and Ilgaz , C. 2004 . The morphology and size of blood cells of some viperid snakes from Turkey . Amphibia–Reptilia , 25 : 465 – 470 .
- Azevedo , A and Lunardi , LO. 2003 . Cytochemical characterization of eosinophilic leukocytes circulating in the blood of the turtle (Chrysemys dorbigni) . Acta Histochemica , 105 : 99 – 105 .
- Bonadiman , SF , Miranda , FJB , Ribeiro , MLS , Rabelo , G , Lainsond , R , Silva , EO and DaMatta , RA. 2010 . Hematological parameters of Ameiva ameiva (Reptilia: Teiidae) naturally infected with hemogregarine . Confirmation of monocytosis. Veterinary Parasitology , 171 : 146 – 150 .
- Bounous , DI , Dotson , TK , Brooks , RL and Ramsay , EC. 1996 . Cytochemical staining and ultrastructural characteristics of peripheral blood leukocytes from the yellow rat snake (Elaphe obsoleta quadrivittata) . Comparative Haematology International , 6 : 86 – 91 .
- Campbell , TW. 1996 . “ Clinical pathology ” . In Reptile medicine and surgery , Edited by: Mader , DR . 248 – 257 . Philadelphia, PA : WB Saunders Company .
- Canfield , PJ. 1998 . Comparative cell morphology in the peripheral blood film from exotic and native animals . Australian Veterinary Journal , 76 : 793 – 800 .
- Cannon , MS , Freed , DA and Freed , PS. 1996 . The leukocytes of the roughtail gecko Cytopodion scabrum . A bright-field and phase-contrast study. Anatomia, Histologia, Embryologia , 25 : 11 – 14 .
- Christopher , MM , Berry , KH , Wallis , IR , Nagy , KA , Henen , BT and Peterson , CC. 1999 . Reference intervals and physiological alterations in hematologic and biochemical values of free-ranging desert tortoises in the Mojave desert . Journal of Wildlife Diseases , 35 : 212 – 238 .
- Crawley , MJ. 1993 . GLIM for ecologists , Oxford : Blackwell Scientific Publications .
- Cuchens , MA and Clem , LW. 1979 . Phylogeny of lymphocyte heterogeneity. IV. Evidence for T-like and B-like cells in reptiles . Developmental and Comparative Immunology , 3 : 465 – 475 .
- Dickinson , VM , Jarchow , JL and Trueblood , MH. 2002 . Hematology and plasma biochemistry reference range values for free-ranging desert tortoises in Arizona . Journal of Wildlife Diseases , 38 : 143 – 153 .
- Divers , SJ and Redmayne , EKA. 1996 . Haematological and biochemical values of 10 green iguanas (Iguana iguana) . Veterinary Record , 138 : 203 – 205 .
- Duguy , R. 1967 . Le cycle annuel des éléments figurés du sang chez Emys orbicularis L., Lacerta muralis Laur. et Natrix maura L. Bulletin de la Societé . Zoologique Françaises , 92 : 23 – 37 .
- Eliman , MM. 1997 . Hematology and plasma chemistry of the Inland Bearded Dragon, Pogona vitticeps . Bulletin of the Association of Reptile and Amphibian Veterinarians , 7 : 23 – 25 .
- Frye , FL. 1991 . “ Hematology as applied to clinical reptile medicine ” . In Biomedical and surgical aspect of captive reptile husbandry , Edited by: Frye , FL . 209 – 279 . Malabar, FL : Krieger Publishing Company .
- Galeotti , P , Sacchi , R , Fasola , M , Pellitteri-Rosa , D , Marchesi , M and Ballasina , D. 2005 . Courtship displays and mounting calls are honest, condition-dependent signals that influence mounting success in Hermann's tortoises . Canadian Journal of Zoology , 83 : 1306 – 1313 .
- Keller , JM , Kucklick , JR , Stamper , MA , Harms , CA and McClellan-Green , PD. 2004 . Associations between organochlorine contaminant concentrations and clinical health parameters in loggerhead sea turtles from North Carolina, USA . Environmental Health Perspectives , 112 : 1074 – 1079 .
- Knotek , Z , Hauptman , K , Knotkova , Z , Hajkova , P and Tichy , F. 2002 . Renal disease haemogram and plasma biochemistry in green iguana . Acta Veterinaria Brno , 71 : 333 – 340 .
- Knotek , Z , Knotkova , Z , Doubek , J , Pejrilova , S and Hauptman , K. 2003 . Plasma biochemestry in female Green iguana (Iguana iguana) with calcium metabolism disorders . Acta Veterinaria Brno , 72 : 183 – 189 .
- Knotkova , Z , Doubek , J , Knotek , Z and Hajkova , P. 2002 . Blood cell morphology and plasma biochemistry in Russian tortoises (Agrionemys horsfieldii) . Acta Veterinaria Brno , 71 : 191 – 198 .
- Kolle , P , Donhauser , J , Krause , D and Hoffmann , R. 2001 . Blood values of European tortoises (Testudo hermanni, Testudo graeca, Testudo marginata, Agrionemys horsfieldii) . Tierarztliche Praxis , 29 : 386 – 390 .
- Lamirande , EW , Nichols , DK , Owens , JW , Gaskin , JM and Jacobson , ER. 1999 . Isolation and experimental transmission of a reovirus pathogenic in rat snakes (Elaphe species) . Virus Research , 63 : 135 – 141 .
- LeBlanc , CJ , Heatley , JJ and Mack , EB. 2000 . A review of the morphology of lizard leukocytes with a discussion of the clinical differentiation of bearded dragon, Pogona vitticeps, leukocytes . Journal of Herpetological Medicine and Surgery , 10 : 27 – 30 .
- Lopez-Olivera , JR , Montane , J , Marco , I , Martinez-Silvestre , A , Soler , J and Lavin , S. 2003 . Effect of venipuncture site on hematologic and serum biochemical parameters in marginated tortoise (Testudo marginata) . Journal of Wildlife Diseases , 39 : 830 – 836 .
- Mader , DR. 2000 . “ Normal hematology of Reptiles ” . In Veterinary hematology , Edited by: Feldman , BF , Zinkl , JG and Jain , NC . 1126 – 1132 . Philadelphia, PA : Lippincott Williams & Wilkins .
- Martinez-Silvestre , A , Marco , I , Rodriguez-Dominguez , MA , Lavin , S and Cuenca , R. 2005 . Morphology, cytochemical staining, and ultrastructural characteristics of the blood cells of the giant lizard of El Hierro (Gallotia simonyi) . Research in Veterinary Sciences , 78 : 127 – 134 .
- McLean , GS , Lee , SK and Wilson , KF. 1973 . A simple method of obtaining blood from lizards . Copeia , 1973 : 338 – 339 .
- Norris , K and Evans , MR. 2000 . Ecological immunology: Life history trade-offs and immune defence in birds . Behavioral Ecology , 11 : 19 – 26 .
- O'Connor , MP , Grumbles , JS , George , RH , Zimmerman , LC and Spotila , JR. 1994 . Potential hematological and biochemical indicators of stress in free-ranging desert tortoises and captive tortoises exposed to a hydric stress gradient . Herpetological Monographs , 8 : 5 – 26 .
- Ots , I , Murumägi , A and Hõrak , P. 1998 . Haematologiacal health state indices of reproducing Great Tits: Methodology and sources of natural variation . Functional Ecology , 12 : 700 – 707 .
- Pejrilova , S , Knotkova , Z , Knotek , Z and Vrbas , V. 2004 . Age-related changes of the haematological profile in green iguana (Iguana iguana rhinolopha) . Acta Veterinaria Brno , 73 : 305 – 312 .
- Pica , A , Della Corte , F , Grimaldi , MC and D'Ippolito , S. 1986 . Blood cells of the common lizard (Podarcis s. sicula Raf.): Morphocytochemistry, DNA amount of red cells and hemopoiesis . Italian Journal of Anatomy and Embryology , 91 : 301 – 320 .
- Puerta , M , Abelenda , M , Salvador , A , Martin , J , Lopez , P and Veiga , JP. 1996 . Haematology and plasma chemistry of male lizards, Psammodromus algirus. Effects of testosterone treatment . Comparative Haematology International , 6 : 102 – 106 .
- R Development Core Team 2007. R: A language and environment for statistical computing. Vienna, R Foundation for Statistical Computing, ISBN 3-900051-07-0, URL http://www.R-project.org (http://www.R-project.org)
- Sacchi , R , Pupin , F , Zuffi , MAL , Scali , S , Boncompagni , E , Binda , A , Galeotti , P and Fasola , M. 2007 . Blood cell morphology of the Moorish gecko, Tarentola mauritanica . Amphibia–Reptilia , 28 : 503 – 508 .
- Saint-Girons , MC. 1970 . “ Morphology of the circulating blood cells ” . In Biology of Reptilia. Vol. 3 , Edited by: Gans , C and Parson , TS . 73 – 91 . New York, NY : Academic Press .
- Salakij , C , Salakij , J , Apibal , S , Narkkong , N-A , Chanhome , L and Rochanapat , N. 2002a . Hematology, Morphology, cytochemical staining, and ultrastructural characteristics of blood cells in King Cobras (Ophiophagus hannah) . Veterinary Clinical Pathology , 31 : 116 – 126 .
- Salakij , C , Salakij , J , Suthunmapinunta , P and Chanhome , L . 2002b . Hematology, morphology and ultrastructure of blood cells and blood parasites from Puff-faced watersnakes (Homalopsis buccata) . Kasetsart Journal , 36 : 35 – 43 .
- Sevinc , M and Ugurtas , IH. 2004 . The morphology and size of blood cells of Lacerta rudis bithynica . Asiatic Herpetological Research , 9 : 122 – 129 .
- Sheldon , BC and Verhulst , S. 1996 . Ecological immunology: Costly parasite defences and trade-offs in evolutionary biology . Trends in Ecology & Evolution , 11 : 317 – 321 .
- Strik , NI , Alleman , R and Harr , KE. 2007 . “ Circulating inflammatory cells ” . In Infectious diseases and pathology of reptiles , Edited by: Jacobson , ER . 167 – 218 . New York, NY : Taylor & Francis .
- Tosunoglu , M , Goçmen , B , Atatur , MK and Cevik , IE. 2001 . Morphological and serological investigations on Lacerta laevis Gray, 1838 (Sauria: Lacertidae) . population from Anatolia. Zoology of the Middle East , 23 : 55 – 60 .
- Ugurtas , IH , Sevinc , M and Yildirimhan , HS. 2003 . Erythrocyte size and morphology of some tortoises and turtles from Turkey . Zoological Studies , 42 : 173 – 178 .