Abstract
Six native bivalves of commercial interest, Aequipecten tehuelchus, Mytilus sp., Aulacomya atra, Ostrea puelchana, Protothaca antiqua and Pododesmus rudis, were examined on account of shell-boring spionid polychaetes in northern Patagonia, Argentina. Adults of Polydora rickettsi were found boring into shells of all molluscs but Mytilus sp., whereas Dipolydora cf. giardi was found only in shells of A. atra. The intensity of infestation of molluscs was low, and the prevalence of infestation by P. rickettsi varied from 20 to 54%, depending on the host species. Both boring spionids are reported for the first time from the Patagonian coast, and also as shell-borers of the examined molluscs.
Keywords:
Introduction
Adults of some species of Polydora and related genera (Annelida: Spionidae) bore into various calcareous substrata, including shells of commercially important molluscs such as oysters, scallops and mussels (Blake & Evans Citation1973; Lauckner Citation1983; Radashevsky et al. Citation2006). The effect of these polychaetes on the host mollusc depends on the intensity of infestation and species of the borer. Weak infestation by polychaetes is usually not significant to the mollusc, but heavy infestation can cause serious shell damage, retarded body growth, gonad reduction, and even death of the host. As a consequence, infestation by polydorin shell-borers is often a serious concern for mollusc fisheries and aquaculture (Bailey-Brock & Ringwood Citation1982; Bower et al. Citation1992; Handley Citation1997; Handley & Bergquist Citation1997; Bower & MacGladdery Citation2003; Simon et al. Citation2006).
The shapes of the burrows formed by polydorin spionids in shells are in some cases species-specific. They may be U-shaped, pear-shaped, Y-shaped, or have multiple branches; some polydorins induce the formation of mud blisters on the inner surface of the shells, which is most harmful to the hosting molluscs (Haigler Citation1969; Blake & Evans Citation1973; Zottoli & Carriker Citation1974; Sato-Okoshi & Okoshi Citation1997, Citation2000; Sato-Okoshi Citation1999; Read & Handley Citation2004; Simon et al. Citation2006). Mud blisters result from secretions of conchiolin and successive calcite layers that the mollusc produces as a mechanism to isolate the worm (Kent 1979; Lauckner Citation1983). Each burrow or mud blister opens to the exterior via two joined boreholes, forming a characteristic ‘8-shaped’ opening. Walls of the burrows are lined with fine silt, and usually a short silty tube projects from each borehole. Worms use burrows only as shelter. While feeding they poke the anterior end out of one of the tube entrances, and extend the palps to collect particles suspended in the water or deposited on the surrounding surface.
Polydorin infestation of commercially important bivalves is a matter of concern in northern Argentine Patagonia, where oyster and mussel aquaculture are incipient activities (Ciocco et al. Citation2005). Artisanal fisheries in the region target wild populations of a variety of molluscs, mainly the scallop Aequipecten tehuelchus (d′Orbigny, 1846). Mud blisters, which reduce the market price of scallops, were attributed in the past to Polydora websteri Hartman in Loosanoff & Engle, 1943 (Orensanz Citation1986; Ciocco Citation1990; Cremonte et al. Citation2005; Schejter & Bremec Citation2007). The identity of the boring polychaetes, however, was never substantiated by detailed descriptions. Polydorin infestation of other molluscs from the region has not been documented.
The objective of the present study was to identify the spionid polychaetes boring into shells of molluscs of commercial interest and to document the prevalence and intensity of infestations of molluscs in northern Argentine Patagonia.
Materials and methods
Bivalves of commercial interest were collected in San José and Nuevo Gulfs (Chubut Province, northern Argentine Patagonia; ) from January to October 2010. Molluscs of commercial size were collected in shallow water using SCUBA equipment, transported to the laboratory (Centro Nacional Patagónico, Puerto Madryn), and kept in aquaria with aerated seawater up to 2 days before examination. Mollusc shells were measured with calipers and broken into small fragments with hammer and pliers; polychaetes were removed under a light microscope in the laboratory. Adult worms were relaxed in isotonic magnesium chloride and examined alive. After examination they were fixed in 10% formaldehyde solution in seawater, rinsed in fresh water, and transferred to 70% ethanol. Bivalves from different sites were pooled to estimate the prevalence and intensity of infection. Prevalence was calculated as the percentage of valves infested, and intensity as the number of worms per valve. Samples were deposited in the Invertebrate Collection of Museo de Ciencias Naturales de La Plata (MLP, La Plata, Buenos Aires, Argentina) and in the Invertebrate Collection of Centro Nacional Patagónico (CNP, Puerto Madryn, Chubut, Argentina). Catalogue number and number of specimens are written in parenthesis.
Results
A total of 309 molluscs, belonging to 6 bivalve species, were examined. Those included the Tehuelche scallop Aequipecten tehuelchus (d'Orbigny, 1846), the ribbed mussel Aulacomya atra (Molina, 1782), the blue mussel Mytilus sp., the Puelche oyster Ostrea puelchana d'Orbigny, 1842, the false oyster Pododesmus rudis (Broderip, 1834), and the striped clam Protothaca antiqua (King & Broderip, 1831). Prevalence of infestation varied substantially among bivalve species. Tehuelche scallops were the most infected, whereas no polydorins were found boring into the shells of blue mussels ().
Table I. Infestation of bivalve molluscs by spionid polychaetes in northern Patagonia. Bivalves are arranged according to the prevalence of the infestation. Shell size (height) is given with mean and range. Prevalence and intensity were calculated per valve
Adults of two polydorin species were found boring in shells of the molluscs. Morphology of the examined polychaetes and their burrows in molluscs is described below.
Taxonomic accounts
Spionidae Grube, 1850
Polydora rickettsi Woodwick, 1961
,
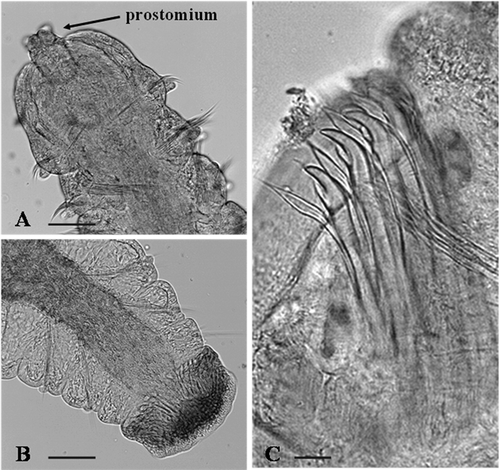
Figure 2. Adult morphology of Polydora rickettsi. A, anterior end, dorsal view; B, modified chaetae of segment 5; C, pygidium. Scale bars: A, C, 50 μm. B, 30 μm.
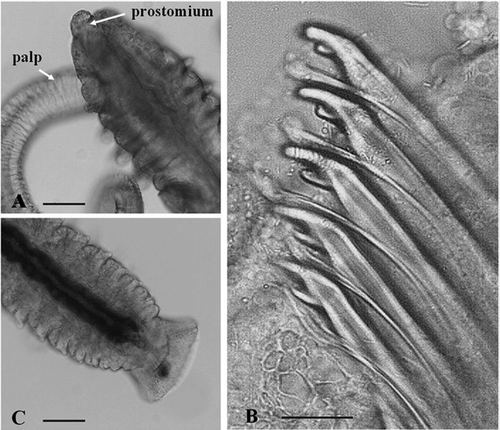
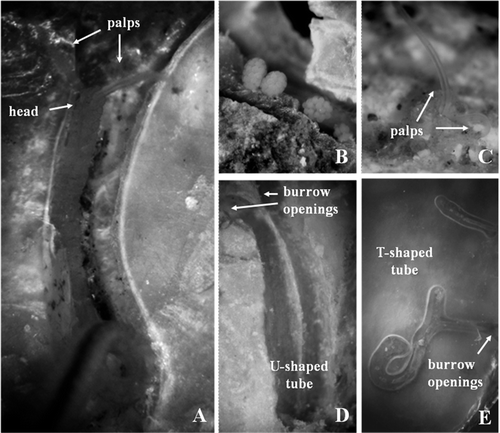
Figure 3. Polydora rickettsi in life. A, individual inside its burrow, in shell of Pododesmus rudis; B, egg capsules inside a burrow in a shell of A. tehuelchus; C, palps projecting from the shell of Protothaca antiqua; D, U-shaped burrow in a shell of A. tehuelchus; E, T-shaped burrow in a shell of P. rudis.
Polydora rickettsi Woodwick Citation1961: 78–81, figs 1–7. Blake Citation1983: 257. Radashevsky & Cárdenas Citation2004: 243–254, figs 2–6. Radashevsky et al. Citation2006: 22–24.
Polydora cf. rickettsi: Sato-Okoshi & Takatsuka Citation2001: 489–490, –D.
Material
San José Gulf: San Román, 42°15′S, 64°12′W, 10–15 m, coll. N. De Garín, R. Vera and M. A. Diaz, 17 March 2010, from shells of Aequipecten tehuelchus (MLP 6495, 3), and 10 September 2010, from shells of Aulacomya atra (CNP INV5_001, 2). Punta Conos, 42°20′S, 64°02′W, 10–20 m, coll. N. De Garín, R. Vera, M. A. Diaz and N. Ortiz, 27 April 2010, from shells of Aulacomya atra (MLP 6496, 1) and Protothaca antiqua (MLP 6497, 2). Fracasso Beach, 42°25′S, 64°07′W, 10–15 m, coll. N. De Garín, R. Vera and M. A. Diaz, 17 March 2010 (MLP 6498, 7) and 25 October 2010 (CNP INV5_002, 4) from shells of Aequipecten tehuelchus, 27 April 2010, from shells of Ostrea puelchana (MLP 6499, 1), 22 July 2010, from shells of Pododesmus rudis (MLP 6500, 5), 28 April 2010 from shells of Protothaca antiqua (MLP 6501, 2) and Aequipecten tehuelchus. Punta Gales, 42°25′S, 64°20′W, 10–15 m, coll. N. De Garín, R. Vera and M. A. Diaz, 20 February 2010, from shells of Aequipecten tehuelchus (MLP 6502, 7), 17 March 2010 (MLP 6503, 3) and 25 October 2010 (CNP INV5_003, 1) from shells of Aulacomya atra.
Adult morphology
Up to 12 mm long and 0.2 mm wide for 96 chaetigers. Body pale to light tan in life; black pigment diffused on pygidium in some individuals. Worms boring in different hosts exhibiting similar pigmentation. Prostomium rounded to blunt on anterior margin. One to two pairs of eyes present. Occipital antenna absent. Caruncle extending to end of chaetiger 3. Chaetiger 1 with short capillaries in neuropodia and small postchaetal lamellae in both rami; notochaetae absent. Posterior notopodia with capillaries only. Chaetiger 5 greatly modified, with up to three dorsal superior geniculate capillaries, five heavy spines alternating with bilimbate-tipped companion chaetae and arranged in a slightly curved diagonal row, and a tuft of ventral capillaries. Heavy spines falcate, with lateral tooth and longitudinal flange laterally on main fang (). Hooks in neuropodia from chaetiger 7, up to 9 in a series, not accompanied by capillaries; hooks bidentate, with weak constriction on shaft. Branchiae from chaetiger 7, full-sized from chaetigers 10 to 11, gradually diminishing in size along posterior part of the body, and absent from 16 to 32 posterior-most chaetigers. Pygidium cup-shaped, with dorsal incision ().
Habitat
Adults of P. rickettsi () were found boring in all examined bivalves except Mytilus sp. Worms occurred in mud blisters and in U-shaped burrows (,D) in shells of A. tehuelchus, T-shaped burrows in P. rudis (), and irregular burrows in other examined bivalves. The palps of worms were seen projecting out of one of the tubes for feeding (). Egg capsules were found inside the burrows in shells of Tehuelche scallops in January (). Prevalence and intensity of infestation varied among molluscs, infestation being most severe in the Tehuelche scallop (Prevalence: 54%, intensity: 1–9).
Remarks
Polydora rickettsi was first described from Cape San Lucas, Baja California, Mexico by Woodwick (Citation1961). In South America, Sato-Okoshi and Takatsuka (Citation2001) reported P. cf. rickettsi boring into shells of the oysters Crassostrea gigas and Ostrea chilensis in Chile, and Radashevsky and Cárdenas (Citation2004) reported P. rickettsi boring into the shells of various molluscs and the barnacle Austromegabalanus psittacus (Molina, 1782), also in Chile. Besides, Radashevsky et al. (Citation2006) reported this species as boring into the shells of the scallop Nodipecten nodosus (Linnaeus, 1758) in Brazil, but this identification needs to be verified.
In northern Patagonia, we found P. rickettsi boring into shells and causative variable damages to five species of commercially important bivalves: the scallop A. tehuelchus, the ribbed mussel A. atra, the oyster O. puelchana, the clam P. antiqua and the anomiid P. rudis (). Individuals from northern Patagonia appear quite similar to P. rickettsi from Chile described by Sato-Okoshi and Takatsuka (Citation2001) and Radashevsky and Cárdenas (Citation2004). All have a truncate prostomium, non-pigmented palps, and a small disc-like pygidium, and share the same type of modified spines on segment 5.
Dipolydora cf. giardi (Mesnil, Citation1896)
? Polydora giardi Mesnil Citation1896: 195–202, pl. 13 figs 1–12. Fauvel Citation1927: 50–52, fig. 17h–m.
? Dipolydora giardi: Radashevsky & Petersen Citation2005: 25–36, fig. .
Material
San José Gulf: Punta Gales, 42°25′S, 64°20′W, 10–15 coll. N. De Garín, R. Vera and M. A. Diaz, 17 March 2010, from shells of Aulacomya atra (MLP 6504, 4).
Adult morphology
Up to 2.5 mm long and 0.15 mm wide for 52 chaetigers. Body pigmentation absent. Prostomium incised to bifid anteriorly. Eyes and occipital antenna absent (). Caruncle extending posteriorly to end of chaetiger 3. Chaetiger 1 with short capillaries and small postchaetal lamellae in both rami. Posterior notopodia with only capillaries. Chaetiger 5 greatly modified, with up to 2 dorsal superior geniculate capillaries, 5 heavy spines alternating with bilimbate-tipped companion chaetae () and arranged in a slightly curved diagonal row, and 4 ventral capillaries. Heavy spines falcate, with lateral tooth. Hooded hooks in neuropodia from chaetiger 7, up to 4 in a series, accompanied by inferior capillary chaetae through chaetigers 7–10; hooks bidentate, without constriction on shaft. Branchiae usually from chaetiger 10, occasionally from chaetiger 11, full-sized from chaetiger 12, absent from posterior half of body. Pygidium small, cup-shaped, with one ventral lobe and two smaller dorsal lobes (). Females and males with mature gametes were observed in March and April.
Habitat
Adults of D. cf. giardi were found in burrows of irregular shape in shells of A. atra. Up to 5 polychaetes were present in one shell and about 45% of examined molluscs were infested. The infested molluscs were usually encrusted with calcareous algae and other epibionts.
Remarks
Dipolydora giardi was first described as a borer of coralline algae from northern France by Mesnil (Citation1896). In South America, it was reported from Chile by Blake (Citation1983) and Sato-Okoshi and Takatsuka (Citation2001). Radashevsky and Petersen (Citation2005) redescribed the species, re-examined materials reported by previous authors and concluded that the geographical distribution of D. giardi was probably restricted to European waters. The latter authors paid attention to the simultaneous hermaphroditism reported by Mesnil (Citation1896, p. 200), but assumed that this kind of sexuality might have been described incorrectly.
Individuals from northern Patagonia appear quite similar to D. giardi from Europe in that they also have an incised to bifid prostomium, no eyes, no occipital antenna, caruncle extending to the end of chaetiger 3, pygidium with three small lobes, major falcate spines of chaetiger 5 with large lateral tooth on one side and a smaller accessory spur on the other side. However, Mesnil's report of hermaphrodites (in contrast to separate sexes found in Argentina) and a disjoint geographical distribution bring into doubt the identity of the South American individuals. Although the disjoint distribution may be explained as a result of unintentional transportation, we cautiously refer to the Argentinean specimens as Dipolydora cf. giardi.
Discussion
Polydorin infestation of commercially important bivalves is a matter of concern for shellfisheries from Argentine Patagonia (Ciocco et al. Citation2005). San José Gulf () is the most important producing area of coastal bivalve, which are harvested by coastal gatherers and commercial divers (Orensanz et al. Citation2006). Among the six species investigated, Tehuelche scallops are the most significant; the annual catch has fluctuated historically around 1000 m. Pododesmus has no commercial value, but is consumed occasionally by recreational gatherers. All the species occur in the same fishing grounds mixed in variable proportions. Infestation of scallops by polydorins increases with age and depresses growth rate (Ciocco Citation1990). Slow growth rate, in turn, favours settlement of polydorins (Orensanz Citation1986; Orensanz et al. Citation1991, fig. ).
The few studies addressing infestation of commercial bivalves by polydorins in the southwestern Atlantic have identified P. websteri as the causing agent in both the Tehuelche scallop, a shallow subtidal, warm-temperate species (Orensanz Citation1986, in footnote; Ciocco Citation1990), and the Patagonian scallop (Zygochlamys patagonica), a cold-temperate species that sustains a large offshore industrial trawl fishery (Schejter & Bremec Citation2007). Polydora websteri was not, however, found in our study but, instead, two other polydorins, P. rickettsi and D. cf. giardi, were identified for the first time as shell-borers in northern Patagonia. Unfortunately, the material identified in earlier studies as P. websteri was not saved, and thus was not available for re-examination. Presently it is not possible to ascertain whether these earlier records were misidentifications of P. rickettsi, or the presence of the latter in Argentine Patagonia is a result of a recent introduction. Further study is required to clarify this issue.
Prevalence of infestation by P. rickettsi varied from 20 to 54%, depending on the host species, and the infestation rate of Aulacomya atra by D. cf. giardi was 45%. These values are low relative to other populations of molluscs that have been studied (e.g. Bower et al. Citation1992; Radashevsky & Olivares Citation2005; Moreno et al. Citation2006). The intensity of infestation of molluscs was low in all cases except in A. tehuelchus, for which heavy polydorin infestation had been reported before (Orensanz Citation1986; Ciocco Citation1990; Orensanz et al. Citation1991). Lack of infestation in Mytilus sp. may be related to the thick, smooth periostracum of this species, which contrasts with the rough surface of the valves of the other species.
Control of polydorin infestation in aquaculture requires an adequate understanding of their reproductive biology (e.g. Handley & Bergquist Citation1997; Simon & Booth Citation2007) and proper taxonomic identification. While infestations may not be controllable in the case of wild harvested host populations, their dynamics can be a consideration in the design of harvest strategies, e.g. by incorporating the dynamics of infestation into yield-per-recruit analysis to account for age- and/or location-dependent reductions in meat yield and growth rate. Results from this study provide scientific support needed in order to address those problems.
Acknowledgements
We wish to express our sincere gratitude to Norberto De Garín, Ricardo Vera, Miguel A. Diaz, Néstor Ortiz, José Ascorti, Soledad Zabala, José Fernandez Alfaya and Fabián Quiroga for their assistance in the field. María Emilia Diez is a pre-doctoral fellow of CONICET and the Ministry of Education, Chubut Province. Three anonymous reviewers contributed substantially to improve the original MS. Financial support was provided by the Agencia Nacional de Promoción Científica y Tecnológica (Projects PICT 1374 and PICT 1674), and by the Russian Foundation for Basic Research (Project 09-04-01235).
References
- Bailey-Brock , JH and Ringwood , A. 1982 . Methods for control of the mud blister worm, Polydora websteri, in Hawaiian oyster culture . Sea Grant Quarterly , 4 : 1 – 6 .
- Blake , JA. 1983 . Polychaetes of the family Spionidae from South America, Antarctica, and adjacent seas and islands . Biology of the Antarctic Seas XIV. Antarctic Research Series , 39 : 205 – 288 .
- Blake , JA and Evans , JW. 1973 . Polydora and related genera as borers in mollusc shells and other calcareous substrates . The Veliger , 15 : 235 – 249 .
- Bower , SM , Blackbourn , J , Meyer , GR and Nishimura , DJH. 1992 . Diseases of cultured japanese scallops (Patinopecten yessoensis) in British Columbia, Canada . Aquaculture , 107 : 201 – 210 .
- Bower SM, McGladdery SE. 2003. Synopsis of Infectious Diseases and Parasites of Commercially Exploited Shellfish. http://www-sci.pac.dfo-mpo.gc.ca/shelldis/title_e.htm (http://www-sci.pac.dfo-mpo.gc.ca/shelldis/title_e.htm) (Accessed: 26 June 2006 ).
- Ciocco , NF. 1990 . Infestación de la vieyra tehuelche (Chlamys tehuelcha (d'Orbigny)) por Polydora websteri Hartman (Polychaeta: Spionidae) en el Golfo San José (Chubut, Argentina): un enfoque cuantitativo . Biología Pesquera , 19 : 9 – 18 .
- Ciocco , NF , Lasta , ML , Narvarte , M , Bremec , C , Bogazzi , E Valero , J . 2005 . “ Argentina ” . In Scallops: Biology, ecology and aquaculture. Chapter 26. , 2nd , Edited by: Shumway , SE and Parsons , GJ . 1251 – 1292 . Argentina : Elsevier .
- Cremonte , F , Figueras , A and Burreson , EM. 2005 . A histopathological survey of some commercially exploited bivalve molluscs in northern Patagonia, Argentina . Aquaculture , 249 : 23 – 33 .
- Fauvel , P. 1927 . Polychètes sédentaires . Addenda aux Errantes, Archiannélides, Myzostomaires. Faune de France , 16 : 1 – 494 .
- Haigler , SA. 1969 . Boring mechanism of Polydora websteri inhabiting Crassostrea virginica . American Zoologist , 9 : 821 – 828 .
- Handley , SJ. 1997 . Optimizing subtidal oyster production, Marlborough Sounds, New Zealand: Spionid polychaete infestations, water depth, and spat stunting . Journal of Shellfish Research , 16 : 143 – 150 .
- Handley , SJ and Bergquist , PR. 1997 . Spionid polychaete infestations of intertidal Pacific oysters Crassostrea gigas (Thunberg), Mahurangi harbour, northern New Zealand . Aquaculture , 153 : 191 – 205 .
- Kent , RML. 1981 . The effect of Polydora ciliata on the shell strength of Mytilus edulis . Journal du Conseil. Conseil Permanent International pour l'Exploration de la Mer , 39 : 252 – 255 .
- Lauckner , G. 1983 . “ Diseases of Mollusca: Bivalvia ” . In Diseases of marine animals , Edited by: Kinne , O . 477 – 961 . Hamburg : Biologische Anstalt Helgoland .
- Mesnil , F. 1896 . Études de morphologie externe chez les Annélides . I. Les Spionidiens des côtes de la Manche. Bulletin scientifique de la France et de la Belgique , 29 : 110 – 287 .
- Moreno , RA , Neill , PE and Rozbaczylo , N. 2006 . Native and non-indigenous boring polychaetes in Chile: A threat to native and commercial mollusc species . Revista Chilena de Historia Natural , 79 : 263 – 278 .
- Orensanz , JM. 1986 . Size, environment and density: The regulation of a scallop stock and its management implications . Canadian Special Publication in Fisheries and Aquatic Sciences , 92 : 195 – 227 .
- Orensanz , JM , Parma , AM , Ciocco , N and Cinti , A. 2006 . “ Achievements and setbacks in the commercial diving fishery of San José Gulf, Argentine Patagonia ” . In Fisheries management: Progress towards sustainability , Edited by: McClanahan , TR and Castilla , JC . 68 – 87 . Oxford : Blackwell .
- Orensanz , JM , Pascual , M and Fernandez , M. 1991 . “ Scallops: Biology, ecology and aquaculture ” . In Fisheries and aquaculture , Edited by: Shumway , S . 981 – 999 . Argentina : Elsevier .
- Radashevsky , VI and Cárdenas , CA. 2004 . Morphology and biology of Polydora rickettsi (Polychaeta: Spionidae) from Chile . New Zealand Journal of Marine and Freshwater Research , 38 : 243 – 254 .
- Radashevsky , VI , Lana , PC and Nalesso , RC. 2006 . Morphology and biology of Polydora species (Polychaeta: Spionidae) boring into oyster shells in South America, with the description of a new species . Zootaxa , 1353 : 1 – 37 .
- Radashevsky , VI and Olivares , C . 2005 . Polydora uncinata (Polychaeta: Spionidae) in Chile: An accidental transportation across the Pacific . Biological Invasions , 7 : 489 – 496 .
- Radashevsky , VI and Petersen , ME. 2005 . On the morphology and distribution of Dipolydora giardi and status of D. trilobata (Annelida: Spionidae) . Zootaxa , 1086 : 25 – 36 .
- Read , G and Handley , S. 2004 . New alien mudworm now becoming a pest in longline mussels . Marine Biosecurity , 12 : 30 – 31 .
- Sato-Okoshi , W. 1999 . Polydorid species (Polychaeta: Spionidae) in Japan, with descriptions of morphology, ecology and burrow structure. 1 Boring species . Journal of Marine Biology Association of the UK , 79 : 831 – 848 .
- Sato-Okoshi , W and Okoshi , K. 1997 . Survey of the genera Polydora, Boccardiella and Boccardia (Polychaeta, Spionidae) in Barkley Sound (Vancouver Island, Canada), with special reference to boring activity . Bulletin of Marine Science , 60 : 482 – 493 .
- Sato-Okoshi , W and Okoshi , K. 2000 . Structural characteristics of self-excavated burrows by boring polydorid species (Polychaeta, Spionidae) . Bulletin of Marine Science , 67 : 235 – 248 .
- Sato-Okoshi , W and Takatsuka , M. 2001 . Polydora and related genera (Polychaeta, Spionidae) around Puerto Montt and Chiloé Island (Chile), with description of a new species of Dipolydora . Bulletin of Marine Science , 68 : 485 – 503 .
- Schejter , L and Bremec , C. 2007 . Benthic richness in the Argentine continental shelf: The role of Zygochlamys patagonica (Mollusca: Bivalvia: Pectinidae) as settlement substrate . Journal of the Marine Biological Association of the UK , 87 : 917 – 925 .
- Simon , CA and Booth , AJ. 2007 . Population structure and growth of polydorid polychaetes that infest cultured abalone Haliotis midae . African Journal of Marine Science , 29 : 499 – 509 .
- Simon , CA , Ludford , A and Wynne , S. 2006 . Spionid polychaetes infesting cultures abalone, Haliotis midae, in South Africa . African Journal of Marine Science , 28 : 167 – 171 .
- Woodwick , KH. 1961 . Polydora rickettsi, a new species of spionid polychaete from Lower California . Pacific Science , 15 : 78 – 81 .
- Zottoli , RA and Carriker , MR. 1974 . Burrow morphology, tube formation, and microarchitecture of shell dissolution by the spionid polychaete Polydora websteri . Marine Biology , 27 : 307 – 316 .