Abstract
The objective of the present study was to identify the cell types of the endocrine pancreas by tinctorial, immunocytochemical and ultrastructural characteristics in Lissemys punctata punctata turtles. (a) B-cells were identified by fine light purple cytoplasmic granules tinctorially, insulin antibody reaction (brown colour), and mitochondrial zone with crystalline-like secretory granules ultrastructurally. (b) A-cells were characterized by very fine orangeophilic granules tinctorially, glucagon antibody reaction (bluish colour), and rough endoplasmic reticular (RER) and mitochondrial zones with larger secretory granules ultrastructurally. Other cell types were identified from ultrastructural characteristics. (c) D-cells had RER zone with clustered large secretory granules. (d) PP-cells showed fusiform shape with tapering ends, RER and mitochondrial zones, and small secretory granules. (e) Vth-cell type was characterized by triangular shape with mitochondrial zone and clustered secretory granules. (f) VIth-cell type was identified by pyriform shape, mitochondrial zone, and least population of clustered secretory granules. We conclude that the occurrence of several types of pancreatic endocrine cells may be related to the adaptation of Lissemys turtles.
Introduction
Some information is available on the endocrine pancreas of chelonian turtles and tortoises. Endocrine cells of the pancreas are generally located adjacent to the pancreatic ducts known in different families of freshwater turtles, such as Chrysemys dorbigni (Family Emydidae) and Phrynops hilarii (Family Chelidae) (Cardeza Citation1957) and Geoemyda pulcherrima (Family Geoemydidae) (Miller Citation1962), belonging to the order Chelonia. Cardeza (Citation1957) has also reported that occasionally large islets are seen with long projections from the acinar tissues. Non-B-cells, occurring in groups, are found in the exocrine pancreas of different families (Emydidae and Chelidae) of turtles. In Chrysemys picta belonging to the Family Emydidae, the endocrine part of the pancreas is covered by connective tissue and two types of islets are present: the larger type at the cranial end of the pancreas and the smaller type throughout the gland. Beta and alpha immunoreactive cells have been identified in the endocrine pancreas of Pseudemys scripta elegans (Family Emydidae) (Miller Citation1962). A-cells have been recorded outside the pancreatic islets in different families of turtles: Testudo sp. Russian tortoises (Family Testudinidae), Emys sp. (Family Emydidae) (Titlbach Citation1966), Testudo horsfieldi (Family Testudinidae) and Clemmys caspica (Family Geoemydidae) (Yaglov Citation1976). Three types of endocrine cells – namely B-, A- and D-cells – are present in the endocrine pancreas; A-cells are fewer than B-cells, and D-cells much fewer than A-cells found in Chrysemys picta (Family Emydidae) (Cardeza Citation1957). Subsequently, four types of endocrine cells – namely, insulin-, glucagon-, somatostatin- and pancreatic polypeptide-IR cells. The latter findings corresponded to B-, A-, D- and PP-cells, respectively, confirmed by ultrastructural studies of the endocrine pancreas of Pseudemys scripta elegans (Family Emydidae) (Agulleiro et al. Citation1985), Testudo graeca (Family Testudinidae) and Mauremys caspica (Family Geoemydidae) (Garcia Ayala et al. Citation1989). The pancreatic islets of these three species of turtles contain a small, compact core of insulin cells, with glucagon cells located peripherally and some somatostatin cells are intermingled. Sometimes PP-cells are found next to the insulin cells in the splenic lobe of M. caspica. Immunocytochemical study also showed the same distribution pattern of insulin and glucagon cells, as insulin-IR and glucagon-IR cells, in the endocrine pancreas of M. caspica. Additionally, somatostatin-IR and PP-IR cells remain associated with glucagon-IR cells. Endocrine cells are also present within the epithelium of the excretory ducts of the exocrine pancreas of M. caspica. However, in T. graeca (Family Testudinidae), PP-cells are absent in the splenic lobe and abundantly present in isolation in the duodenal lobe of the pancreas. A fifth cell type, characterized by euchromatic nucleus with electron-dense nucleolus and nuclear bodies, and secretory granules containing an eccentric dense core with wide peripheral halo, was detected in the endocrine pancreas of T. graeca (Family Testudinidae) in summer (Garcia Ayala et al. Citation1987). Epple & Brinn (Citation1987) have reviewed cell types of the endocrine pancreas of turtles. The gastroenteropancreatic (GEP) endocrine system, studied by immunocytochemical technique, showed six types of immunoreactive endocrine cells – namely, insulin-(B), glucagon-(A), somatostatin-(D), pancreatic polypeptide-(PP), secretin, and peptide tyrosine tyrosine (PYY) – in the pancreas of two species of turtles, Testudo graeca (Family Testudinidae) and Mauremys caspica (Family Geoemydidae) (Perez-Tomas et al. Citation1989). Lozano et al. (Citation2000), using the immunogold antibody method, have identified six types of immunoreactive cells – namely, insulin-(B), glucagon-(A), Salmon (S) somatostatin-(SST-14, SST-25 and SST-28), pancreatic polypeptide-(PP), peptide tyrosine tyrosine (PYY) and neuropeptide tyrosine (NPY) like immunoreactivities – in the endocrine pancreas of the turtle Pseudemys scripta elegans belonging to the Family Emydidae. The latter authors have identified cell types from ultrastructural characteristics of the secretory granules. Insulin-IR B-cells contain crystalline-like secretory granules with an electron-dense core and wide peripheral halo. Glucagon-IR A-cells have round secretory granules with a large electron-dense core surrounded by a narrow peripheral halo. The SST-28/SST-14-immunogold-labelled cells showed round or ovoid electron-dense granules. The secretory granules of the glucagon/NPY-like cells have a pyriform, round or oval electron-dense core, and are smaller than in the glucagon-IR A-cells. There is only a single report that in freshwater turtles Lissemys punctata punctata belonging to the Family Trionychidae, islets are present throughout the gland, consisting of a single cell to small or large groups of cells, depending on the seasonal cycle. Beta cells are found during the different phases of the reproductive cycle (Chandavar & Naik Citation2004). Most of the earlier studies of endocrine pancreas were carried out in primitive and intermediate groups of turtles evolved. Study of endocrine pancreas, including their cell types, of recently evolved turtles (Lissemys) belonging to the Family Trionichidae was neglected. Moreover, earlier pancreatic endocrine cell types were not studied by tinctorial, immunocytochemical and ultrastructural levels. In the current study, endocrine cell types of the pancreas were investigated, considering all these parameters together, in Lissemys punctata punctata.
Materials and methods
Animals
Five freshwater adult soft-shelled turtles, Lissemys punctata punctata (Bonnoterre) of either sex (weighing between 800 and 900 g), were collected (long before the imposition of the animal care act in India) from a local natural population near Calcutta in September and kept in the polythene cage (90 cm × 60 cm × 60 cm) in the laboratory with controlled temperature (25°C) and photoperiod (11L:13D), food (small apple-snail Pila globosa and shrimps) and water ad libitum for 10 days for acclimatization. Turtles were anaesthetized with sodium barbital injection; splenic and duodenal lobes of the pancreas were dissected free, and immersed in fixatives for light and electron microscopic studies.
Light microscopy
Tissues were immersed in Parakkal's (Citation1961) chrome–alum–Bouins fixative for 24 h and processed for routine microtomy. Five-micrometre thick paraffin sections were cut by microtome (BIOCUT, Cambridge, UK) from the splenic and duodenal lobes of the pancreas. Unstained serial sections were examined under microscope for selection of larger islets and were stained with aldehyde fuchsin trichrome (AFT) technique (Epple Citation1967a,Citationb) for studying islet size, distribution pattern and tinctorial characteristics of cell types, especially B (β)-, A (α)- and D (δ)-cells located in the islets, and were photographed by photomicroscope (Reichert, Austria). B-, A- and D-cells were identified from their tinctorial properties.
Immunocytochemistry
Immunoreactivities of B- and A-cells were studied from the splenic lobe of the pancreas. A small piece of the pancreas (1 cm × 1 cm) was immersed in Bouin's fluid for 18 h and processed for routine microtomy. Five-micrometre thick paraffin sections were cut by a BIOCUT (Cambridge, UK) microtome and stained for immunocytochemical demonstrations of insulin (B) and glucagon (A) cells, using respective mammalian primary antisera, by the peroxidase–antiperoxidase (PAP) method of Sternberger (Citation1986) with some modifications as follows. Paraffin sections were deparaffinized with xylene, hydrated through descending grades of ethanol and then incubated in 1 × Tris-buffered saline–Tween-20 (1 × TBST), containing 0.2% bovine serum albumin (BSA) at pH 7.4 in a humidified chamber for 1 h at room temperature (25°C) to block the non-specific reactive sites. For insulin-immunoreactive (insulin-IR) B-cells, sections were washed in PBS several times and incubated with primary specific mammalian antisera (Guineapig Anti-Bovine insulin whole antiserum) Sigma, product no. I-6136, Lot No. 038H4824) in 1 × TBST containing 0.1% BSA (dilution 1:300) in a humidified chamber at room temperature (25°C) for 2 h. Sections were washed in 1 × TBST several times and again incubated with secondary antibody [protein A-horse radish peroxidase (HRP)] complex (GENE 1, cat no. HP08, lot no. 82065) (1 ml) in 1 × TBST containing 0.1% BSA (dilution 1:1000) for 1 h at 37°C. Control sections were incubated without antibody. All sections were washed in buffered 1 × TBST containing 0.1% BSA for several times. Insulin-immunoreactive (insulin-IR) B-cells were demonstrated by peroxidase–antiperoxidase activity using 0.05% 3,3-diaminobenzidine (DAB, Sigma, product no. P5637) (chromogenic substrate for peroxidase), 0.01% H2O2 in 1 × TBST containing 0.1% BSA at room temperature (25°C) for 5 min. The reaction was stopped by washing with 1 × TBST containing 0.1% BSA. Subsequently sections were mounted in DPX and examined under microscope. Insulin-IR cells were photographed by photomicroscope (Reichert, Austria). Immunostaining was not observed in the control pancreas sections without primary antibody.
For glucagon-IR(A)-cells, pancreas sections after hydration were treated with specific primary mammalian glucagon antibody (Monoclonal Anti-Glucagon, clone K796B10, Mouse Ascites Fluid, Sigma, product no. G2654, Lot No. 016K4785) in 1 × TBST containing 0.1% BSA (dilution 1:4000) in a humidified chamber at 25°C for 1 h and washed in 1 × TBST containing 0.1% BSA. Sections were again incubated with secondary antibody (Goat anti-mouse IgG-alkaline phosphatase complex) (Santa Cruz Biotechnology, SC-2008, lot no. L0604) in 1 × TBST containing 0.1% BSA (dilution 1:40) in a humidified chamber for 1 h at 37°C. Sections were washed in PBS containing 0.1% BSA several times. Alkaline phosphatase activity was demonstrated in glucagon-IR A-cells, using 33 μl of nitroblue tetrazolium (NBT) and 16.5 μl of 5-Bromo-4-chloro-3-indolyl phosphate (BCIP) in alkaline phosphatase (AP) buffer (100 mM Tris–HCl, 100 mM NaCl, 5 mM MgCl2, 0.05% Tween 20, pH 9.5) at room temperature (25°C) for 5 min. The reaction was stopped by washing in PBS containing 0.1% BSA. Sections were mounted in DPX and examined under microscope. Sections were photographed as follows for insulin-IR cells. Immunostaining was not observed in the control pancreas sections without primary antisera. Immunocytochemical studies of other endocrine cells of the pancreas (D, PP, Vth and VIth types) of Lissemys turtles were not included.
Transmission electron microscopy (TEM)
A small piece (1 mm × 1 mm) of the pancreas, each from the splenic and duodenal lobes, were immersed in a freshly prepared fixative, containing 2.5% glutaraldehyde and 2% paraformaldehyde in 0.1M phosphate buffer (pH 7.4) for 8 h at 4°C. After washing, specimens were post-fixed in 1% buffered OSO4 for 1 h at 4°C. Tissues were dehydrated in ascending grades of acetone, infiltrated and embedded in araldite CY212. Thick sections (0.5–1.0 μm) were cut by Ultracut E (Reichert, Austria), stained with toluidine blue and observed under a light microscope. Ultrathin sections (60–70 nm), cut by ultratome E, were contrasted in uranyl acetate and led citrate, and viewed under Morgagni 268 D TEM (Fei Company, Eindoven, The Netherlands) at an operative voltage of 80 KV.
Various cell types of the endocrine pancreas (B-, A-, D-, PP-, Vth- and VIth-types) were identified from their ultrastructural characteristics together with the cytometric analysis under transmission electron microscope.
Quantitative cytometric analysis
Cell area and nuclear area of each endocrine cell type (B-, A-, D-, PP-, Vth- and VIth-) of the pancreas were measured from transmission electronmicrographs of the cell types by planimeter (Allbrit Disc Planimeter, Great Britain). Ten cells for each type were studied at the same magnification (×10,000) for each specimen and five specimens were considered. The C/N ratio of each cell type was determined dividing the cell area by the nuclear area. Diameters of small (< 200 nm) and large (>200 nm) cytoplasmic secretory granules of each cell type were measured (nm) under TEM (magnification ×10,000) using the software (Soft imaging system, GmBH, Münster, Germany) provided with the microscope. For the study of the population of secretory granules, all granules present in each cell type were counted from each section under TEM. Eight to 10 such sections were considered for each intact cell type of each specimen, and five specimens were studied. Populations of cytoplasmic granules present in each cell type were averaged and expressed in number per cell. Populations of each cell type were also counted under TEM at lower magnification (×730). One hundred cells, consisting of various cell types, were counted from random sections of the pancreas of each specimen and five specimens were considered. Differential cell populations were calculated in percent. All the data (different cell population percent, cell area, nuclear area and diameters of small and large secretory granules) were statistically analysed by Student's t-test (Snedecor & Cochrane Citation1989) and presented as mean ± SE.
Results
The pancreas of Lissemys turtles is a compact mass, brownish in colour, localized in the duodenal region of the gut with a small part attached to the splenic region ().
Figure 1. (a) Showing a schematic diagram of the localization of the pancreas in Lissemys turtles. Sections of the splenic lobe of turtle pancreas, stained by AFT method, under light microscope showing: (a1 ) endocrine cells (A, B with arrows) with lightly stained fine cytoplasmic granules and exocrine cells (exc) with intensely stained large cytoplasmic granules. A small B-cell islet [B(I) with arrow] predominantly with B-cells, surrounded by a thin layer of greenish connective tissue (ct) (arrow) near the blood capillaries (bc); B-cells with fine blue cytoplasmic granules and A-cells with very fine orangeophilic cytoplasmic granules. B-cells in small islet [B(I)] or in small group [B(G)], and A-cells freely or in small group A(G). (b) Both B- and A-cells in small groups. One D-cell with scanty bluish-green cytoplasmic granules intermingled with orangeophilic A-cells (arrows) in the splenic lobe of the pancreas of turtle. Profuse quantity of green stained collagenous tissue (ct) associated with blood capillaries (bc). (c) One A-cell islet surrounded by green stained collagenous tissues (ct) showing mostly orangeophilic A-cells and one transforming endocrine cell (TEC (?)) with abundance of large orange–blue granules, compared to blue large granules in the exocrine cells (exc) of the pancreas. (d) One small A-islet (between the arrows) with exclusively orangeophilic granules within the epithelium of the pancreatic duct (dt) with lumen (l) surrounded by huge quantity of green stained collagenous tissue (ct). Scale bars: a1–d, 20 μm.
![Figure 1. (a) Showing a schematic diagram of the localization of the pancreas in Lissemys turtles. Sections of the splenic lobe of turtle pancreas, stained by AFT method, under light microscope showing: (a1 ) endocrine cells (A, B with arrows) with lightly stained fine cytoplasmic granules and exocrine cells (exc) with intensely stained large cytoplasmic granules. A small B-cell islet [B(I) with arrow] predominantly with B-cells, surrounded by a thin layer of greenish connective tissue (ct) (arrow) near the blood capillaries (bc); B-cells with fine blue cytoplasmic granules and A-cells with very fine orangeophilic cytoplasmic granules. B-cells in small islet [B(I)] or in small group [B(G)], and A-cells freely or in small group A(G). (b) Both B- and A-cells in small groups. One D-cell with scanty bluish-green cytoplasmic granules intermingled with orangeophilic A-cells (arrows) in the splenic lobe of the pancreas of turtle. Profuse quantity of green stained collagenous tissue (ct) associated with blood capillaries (bc). (c) One A-cell islet surrounded by green stained collagenous tissues (ct) showing mostly orangeophilic A-cells and one transforming endocrine cell (TEC (?)) with abundance of large orange–blue granules, compared to blue large granules in the exocrine cells (exc) of the pancreas. (d) One small A-islet (between the arrows) with exclusively orangeophilic granules within the epithelium of the pancreatic duct (dt) with lumen (l) surrounded by huge quantity of green stained collagenous tissue (ct). Scale bars: a1–d, 20 μm.](/cms/asset/fcd28f84-4297-4098-b223-1b0654711a6b/tizo_a_573506_o_f0001g.jpg)
Light microscopy
In histological sections of the pancreas, the endocrine cells were located near the blood capillaries ( ). The endocrine cells (B, A and D) were identified by their lightly stained fine cytoplasmic granules compared to the intensely stained coarse cytoplasmic granules found in the exocrine cells (exc) after staining with AFT technique (). A thin layer of collagenous connective tissue (ct) fibres, identified by green staining with AFT technique (Epple Citation1967a) was observed surrounding the endocrine islets of the pancreas of Lissemys turtles (,c). Moreover, an abundance of collagenous fibres (ct) was present near the blood capillaries in association with endocrine cells (,c). The endocrine cells were abundant mostly in the splenic lobe, and formed small or large islets (predominantly with B-cells) seen adjacent to the blood capillaries (–d). Generally, there were two cell types (B and A), identified from their tinctorial characteristics. A-cells were found in different locations, within the islets (,d), adjacent to blood capillaries of the exocrine tissues, or in isolation in the splenic lobe. The B-cells were oval in shape with round nucleus and fine, light purple granules present in the cytoplasm identified by aldehyde fuchsin stain (, ). The A-cells were oval in shape with oval nucleus and were characterized by very fine, orangeophilic cytoplasmic granules seen after staining with Ponceau de xyledine by AFT (, ). Some islets showed predominantly B-cells ( 1,b) and some other islets predominantly A-cells (). Both B- and A-cells were present in the same islet (,c). One exocrine cell, probably by its transformation into endocrine cell (TEC?) with very large orange and blue studded granules was seen intermingled with the A-cell islet ().
Table I. Tinctorial characteristics of B- and A-cells in the endocrine pancreas of Lissemys turtles
D-cells, identified by scanty, lightly stained bluish-green cytoplasmic granules by AFT (), were seen intermingled with A-cells. Small A-cell islet identified by AFT was observed within the epithelium of the pancreatic duct (). There was no definite distribution pattern of B-, A- or D-cells seen in any islet or group of endocrine cells in the splenic or duodenal lobe of the pancreas.
Immunocytochemistry
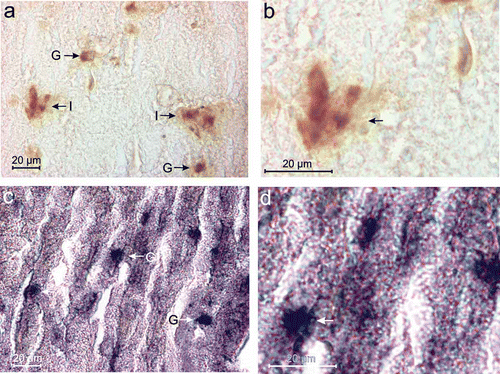
Insulin-immunoreactive (Insulin-IR) B-cells were identified by brownish/deep brown cytoplasmic granules (,b) and these cells were distributed mostly in islets (I) and groups (G) () in the splenic lobe of the pancreas of Lissemys turtles. However, glucagon-immunoreactive (Glucagon-IR) A-cells were identified by bluish/deep blue cytoplasmic granules (,d) and were scatteredly distributed both in groups and in isolation in the splenic lobe of the pancreas.
Figure 2. (a) Insulin-immunoreactive (Insulin-IR) B-cells with brownish/deep brown reactive sites are seen in islets (I) or groups (G) in the splenic lobe of the pancreas of Lissemys turtles. (b) An enlarged view of showing deep brown reactive sites in the cytoplasm of insulin-IR B-cells (arrows) in the splenic lobe of turtle pancreas. (c) Glucagon-immunoreactive (Glucagon-IR) A-cells with deep blue reactive sites are seen in small groups (G) and in isolation in the splenic lobe of the pancreas of turtle. (d) An enlarged view of showing deep blue reactive sites in the cytoplasm of glucagon-IR A-cells (arrow) of the splenic lobe of turtle pancreas. Scale bars: a–d, 20 μm.
Electron microscopy
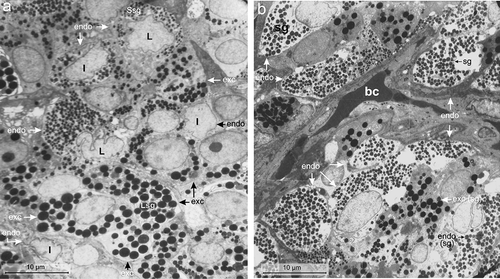
Under electron microscope, the endocrine cells of the pancreas of Lissemys turtles showed large indented/lobulated nucleus with smaller secretory granules compared to the small round/oval nucleus with larger secretory granules of exocrine cells. The nuclei were euchromatic in endocrine cells unlike hyperchromatinic in exocrine cells (). The endocrine cells were distributed mostly near the blood capillaries, (). Moreover, the secretory granules, mitochondria or rough endoplasmic reticulum (RER), each was aggregated in one corner of the endocrine cells unlike those evenly distributed in the exocrine cells. There were six cell types (A, B, D, PP, Vth and VIth) found in the endocrine pancreas of Lissemys turtles. No perceptible differences in the distribution pattern and morphology of the endocrine cells of the pancreas were observed between male and female turtles.
Figure 3. (a) Electron micrograph showing indented (I) or lobulated (L) nucleus with smaller secretory granules (Ssg) in the endocrine cells (endo) (arrow) compared to those of larger secretory granules (Lsg) of the exocrine cells (exc) in the splenic lobe of the pancreas of Lissemys turtles. Scale bar: 10 μm. (b) Electron micrograph showing distribution of endocrine cells (endo) mostly near the blood capillaries (bc). Secretory granules (sg) are smaller in the endocrine cells (endo) than in exocrine cells (exc). Scale bar: 10 μm.
B-cell
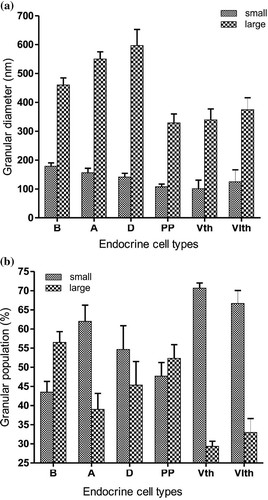
The B-cell population was highest (40 ± 2%) among all the endocrine cells (). These cells were large (with an average cell area of 51.41 ± 1.8 μm2), oval in shape and contained a very large (with an average nuclear area of 20.94 ± 3.17μm2) central, euchromatic, lobulated nucleus (, ). The C/N ratio of B-cells was lower (2.56 ± 0.86:1) than A-cells (3.82 ± 0.54:1) (). The RER was short and less abundant (,c). There was a large number of mitochondria (mt) aggregated in one corner of the B-cell cytoplasm (,b) and they were mostly oval in shape with conspicuous cristae (, ). The Golgi apparatus (G) was scanty with concentric vesicles (, ). The secretory granules were moderately abundant (94 ± 8.34/cell) () and homogenously distributed in the cell cytoplasm, barring the mitochondrial region () as in A-cells (). The secretory granules (sg) were oval in shape and showed a crystalline-like electron-dense core with wide electron-lucent peripheral halo (, ), or a negligible electron-dense core with very wide peripheral halo (). Their size varied between 131 and 526 nm. The diameters of the small and large secretory granules were 178.6 ± 11.63 nm and 460.16 ± 24.46 nm, respectively (, ). The population (%) of small secretory granules was lower (43.5 ± 2.82%) than large (56.5 ± 2.82%) granules (). A few vacuoles (v) were also seen in the cytoplasm (). A few B cells (?) were seen intermingled within the epithelium (ep) of the pancreatic duct with few endocrine cells (endo) seen close to the duct ().
Table II. Cellular and intracellular characteristics of endocrine cells in the pancreas under TEM in Lissemys turtles
Table III. Ultrastructural characteristics of the secretory granules of the endocrine cells of the pancreas of Lissemys turtles
Figure 4. Electron micrographs of the B-cell of the endocrine pancreas of the splenic lobe of Lissemys turtles showing (a) an intact large, oval B-cell close to blood capillaries (bc), with a very large indented euchromatic nucleus (n), mitochondrial region (mt) and moderate abundance of homogenously distributed secretory granules (sg) barring the mitochondrial region. (b) An enlarged view of showing conspicuous mitochondrial (mt) region with few rough endoplasmic reticulum (RER) at the periphery. (c) Short RER is seen very close to the oval mitochondria (mt) with conspicuous cristae. (d) Golgi bodies (G) with concentric vesicles are seen surrounding a secretory granule (sg). (e) Showing secretory granules (sg) with crystalline-like electron-dense core and very wide electron-lucent peripheral halo. (f) Other secretory granules showing negligible electron-dense part and very wide halo. (g) Showing small vacuoles (V) close to the secretory granules (sg). (h) Some B-cells (?) are intermingled in the epithelium (ep) of the pancreatic duct with few endocrine cells (endo) close to the duct. Scale bars: a, 2 μm. b, 1 μm. c–f, 0.25 μm. g, 0.5 μm. h, 5 μm.
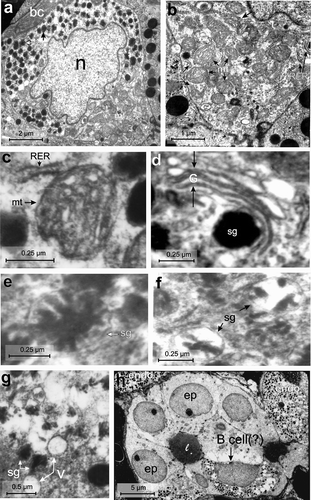
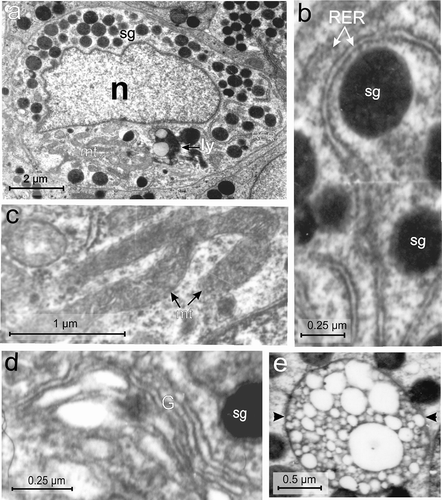
Figure 5. Electron micrographs of the A-cell of the endocrine pancreas of the splenic lobe of Lissemys turtles showing (a) an intact A-cell with elongated and indented euchromatic nucleus (n), RER region, mitochondrial region (mt), lysosomes (ly) and moderate abundance of homogeneously distributed electron-dense secretory granules (sg) barring the mitochondrial region. (b) Showing long filamentous RER, encircling the secretory granules (sg). (c) Long filamentous and branched mitochondria (mt) with inconspicuous cristae are seen in the cytoplasm. (d) Golgi apparatus (G) with concentric vesicles surrounding a secretory granule (sg) is seen. (e) Showing a multivescicular body (arrows) with numerous empty vesicles of various sizes. Scale bars: a, 2 μm. b, d, 0.25 μm. c, 1 μm. e, 0.5 μm.
A-cell
The population of A-cells was lower (32 ± 1.52%) than B-cells and second highest amongst all endocrine cells (). The A-cell was also large (with an average cell area of 47.57 ± 1.76 μm2) and oval in shape with a small (average nuclear area of 12.73 ± 1.23 μm2), elongated, euchromatic and indented nucleus (, ). The C/N ratio was higher (3.82 ± 0.54:1) than in other cell types (). The cytoplasm showed RER, mitochondrial zone (mt) (), and Golgi apparatus (G) with cisternae () and scanty lysosomes (ly). The RER was long and abundant with conspicuous cisternae, located surrounding the secretory granules (, ). The mitochondria (mt) were abundant, long, filamentous and branched (intercalated) with small inconspicuous cristae (, ). The Golgi apparatus (G) showed concentric vesicles surrounding the secretory granules (). The secretory granules (sg) were moderately abundant (102 ± 18.8/cell), and homogeneously distributed in the cytoplasm barring the mitochondrial zone (, ). The secretory granules (sg) were round and electron-dense with smooth surface (, ). The diameter of secretory granules ranged between 104 and 666 nm. The diameters of small and large secretory granules were 156 ± 15.32 nm and 550 ± 25.1 nm, respectively (, ). Populations of small granules were higher (62 ± 4.23%) than the larger type (39 ± 4.16%) (). Some A-cells showed multivesicular bodies with numerous empty vesicles of various size (indicated by arrow) ().
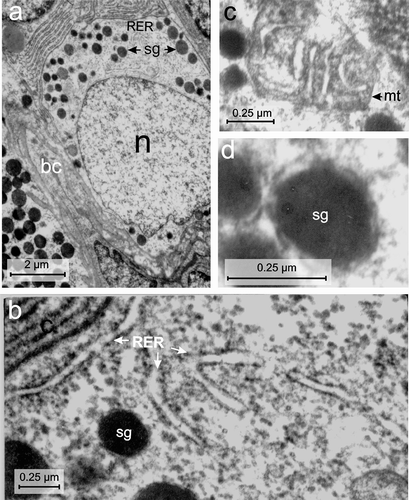
Figure 6. Electron micrographs of the D-cell of the endocrine pancreas of the splenic lobe of Lissemys turtles showing (a) an intact oval D-cell adjacent to the blood capillaries (bc), containing a large oval euchromatic nucleus (n) and moderate number of electron-dense secretory granules (sg) of various size, aggregated in small groups with a RER region. (b) Branched RER with dilated cisternae is seen. (c) Showing mitochondria (mt) with conspicuous cristae. (d) Secretory granules (sg) with wide electron-dense core and narrow electron-lucent peripheral halo are seen. Scale bars: a, 2 μm. b–d, 0.25 μm.
D-cell
The D-cell population was relatively lower (13.74 ± 0.89%) than B- and A-cells (Table II). The D-cells were also large (with an average cell area of 47.16 ± 10.19 μm2), oval in shape, with a large (average nuclear area of 19.85 ± 4.56 μm2), round, partly indented, euchromatic nucleus (n) (, Table II). The C/N ratio was lower (2.4 ± 0.91:1) than A-cells (Table II). These cells also showed RER zone in the cell cytoplasm (). These were long and branched with conspicuous cisternae (, Table II). The mitochondria (mt) were scanty and oval in shape with conspicuous cristae (, Table II). The Golgi apparatus was also scanty. The population of secretory granules was moderately abundant (74 ± 7.9/cell) (), located in small groups (sg) at one corner of the cell cytoplasm (). These were round in shape and showed wide electron-dense core with a narrow electron-lucent peripheral halo (, ). Their size varied between 104 and 759 nm. Diameters of small and large secretory granules were 141.2 ± 12.94 nm and 596.8 ± 55.76 nm, respectively (, ). Populations of small granules were higher (54.66 ± 6.17%) than the large granules (45.33 ± 6.17%) ().
PP-cell
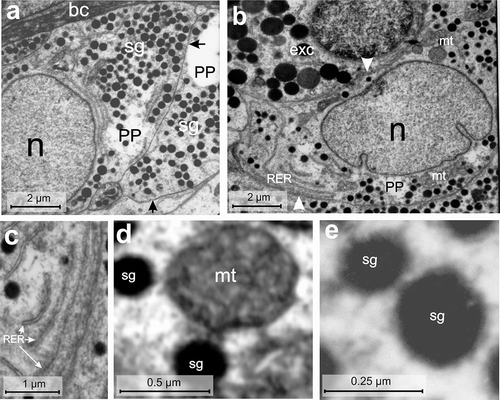
The PP-cell population was further lower (9.15 ± 0.49%) than the B-, A- or D-cells (Table II). The PP-cells were fusiform in shape, mostly with tapering ends, and small in size (average cell area of 32.27 ± 2.43 μm2) with a small (average nuclear area of 13.3 ± 1.79 μm2), partly indented, euchromatic nucleus (n) (). The C/N ratio (2.63 ± 0.91:1) was higher than B- or D-cells (Table II). The cytoplasm showed RER and small mitochondrial zones (mt) (). The RER was long and narrow with conspicuous cisternae (). Mitochondria (mt) were generally round with conspicuous cristae (). The Golgi bodies were scanty. The secretory granules (sg) were moderately abundant (67 ± 15.85/cell) () and distributed mostly in the tapering ends of the cell cytoplasm (). The secretory granules (sg) were round with a wide electron-dense core and a thin layer of peripheral halo (, ). Their size varied between 80 and 416 nm in diameter. The diameters of small and large secretory granules were 107.6 ± 9.25 nm and 328.8 ± 30.78 nm, respectively (, ). Populations of small granules were lower (47.66 ± 3.58%) than large granules (52.33 ± 3.58%) ().
Figure 7. Electron micrographs of the PP-cell of the endocrine pancreas of the splenic lobe of Lissemys turtles showing (a) two fusiform cells (arrows) with tapering ends adjacent to the blood capillaries (bc), large euchromatic oval nucleus (n) (partly seen) and moderate abundance of small electron-dense secretory granules (sg). (b) A PP-cell is seen adjacent to an exocrine cell (exc) (arrowhead) with an intact indented nucleus (n), RER region and mitochondrial regions (mt). (c) An enlarged view of RER with dilated cisternae in the cell cytoplasm is seen. (d) A magnified view of an oval mitochondrium (mt) showing conspicuous cristae adjacent to few secretory granules (sg). (e) The secretory granules (sg) with a wide electron-dense core and a thin layer of peripheral halo are seen. Scale bars: a, b, 2 μm. c, 1 μm. d, 0.5 μm. e, 0.25 μm.
Vth-cell type
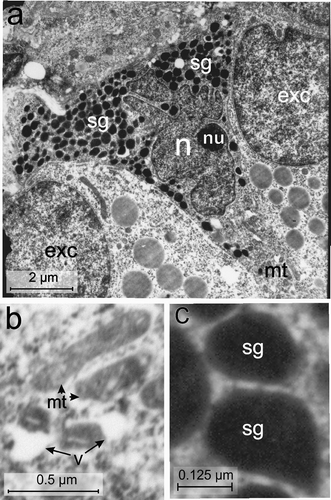
Under electron microscope the fifth cell-type () was scarce and its population was very low (2.26 ± 0.36%). They were scattered in the splenic lobe of the pancreas. These cells were smallest among all other endocrine cells, with an average cell area of 26.15 ± 1.55 μm2 and triangular in shape with a very small nucleus (average nuclear area of 8.41 ± 2.08 μm2) (, Table II). The C/N ratio (3.35 ± 0.82: 1) was almost same as in A-cells (Table II). The nucleus (n) was extremely indented and hyperchromatinic with a large single round nucleolus (nu), located near the nuclear membrane (). The RER was scanty and mitochondria were abundant in the mitochondria zone (mt) located in one corner of the triangular cell. The mitochondria (mt) were oval or elongated in shape with conspicuous cristae (). The Golgi apparatus was scanty. A few vacuoles (v) were seen in the cytoplasm (). The secretory granules (sg) were abundant (185 ± 40.26/cell), and aggregated in one corner of the cell (, Table III). They were mostly pear-shaped with a wide electron-dense core and a narrow peripheral halo (, Table III). The granule size varied between 48 and 384 nm. Diameters of small and large secretory granules were 101 ± 29.54 nm and 339 ± 38.25 nm, respectively (, Table III). Populations of small granules were significantly higher (70.66 ± 1.33%) than the large (29.33 ± 1.33%) granules ().
Figure 8. Electron micrographs of the Vth-cell type of the endocrine pancreas of the splenic lobe of the Lissemys turtles showing (a) an intact triangular in shape, located adjacent to exocrine cells (exc) with indented hyperchromatinic nucleus (n), containing a prominent nucleolus (nu) near the nuclear membrane, mitochondrial region (mt) and abundance of secretory granules (sg) aggregated in two corners of the cell. (b) Oval and elongated mitochondria (mt) with conspicuous cristae and vacuoles (v) are seen in the cell cytoplasm. (c) Pear-shaped secretory granules (sg) showing wide electron-dense core with a narrow peripheral halo. Scale bars: a, 2 μm. b, 0.5 μm. c, 0.125 μm.
VIth-cell type
The sixth cell type () was also scarce and scattered in the splenic lobe of the endocrine pancreas. Their population was also very low (2.85 ± 0.14%), like that of the Vth cell type. These cells were pyramidal in shape and smaller in size (average cell area of 34.25 ± 7.26 μm2) with larger nuclear size (average nuclear area of 14.8 ± 3.45 μm2) than the Vth cell type. The C/N ratio (2.32 ± 0.04:1) was nearly the same as in D-cells (Table II). The RER and Golgi apparatus were scanty. The mitochondria (mt) were abundant and aggregated in small groups () and were round/oval in shape with conspicuous cristae (, Table III). The secretory granules (sg) were least in number (17 ± 6.43/cell) (Table III) and aggregated in 2–3 small groups (). They were oval in shape with a wide electron-dense core and a narrow peripheral halo (, Table III). Their size varied between 83 and 440 nm. The diameters of small and large secretory granules were 124.5 ± 41.5 nm and 374.5 ± 41.5 nm, respectively (Table III, ). The populations of small granules were much higher (66.66 ± 3.38%) than the large granules (33.34 ± 3.6%) ().
Figure 9. Electron micrographs of the VIth-cell type of the endocrine pancreas of the splenic lobe of Lissemys turtles showing (a) a very small, pyramidal in shape, with a large euchromatic indented nucleus (n), mitochondrial region (mt) and scanty secretory granules (sg), aggregated in small groups. (b) Several round/oval mitochondria with conspicuous cristae are seen. (c) Showing oval secretory granules (sg) with wide electron-dense core and narrow peripheral halo. Scale bars: a, 2 μm. b, 1 μm. c, 0.25 μm.
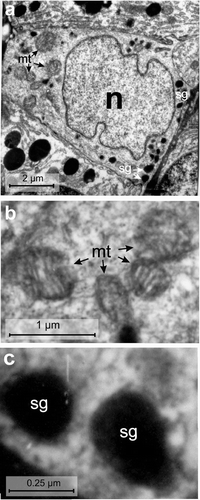
Figure 10. (a) Histograms (representing mean ± SE values) showing diameters (nm) of small and large secretory granules of different endocrine cells (B, A, D, PP, Vth and VIth) in the splenic lobe of the pancreas of Lissemys turtles. (b) Histograms (representing mean ± SE values) showing populations (%) of small and large secretory granules of different endocrine cell types (B, A, D, PP, Vth and VIth) in the splenic lobe of the pancreas of Lissemys turtles.
Discussion
The present study reveals that pancreatic islets and blood capillaries supplying the islets are independently covered by a thin layer of collagenous connective tissue which is stained green by the aldehyde fuchsin trichrome (AFT) technique in Lissemys turtles, as reported earlier in another turtle species, Chrysemes picta (Cardeza Citation1957). However, the connective tissue covering of the pancreatic islets was not found in some other turtle species studied, e.g. Testudo graeca (Garcia Ayala et al. Citation1987) and Lissemys turtles (Chandavar & Naik Citation2004). Such a variation in the occurrence of the connective tissue covering of the pancreatic islets might be due to the lack of study by AFT. Nevertheless, the connective tissue covering may be required to provide mechanical support and protection of the pancreatic islets in situ in turtles. The pancreatic endocrine cells occur mostly in islets or groups in the splenic lobe, but in small groups or in isolation in the duodenal lobe of the pancreas of Lissemys turtles. Some islets consist of predominantly B-cells (B-cell islet) and others with predominantly A-cells (A-cell islet). Both B- and A-cells together with D-cells and/or other types of endocrine cells (mixed islet) may also be present in the same or different islets or groups. Sometimes endocrine cells, especially A-type, seen under light microscope, and B-type, seen under TEM, are also present within the epithelial layer of the pancreatic ducts or ductules of the pancreas of Lissemys turtles as has been reported in another turtle species Testudo graeca (Garcia Ayala et al. Citation1987). Few endocrine cells were observed near the ductule of the splenic lobe of the pancreas of Lissemys turtles. Lozano et al. (Citation2000) have reported that glucagon/NPY cells occur close to the pancreatic ducts of the duodenal lobe in Pseudemys scripta elegans. Under light microscope, the endocrine cells are lightly stained compared to those of the deeply stained acinar cells in Lissemys turtles. Ultrastructurally, the endocrine cells are also clearly distinguishable from the exocrine cells of the pancreas. The endocrine cells are generally large in size with large, indented or lobulated nucleus and numerous small secretory granules, accompanied by blood capillaries. However, exocrine cells are relatively small with small oval nucleus and large secretory granules seen in the Lissemys pancreas.
In the current study, we have identified two major cell types, B- and A-cells, by their tinctorial and immunocytochemical characteristics. B-cells have light purple granules, whereas A-cells are orangeophilic. B- and A-cells can also be identified by insulin and glucagon antibodies, respectively. Ultrastructurally, six types of endocrine cells (B-, A-, D-, PP-, Vth- and VIth-types) are distinguishable in the pancreas of Lissemys turtles. B-cells are generally large with large lobulated nucleus, a mitochondrial zone and abundant crystalline-like secretory granules, whereas A-cells are larger, but with a small indented nucleus, having rough endoplasmic reticular (RER) and mitochondrial zones. D-cells have a large indented nucleus with RER zone. PP-cells are small, fusiform with a partially indented nucleus, RER and mitochondrial zones. Vth endocrine cells are triangular in shape, with an extremely indented nucleus, mitochondrial zone and numerous pear-shaped secretory granules, whereas, the VIth cell type is small, pyriform in shape, containing a large indented nucleus, mitochondrial zone and scanty secretory granules.
Nevertheless, light microscopic, immunocytochemical and ultrastructural findings of various types of endocrine cells of the pancreas of Lissemys turtles highlight certain characteristic features, which are unreported in other chelonians. (a) Ultrastructurally, the endocrine cells are clearly distinguishable by their large size with large indented/lobulated nucleus, located mostly near the blood capillaries, and abundance of small secretory granules unlike the small cell size and nucleus size, and less abundance of very large secretory granules in the exocrine cells of the pancreatic acini. (b) Some islets are predominant with B-cells (B-islet) and others with A-cells (A-islets) and still other islets with both B- and A-cells (mixed islet) observed in the endocrine pancreas of Lissemys turtles. (c) Six types of endocrine cells have been identified, B- and A-cells by tinctorially, immunocytochemically and ultrastructrually; D-, PP-, Vth- and VIth-cell types exclusively by ultrastructural study. (d) Fusiform, triangular or pyramidal shape of PP-, Vth- or VIth-cell types, respectively, are the characteristic features of these pancreatic endocrine cells of Lissemys turtles. (e) Endocrine cell types have differential population with highest number of B-type, followed (sequentially in decreasing order) by A-, D-, PP-, Vth- and VIth-types. (f) Endocrine cells have RER and mitochondrial zones (except the mitochondrial zone in D-cell). (g) Secretory granules are largest in D-cell followed by A-, B-, PP-, VIth- and Vth–types, respectively. Granules are clustered in D-, Vth- and VIth-cell types. Their population is highest in Vth-type and least in VIth-cell type.
RER, mitochondria and secretory granules have localized distributions in the cytoplasm of endocrine cells of Lissemys turtles. Some differences in the distribution pattern of some subcellular organelles have also been reported in other turtle species (Agulleiro et al. Citation1985; Garcia Ayala et al. Citation1987). There are reports that in Testudo graeca, mitochondria are clustered with scarce RER and larger secretory granules (300–700 nm) in the B-cell, indented nucleus with scattered mitochondria and clustered secretory granules (150–450 nm) are present in the A-cell, scarce RER with moderately abundant small secretory granules (100–300 nm) are found in the D-cell. PP-cells have RER with well developed Golgi cisternae, scarce mitochondria and pyriform/ovoid secretory granules (150–400 nm). A Vth-type of endocrine cells containing small secretory granules (150–400 nm) with dense core surrounded by a wide peripheral halo has also been reported to be present in the endocrine pancreas during summer in Testudo graeca (Garcia Ayala et al. Citation1987). Cellular and subcellular ultrastructural differences of the cytoplasmic organelles of different endocrine cell types of the pancreas of Lissemys turtles from those of other turtle species might be due to the differences in the state of activities of these cells and/or to species specificity.
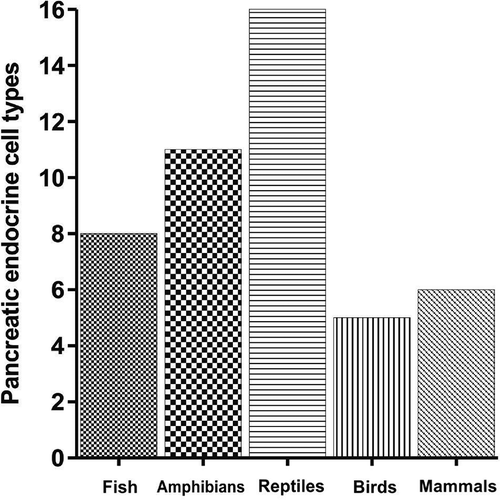
The B-cell population is highest of all the endocrine cells followed by A-, D-, PP- and Vth-/VIth-types observed in the endocrine pancreas of Lissemys turtles. The same pattern of population of endocrine cells has also been reported in the endocrine pancreas of amphibia (Lee et al. Citation2003). However, B- and A-cells are most common and the major pancreatic endocrine cell types found in Lissemys turtles as has been reported in other turtle species, Pseudemys scripta elegans (Lozano et al. Citation2000) and lizard, Anolis (Rhoten & Smith Citation1978), and in all other vertebrates studied. In addition, there are several other types of endocrine cells in turtles and other vertebrates. The pancreatic endocrine cell types are also highly variable in different chelonian species with two types (B- and non-B-cells) in three species of freshwater turtles, Chrysemys dorbigni, Phrynops hilarii (Cardeza Citation1957), Geoemyda pulcherrima (Miller Citation1962), five types (A-, B-, D-, PP- and Vth-type) in Testudo graeca (Garcia Ayala et al. Citation1987) and A-, B-, D-, PP- and glucagon-NPY types in Pseudemys scripta elegans (Lozano et al. Citation2000). Earlier reports indicate that poikilotherms, especially chelonians, have maximum number of pancreatic endocrine cell types (16 types in reptiles) compared to homeothermic vertebrates (six types) (). Chelonians among reptiles (including lizards) represent highest number of pancreatic endocrine cell types (10 types). Such a wide variation of the pancreatic endocrine cell types in chelonians may be related to the wide variety of adaptations (terrestrial, aquatic or both) (Carr Citation1972). Earlier reports also indicate that number of endocrine cell types gradually increased from eight types in fish and 11 types in amphibia to 16 types in reptiles, but subsequently decreased to five types in birds and six types in mammals (). It is likely that pancreatic endocrine cell types are phylogenetically conserved in the course of evolution of different vertebrate animals, because the number of endocrine cell types remains constant throughout the vertebrate kingdom, except in Reptilia which could be due to an adaptational variation, but this needs to be confirmed. Although the current findings of six types of endocrine cells of the pancreas of Lissemys turtles corroborate with those of other species of soft-shelled turtles, whether the cell types of Lissemys turtles correspond to those of other turtles needs to be confirmed. Furthermore, the structure–function interrelationship of these pancreatic endocrine cell types is yet to be ascertained in Lissemys turtles.
Figure 11. Histograms showing pancreatic endocrine cell types in different groups of vertebrate animals.
Functions of B- and A-cells are well known in chelonians (Lozano et al. Citation2000). D- and PP-cells are known to produce somatostatin and pancreatic polypeptide, respectively, in turtles Testudo graeca (Garcia Ayala et al. Citation1987) and Pseudemys scripta elegans (Agulleiro et al. Citation1985; Lozano et al. Citation2000). The Vth-type of pancreatic endocrine cell has been reported to produce histamine containing secretory granules in Testudo graeca (Garcia Ayala et al. Citation1987). However, the precise functions of these pancreatic endocrine cell types, especially of D-, PP-, Vth- and VIth-cells, need to be confirmed in Lissemys turtles.
We conclude that our study of pancreatic endocrine cell types in turtles will help in understanding the physiology of each cell types from a comparative endocrinological standpoint, adaptation as well as pancreatic disease in turtles in the future.
Acknowledgements
Insulin and glucagon antibodies were donated by Professor Debi Prasad Sarkar, Department of Biochemistry, University of Delhi. Transmission electron micrographic work was carried out in the laboratory of Dr T. C. Nag, AIIMS, New Delhi. Dr. Shibsankar Roy, IICB, assisted in immunocytochemical technique and Gautam Ghosh, Department of Zoology, Midnapore College, helped in colour photography. Dr. Tapan Dutta, Assistant Professor of Life Science, Panskura Banamali College, East Midnapore, West Bengal, India, prepared computerised histograms and photoplates of the manuscript.
References
- Agulleiro , B , Garcia Ayala , A and Abad , ME. 1985 . An immunocytochemical and ultrastructural study of the endocrine pancreas of Pseudemys scripta elegans (Chelonia) . General and Comparative Endocrinology , 60 : 95 – 103 .
- Cardeza , AF. 1957 . Histology of the islands of Langerhans in the turtle diabetic from alloxan and from the effects of glucose . Revista de la Sociedad Argentina de Biologia , 33 : 74 – 79 .
- Carr , A. 1972 . The reptiles , New York, NY : Time-Life Books .
- Chandavar , VR and Naik , PR. 2004 . Variation in plasma glucose and pancreatic beta cells in the turtle, Lissemys punctata (order: Chelonia; family: Trionychidae) . Acta Zoologica (Stockholm) , 85 : 113 – 118 .
- Epple , A. 1967a . A staining sequence for A, B, and D cells of pancreatic islets . Stain Technology , 42 : 53 – 61 .
- Epple , A. 1967b . Further observations on amphiphil cells in the pancreatic islets . General and Comparative Endocrinology , 9 : 137 – 142 .
- Epple , A and Brinn , JE. 1987 . The comparative physiology of the pancreatic islets , 223 Heidelberg : Springer-Verlag .
- Garcia Ayala , A , Lozano , MT and Agulleiro , B. 1987 . Endocrine pancreas of Testudo graeca L. (Chelonia) in summer and winter. An immunocytochemical and ultrastructural study . General and Comparative Endocrinology , 68 : 235 – 248 .
- Garcia Ayala , A , Lozano , MT and Agulleiro , B. 1989 . Comparative study on the endocrine cells in the pancreas of Mauremys caspia (Chelonia) in summer and winter . General and Comparative Endocrinology , 75 : 363 – 375 .
- Lee , JH , Ku , SK , Lee , HS and Kitagawa , H. 2003 . An immunohistochemical study of endocrine cells in the pancreas of the Red-bellied frog (Bombina orientalis) . European Journal of Histochemistry , 47 : 165 – 172 .
- Lozano , MT , Garcia Hernandez , MP , Garcia Ayala , A , Elbal , MT and Agulleiro , B. 2000 . Identification of the pancreatic endocrine cells of Pseudemys scripta elegans by immunogold labeling . General and Comparative Endocrinology , 117 : 163 – 172 .
- Miller , MR. 1962 . Observation on the comparative histology of the reptilian pancreatic islets . General and Comparative Endocrinology , 2 : 407 – 414 .
- Parakkal , PF. 1961 . Mordanting fixation as a means of facilitating the staining of pancreatic cells of mouse . Stain Technology , 36 : 33 – 34 .
- Perez-Tomas , R , Ballesta , J , Madrid , LM and Polak , JM. 1989 . Comparative immunohistochemical study of the gastroenteropancreatic endocrine system of three reptiles . General and Comparative Endocrinology , 76 : 171 – 191 .
- Rhoten , WB and Smith , PH. 1978 . Localization of four polypeptide hormones in the saurian pancreas . American Journal of Anatomy , 151 : 595 – 602 .
- Snedecor , GW and Cochran , WG. 1989 . Statistical methods , 7th , Ames, IA : The Iowa State College Press .
- Sternberger , LA. 1986 . Immunocytochemistry , New York, NY : Wiley .
- Titlbach , M. 1966 . Licht- und elektronenmikroskopische Untersuchungen der Langerhans schen Inseln von Schildkroten (Testudo graece, Emys orbicularis L.) . Zditschrift fűr Zellforschung , 70 : 21 – 35 .
- Yaglov , VV. 1976 . Morphology of the endocrine portion of the reptile pancreas . Arkh. Anatomy, Histology and Embryology , 71 : 89 – 93 .