Abstract
In two Messor species, M. meridionalis and M. foreli, glandular source, optimum concentration, longevity and interspecific responses of the trail pheromone were studied in the laboratory. Dufour's gland is the sole origin of the trail pheromone, and has no synergism with the content of the poison gland or the hindgut. Secretions of other gaster complex glands, as well as hindgut contents, in fact, did not evoke trail following. The optimum concentration of trail pheromone was found to be one gland equivalent/30 cm trail. This concentration demonstrated effective longevity for about 1 h. Interspecific trail-following tests indicate that signals mediating trail-following behaviour in these harvesting ants are not strictly species-specific.
Introduction
At the basis of ant colony organization are very efficient communication systems among which signals mediated by semiochemicals play a central role (Vander Meer & Alonso Citation1998). Chemical signals are used to mark the path towards a newly discovered feeding site and to elicit nest mates to leave the nest (Bradshaw & Howse Citation1984; Morgan Citation1984; Billen & Morgan Citation1998). Chemical trail communication allows group foragers to exploit conspicuous food sources efficiently and represents the most prevalent form of recruitment behaviour (Hölldobler & Wilson Citation1990). Trail communication is more commonly based on a multicomponent system, where the secretions of different glands (or a blend of pheromones produced by the same gland) may contribute to the structure of the trail and regulate different behaviours in the process of recruitment (Hölldobler & Wilson Citation1990; Hölldobler Citation1995; Traniello & Robson Citation1995; Jackson et al. Citation2006). Sudd (Citation1959) defined trail-laying as a field activity in which an insect marks a route with scent or odour traces such that other insects of the same community are able to follow it. Wilson (Citation1962) stated that, when an ant discovers a new food source, it returns to the nest laying an odour trail. This directs nest mates to the food, and on their return to the nest they reinforce the trail. This continues until the food source becomes exhausted or overcrowded, when the workers returning to the nest cease to lay a trail. The existing volatile pheromone disperses so the workers are no longer recruited to the source. Trail pheromones in all termites are secreted from the sternal gland. The major component is one of just two compounds: either (Z,Z,E)-dodecatrienol (by Reticulitermies flavipes for example) or (E,E,E)-neocembrene-A (E-6-cembrene A) (by Nasutitermes exitiosus for example) (Pasteels & Bordereau Citation1998). In contrast, among the ants, some 10 different glands are sources of trail pheromone and there is great diversity in the glands and compounds used in different ant genera (Hölldobler & Wilson Citation1990). The glandular sources of ant trail pheromones vary between, and within, the different subfamilies. A large number of glands have been found to be used for trail pheromone production, suggesting possibly many independent origins of this behaviour (Hölldobler & Wilson Citation1990). All of these glands open near the tip of the gaster, except the tibial gland used by species of Crematogaster (Jackson & Morgan Citation1993). Within the genus Messor, both Dufour's and the poison gland could be involved in this process. In M. pergandei (Mayr, 1886) (Blum Citation1974) and M. egyptiacus (Emery, 1878) (Ali Citation2009), trail substances are produced by the poison gland. In M. capitatus (Latreille, 1798), M. structor (Latreille 1798), and probably also in M. ebeninus Santschi 1927, Dufour's gland secretions are involved in the recruitment process and are responsible for the production of the trails (Hahn & Maschwitz Citation1985; Coll et al. Citation1987; Grasso et al. Citation1998). A peculiar situation was found in M. bouvieri Bondroit, 1918 where both poison and Dufour's gland substances are involved in trail communication, although workers showed a strong preference for Dufour's gland secretions (Jackson et al. Citation1989, Citation1991).
To optimize their foraging behaviour, ants select the most rewarding source. This selection is due to a modulation of the quantity of pheromone laid on a trail (Hölldobler & Wilson Citation1990). A specific concentration of trail pheromones is important since concentrations that are too high or too low elicit either no response or repellency (Barlin et al. Citation1976). Pheromones are released mainly from exocrine glands as liquids that evaporate into the surrounding air and form a cloud of vapour about the signalling animal (Bossert & Wilson Citation1963). The chemical nature and distance through which a pheromone may transmit a message is a function of the volatility of the compound, its stability in air, its rate of diffusion, the olfactory efficiency of the receiver, and wind currents (Fitzgerald & Underwood Citation1998). Trail communication systems are strongly affected by a series of social and ecological influences, which determine the properties of the signals, such as their specificity. For example, different degrees of trail signal specificity could be influenced by intra- and interspecific competition, orientation and homing mechanisms, territorial recognition and advertisement (Hölldobler & Carlin Citation1987; Traniello & Robson Citation1995). At the species level, trail pheromones do not differ chemically as much as sex pheromones. However, although different species in a genus may share the main components of their trail pheromones, as for example in the sympatric fire ant Solenopsis spp., workers will only follow those of their own species. Similarly, the recruitment pheromone seems to be the same in all species of the harvester ant Pogonomyrmex, but the Dufour's gland secretion, used to mark persistent trunk routes, has a hydrocarbon blend that varies between species (and other markers may even differ between colonies, and, in some species, between individuals; Hölldobler & Wilson Citation1990).
The genus Messor is a worldwide group of seed-harvesting ants, the dominant ants in deserts and dry grasslands. They collect seeds by a mixture of individual foraging and ‘column retrieval’ (Hölldobler & Wilson Citation1990). Because of their ready availability, and their potential to damage crops (Collingwood Citation1985), the communication behaviour has received some attention. Localized crop damage has been reported in Saudi Arabia and significant economic damage to crops is found in North Africa (Jackson et al. Citation1989). M. meridionalis (André, 1883) is a central Asian species extending westward into the Middle East. M. foreli Santschi, 1923 is a small species and it is a true desert species common in the northern Sahara (Collingwood Citation1985).
The present research is part of a wider study on the ecology and social biology of M. meridionalis and M. foreli. In particular, I provide here for the first time detailed data on sources of signals mediating trail communication, the dosages eliciting maximal trail following to gland extracts and the longevity of gland extract trails and investigate interspecific trail-following responses of these harvesting ants. Trail-following investigations may provide a better understanding of the chemical communication system employed by these species.
Materials and methods
Ants
M. meridionalis (one colony, about 2000 individuals) and M. foreli (one colony, about 1500 individuals) containing a few queens with brood, workers and males were collected from Al-Harik city south Riyadh and reared in the laboratory in artificial nests made of a plastic bottle with some soil. The bottle was placed in a plastic bowl with 60 cm internal diameter and 25 cm vertical wall to serve as a foraging arena. The colonies were kept at a temperature of 25°C, 60% relative humidity, and a constant period of 12 h light per day. Ants were fed with seeds and freshly killed insects, water was also provided. To compensate moisture loss of the soil granules, a few drops of water were added at different periods whenever needed. Ten days prior to the start of the experiment were left for establishment of the colonies within the lab.
Bioassays
Glandular extracts were obtained by dissecting frozen ants in distilled water under a stereomicroscope and placing the appropriate in 100 μl hexane. Colonies used for glandular bioassays were starved for 7 days before the beginning of the tests. Each test was replicated five times.
Aliquots of 100 μl hexane solution of the glandular secretions were applied with a microsyringe along a 30 cm long pencil line drawn on one arm of a ‘V’- shaped filter paper (the arms diverged by an angle of approximately 90°). The control trail (laid down on the other arm of the paper with 100 μl hexane and starting at the same point) was offered simultaneously. Some seeds were provided at the end of the trail, but to prevent trail over marking by returning ants, all the ants that held a seed between the mandibles were immediately aspirated. The sides of the control and experimental trails were regularly alternated, and for each test a new paper was used. Trails were applied 10 min before each test to allow the solvent and alarm-eliciting substances to evaporate (Blatrix et al. Citation2002). The filter paper with the trails was introduced 1–2 cm into the nest entrance and the test started when the first ant emerged from the nest. The behaviour of the ants was observed, and for 5 min a count was made of all the ants that followed the trails from the nest to the end. This test was conducted five times and the cumulative number (mean ± SE) of ants in each arm served for scoring trail-following activity was calculated. At least 20 min elapsed between tests.
In an experiment to determine whether the glands have a synergetic effect, a mixture of both Dufour's and poison glands or Dufour's gland and hind gut in 100 μl hexane was independently applied on workers of each ant species as mentioned above. Also the whole abdomen (gaster) in 100 μl hexane was applied in the same way. Worker ants were allowed in the foraging arena for 20 min. This test was conducted five times and the mean number of ants following the trail was calculated in these three cases and then compared with hexane alone.
The trail pheromone concentration that elicited the maximal worker response was determined using different concentrations of the source gland (0.001, 0.01, 0.1, 1, 5, 10, 20, and 40 glands) in 100 μl hexane. Worker ants were allowed to access the foraging arena for 20 min. The mean number of ants per 30 cm trail was calculated for each concentration and compared to hexane alone (control). Each of the individual concentrations was conducted five times.
To determine the longevity of trail pheromones, the concentration that elicited the maximal activity – obtained from the previous experiment – was applied as above. Ants were allowed access to the foraging arena at different time periods post-application including 0, 15, 30, 45, 60, 75, 90 min. This test was conducted five times and the mean number of ants per 30 cm trail was calculated and compared with hexane alone for each time period.
To test the trail-following response of workers of each species to trails of the other species, we employed trails made with source gland extracts using the methods described above. This test was conducted five times and the mean number of responding ants in each case was calculated.
Statistical analysis
All statistical analyses were undertaken using MINITAB software (MINITAB, State College, PA, Version 13.1, 2002). Data were analysed using the non-parametric equivalent of an ANOVA: Kruskal–Wallis, and then a Tukey's test to differentiate between the groups.
Results
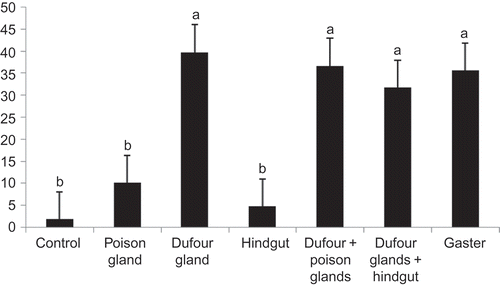
In both Messor meridionalis and M. foreli, the ability of different abdominal glands (Dufour's and poison glands) and hindgut to elicit trail following was tested against a hexane control. Since ants followed the abdominal gland extracts over hexane in all cases, the various abdominal glands were tested against each other ( and ). To assess whether more than one gland was involved in trail signalling, combined extracts of Dufour's gland and poison glands as well as Dufour's gland and hindgut were compared to that of Dufour's gland alone. Results revealed no significant difference between the activity induced by Dufour's gland alone and that induced by the combined extracts or that of the gaster alone. This indicates that the Dufour's gland secretion accounts for all the trail pheromone activity, and that there is no synergism when combined with other sources ( and ).
Figure 1. Mean number of Messor meridionalis workers evoked by hexane (controls), gaster, hindgut contents, poison or Dufour's gland secretions. Error bars represent the standard errors of the mean of five replicates. Similar letters indicate no significant difference, while different letters indicate significant difference.
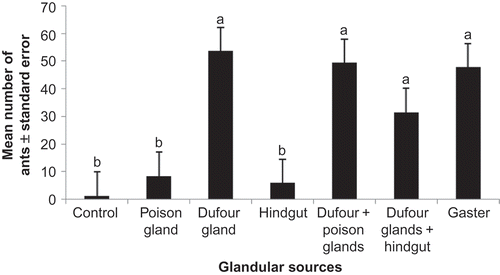
Figure 2. Mean number of Messor foreli workers evoked by hexane (controls), gaster, hindgut contents, poison or Dufour's gland secretions. Error bars represent the standard errors of the mean of five replicates. Similar letters indicate no significant difference, while different letters indicate significant difference.
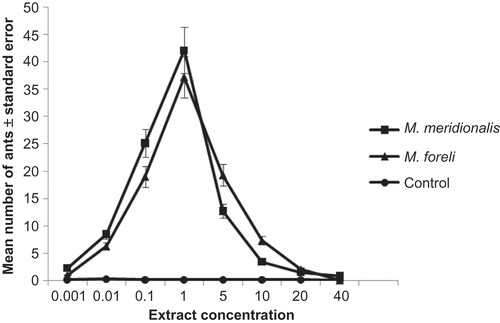
Eight concentrations of gaster extract were tested in this experiment (0.001, 0.01, 0.1, 1, 5, 10, 20, and 40 glands equivalent in 100 μl hexane per 30 cm trail) to determine the dosage eliciting maximal trail following activity. The highest activity of worker ants evoked at 1.0 gaster/30 cm trail. This activity significantly decreased at concentrations below and above this concentration (P < 0.05, n = 5) (), which may suggest it as the concentration that elicits significant response from M. meridionalis and M. foreli workers.
Figure 3. Mean number of Messor workers responding to an artificial trail of different concentrations of Dufour gland extract. Error bars represent the standard errors of the mean of five replicates.
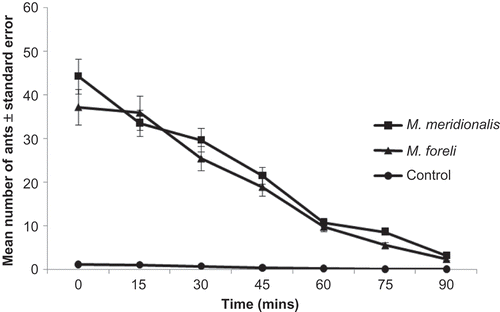
At the beginning of the longevity experiment, the activity of M. meridionalis and M. foreli workers recorded a maximum value (44.32 ± 6.75, 37.2 ± 7.13). Gradually, activity decreased, recording a mean number of ants, 33.6 ± 5.58 and 29.67 ± 5.51 in M. meridionalis and 35.9 ± 6.73 and 25.4 ± 7.4 in M. foreli, after the trail had aged 15 and 30 min. Activity was reversibly correlated with the pheromone time application (), since the activity recorded 3.16 ± 0.28 and 2.31 ± 0.41 in M. meridionalis and M. foreli, respectively, after the pheromone had aged 90 min.
Figure 4. Longevity of the trail pheromone of the two Messor ant species. Error bars represent the standard errors of the mean of five replicates.
In all tests involving M. meridionalis and M. foreli, the heterospecific Dufour's gland secretions induced a strong trail following response by workers (). In particular, workers of the two Messor species did not show any preference in choice between their own trails and those of the other.
Table I. Interspecific responses of M. meridionali s and M. foreli trail extracts
Discussion
To perform most of their tasks, ants mainly rely on signals conveyed by chemical secretions. As a result, the dissection and reconstruction of the strategies and mechanisms underlying ant social organization involve the study of chemical communication (Traniello & Robson Citation1995). Semiochemicals are often necessary to recruit and direct workers where labour is required; therefore, trail communication has a basic role in the foraging ecology of many species (Hölldobler & Wilson Citation1990). The present research provides further information on the chemical communication systems of two species of Messor ants, and will aid us to clarify some aspects of their behavioural ecology related to resource and space partitioning, as well as other community-level interactions.
The results of our glandular bioassays show that the anatomical source of the trail pheromone is Dufour's gland. This is consistent with previous studies on M. structor (Hahn & Maschwitz Citation1985), M. bouvieri (Jackson et al. Citation1989, Citation1991), M. capitatus (Grasso et al. Citation1998), M. minor and M. wasmanni (Grasso et al. Citation1999).
The present research shows that neither the poison gland nor the hindgut had effect on Dufour's gland in the two Messor species. The same results were found in Pheidole jordanica and P. sinaitica by Ali and Mashaly (Citation1997), in Monomorium lepineyi by Mashaly et al. (Citation2010) and in M. niloticum, M. najrane and M. mayri by Mashaly (Citation2010).
The concentration of 1.0 gland trail induced the maximal activity of the trail pheromone following for M. meridionalis and M. foreli. Similarly, the same concentration in both M. lepineyi and M. bicolor was found by Mashaly et al. (Citation2010). The chemical analysis of the gland contents of M. meridionalis and M. foreli workers clearly showed the presence of a mixture of straight-chain hydrocarbons, where M. meridionalis and M. foreli contains 14 and 20 components, respectively, 10 of which are overlapping (Mashaly et al., unpublished data 2011). This could be the basis of the similar behaviour of the two species at the same concentration. Additionally, in M. niloticum, the optimum concentration was 1.0 and 0.1 gaster equivalent (GE)/30 cm trail (Mashaly Citation2010). In Iridomyrmex humilis Mayer, the maximal activity was in response to a trail containing 0.1–1.0 ant (gaster) equivalent per 50 cm. This activity dropped when the concentration was lower or higher than this concentration (Van Vorhis et al. Citation1981). Jones and Blum (Citation1982) and Hölldobler and Wilson (Citation1990) stated that the chemical communication between isolated animals requires the volatile nature of pheromones. Individual trail pheromones can vary in volatility (and stability) and also elicit different behavioural responses that are dependent on concentration, context, and their proportion in mixtures.
In ants, trail pheromone longevity varies between minutes in Aphaenogaster albisetosus (Hölldobler et al. Citation1995) to several weeks in some Eciton species (Torgerson & Akre Citation1970). For species only using chemical communication in foraging, the longevity of the trail could be linked to the feeding ecology (Traniello Citation1989). Short-lived trails can rapidly modulate recruitment to ephemeral food sources, whereas long-lived trails are be more suited to persistent or recurrent food sources (Fitzgerald & Underwood Citation1998). In the two Messor species, the activity of trail pheromone decreased to the lowest level after 1 h. The activity of trail pheromone in Pachycondyla sennaarensis Mayr, 1862 decreased to half of the original activity level after 1 h (Mashaly et al. Citation2011). In Pheidole teneriffana Forel, 1893 (Ali Citation1996) and M. niloticum, M. mayri and M. najrane (Mashaly Citation2010), the optimum dose of the trail completely disappeared after 1 h. In P. jordanica, P. sinaitica and Pheidole sp., the activity of workers decreased to its lowest level after 75–90 min at a concentration of two gasters, while the activity decreased gradually by the time recording just a trace after 165–180 min at a concentration of 5 gasters (Ali & Mashaly Citation1997). In M. lepineyi and M. bicolor, the activity of worker ants decreased to its lowest level after 2 h (Mashaly et al. Citation2010).
The chemical secretions produced by Dufour's gland of the two examined species had similar behavioural effects when offered to heterospecific workers. In fact, the workers of each species were able to recognize and follow artificial trails marked with the Dufour's gland secretions of the other species. This means that the components of Dufour's gland secretions inducing the normal trail-following responses in M. meridionalis and M. foreli seem to be relatively anonymous and not strictly species-specific. The responses displayed by the ants could be caused by a different composition (or amount) of the active substances present in the Dufour's gland of the two species. The basis of the cross-trail-following could be achieved by the overlapping in chemical structure as discussed above.
Initial studies on the topic showed a high specificity of trail pheromones (Wilson & Pavan Citation1959; Wilson Citation1962), but later investigations have clearly shown that this is not a general rule, since within a subfamily (or even within a genus) trail signals may vary widely in specificity (Attygalle & Morgan Citation1985; Hölldobler & Wilson Citation1990; Traniello & Robson Citation1995). Ants belonging to the large and diverse subfamily Myrmicinae may use different glandular sources (poison, Dufour's, tibial glands) to mark their tracks (Robinson et al. Citation1974; Attygalle & Morgan Citation1985; Hölldobler & Wilson Citation1990; Billen & Morgan Citation1998; Mashaly Citation2010). However, whichever gland they use, myrmicines investigated so far show a strong variability of intra- and intergeneric trail specificity, ranging from a total or a partial specificity to a complete anonymity of signals (Attygalle & Morgan Citation1985; Traniello & Robson Citation1995). For example, odour trail pheromones are completely species-specific between Tetramorium caespitum Linne, 1758 and T. guineense Bernard 1953, but the latter could follow trails of other myrmicine genera (Blum & Ross Citation1965). Workers of Crematogaster scutellaris Olivier, 1792 follow the trails of C. laestrygon Emery, 1869, but the latter always prefer their own trace (Gobin & Billen Citation1994). A partial specificity was also found within the genus Solenopsis Westwood, 1840 with some species following each other's artificial trails and other being very selective in their response (Wilson Citation1962; Barlin et al. Citation1976; Jouvenaz et al. Citation1978). Ants of the genus Myrmica Latreille, 1804 show the least species specificity within the myrmicines, since several species follow trails produced from each other's poison gland (Blum Citation1974; Cammaerts et al. Citation1981; Evershed et al. Citation1982). Interspecific trail-following tests in three sympatric species M. capitatus, M. minor and M. wasmanni showed that workers of each species are able to recognize and follow artificial trails obtained from the Dufour's gland secretions of the others (Grasso et al. Citation2002).
In conclusion, short-lasting trail pheromones are secreted from the Dufour's gland in the two Messor ants, M. meridionalis and M. foreli. Also, the concentration of the pheromone had a strong effect on worker activity. Trail-following behaviour in the two Messor ants is not strictly species-specific. Trail-following investigations may provide a better understanding of the chemical communication system employed by these species under such ecological conditions.
Acknowledgements
This work was gratefully supported by the Center of Excellence for Biodiversity Research, College of Science, King Saud University, Riyadh, Saudi Arabia. Also, the author thanks Prof. Dr. C. A. Collingwood (City Museum, Leeds, UK) for confirmation of the ant identification.
References
- Ali , AS. 2009 . Detection of the trail pheromone source and effect of certain factors on trail following activity of some ants , Egypt : MSc thesis, Minia University .
- Ali , MF. 1996 . Source, optimum dose response, longevity and isolation of trail pheromone of the ant Pheidole teneriffana (Forel) (Formicidae: Hymenoptera) . Journal of Egyptian German Society of Zoology , 20 : 69 – 82 .
- Ali , MF and Mashaly , AMA. 1997 . Trail pheromone investigation of some Pheidole ants (Formicidae: Hymenoptera) . Egyptian Journal of Zoology , 28 : 113 – 123 .
- Attygalle , AB and Morgan , ED. 1985 . Ant trail pheromones . Advances in Insect Physiology , 18 : 1 – 30 .
- Barlin , MR , Blum , MS and Brand , JM. 1976 . Fire ant trail pheromones: Analysis of species specificity after gas chromatographic fractionation . Journal of Insect Physiology , 22 : 839 – 844 .
- Billen , J and Morgan , ED. 1998 . “ Pheromone communication in social insects: sources and secretions ” . In Pheromone communication in social insects: Ants, wasps, bees and termites , Edited by: Vander Meer , RK , Breed , MD , Espelie , KE and Winston , ML . 3 – 33 . Boulder, CO : Westview Press .
- Blatrix , R , Schulz , C , Jaisson , P , Francke , W and Hefetz , A. 2002 . Trail pheromone of ponerine ant Gnamptogenys striatula: 4-methylgeranyl esters from Dufour's gland . Journal of Chemical Ecology , 28 : 2557 – 2567 .
- Blum , MS. 1974 . Myrmicine trail pheromones: Specificity, source and significance . Journal of the New York Entomological Society , 82 : 141 – 147 .
- Blum , MS and Ross , GN. 1965 . Chemical releasers of social behavior. V. Source, specificity and properties of the odour trail pheromone of Tetramorium guineense (F.) (Formicidae: Myrmicinae) . Journal of Insect Physiology , 11 : 857 – 868 .
- Bossert , WH and Wilson , EO. 1963 . The analysis of olfactory communication among animals . Journal of Theoretical Biology , 5 : 443 – 469 .
- Bradshaw , JWS and Howse , PE. 1984 . “ Sociochemicals in ants ” . In Chemical ecology of insects , Edited by: Bell , WJ and Cardé , RT . 429 – 473 . London : Chapman and Hall .
- Cammaerts , MC , Evershed , RP and Morgan , ED. 1981 . Comparative study of the Dufour gland secretions of workers of four species of Myrmica ants . Journal of Insect Physiology , 27 : 59 – 65 .
- Coll , M , Hefetz , A and Lloyd , HA. 1987 . Adnexal gland chemistry of Messor ebeninus Forel (Formicidae: Myrmicinae) . Zeitschrift für Naturforschung , 42 : 1027 – 1029 .
- Collingwood , CA. 1985 . Hymenoptera: Fam . Formicidae of Saudi Arabia. Fauna of Saudi Arabia , 7 : 230 – 302 .
- Evershed , RP , Morgan , ED and Cammaerts , MC. 1982 . 3-Ethyl-2, 5-dimethylpyrazine, the trail pheromone from the venom gland of eight species of Myrmica ants . Insect Biochemistry , 12 : 383 – 391 .
- Fitzgerald , TD and Underwood , DLA. 1998 . Communal foraging behavior and recruitment communication in Gloveria sp . Jounal of Chemical Ecology , 24 : 1381 – 1396 .
- Gobin , B and Billen , J. 1994 . Spécificité de la phéromone de piste chez Crematogaster scutellaris et Crematogaster laestrygon . Actes des Colloques Insectes Sociaux , 9 : 35 – 39 .
- Grasso , DA , Mori , A and Le Moli , F. 1998 . Chemical communication during foraging in the harvesting ant Messor capitatus (Hymenoptera, Formicidae) . Insectes Sociaux , 45 : 85 – 96 .
- Grasso , DA , Mori , A and Le Moli , F. 1999 . Recruitment and trail communication in two species of Messor ants (Hymenoptera, Formicidae) . Italian Journal of Zoology , 66 : 373 – 378 .
- Grasso , DA , Mori , A and Le Moli , F. 2002 . Behavioural investigation of trail signals specificity in three sympatric species of Messor ants (Hymenoptera, Formicidae) . Italian Journal of Zoology , 69 : 147 – 151 .
- Hahn , M and Maschwitz , U. 1985 . Foraging strategies and recruitment behaviour in the European harvester ant Messor rufitarsis (F.) . Oecologia , 68 : 45 – 51 .
- Hölldobler , B. 1995 . The chemistry of social regulation: Multicomponent signals in ant societies . The Proceedings of the National Academy of Sciences Online (USA) , 92 : 19 – 22 .
- Hölldobler , B and Carlin , N. 1987 . Anonymity and specificity in the chemical communication signals of social insects . Journal of Comparative Physiology A , 161 : 567 – 581 .
- Hölldobler , B , Oldham , NJ , Morgan , ED and König , WA. 1995 . Recruitment pheromones in the ants Aphaenogaster albisetosus and A. cockerelli (Formicidae: Hymenoptera) . Journal of Insect Physiology , 41 : 739 – 744 .
- Hölldobler , B and Wilson , O. 1990 . The ants , 269 Berlin : Springer .
- Jackson , BD and Morgan , ED. 1993 . Insect chemical communication: Pheromones and exocrine glands in ants . Chemoecology , 4 : 125 – 144 .
- Jackson , BD , Wright , PJ and Morgan , ED. 1989 . 3-Ethyl-2,5 dimethylpyrazine, a component of the trail pheromone of the ant Messor bolivien . Experientia , 45 : 487 – 489 .
- Jackson , BD , Wright , PJ and Morgan , ED. Chemistry and trail following of a harvester ant . Proceedings of the Insect Chemical Ecology Conference, Tabor 1990 . The Hague. pp. 109 – 112 . Academia Prague and SPB Academical Publishers .
- Jackson , DE , Martin , SJ , Holcombe , M and Ratnieks , F. 2006 . Longevity and detection of persistent foraging trails in Pharaoh's ant, Monomorium pharaonis . Animal Behaviour , 71 : 351 – 359 .
- Jones , TH and Blum , MS. 1982 . Ant venom alkaloids from Solenopsis and Monomorium species: Recent developments . Tetrahedron , 38 : 1949 – 1958 .
- Jouvenaz , DP , Lofgren , CS , Carlson , DA and Banks , WA. 1978 . Specificity of the trail pheromone of four species of fire ant, Solenopsis spp . Florida Entomology , 61 : 244
- Mashaly , AMA. 2010 . Monomorium ant's trail pheromones: Glandular source, optimal concentration, longevity and specificity . Journal of Asian Pacific Entomology , 13 : 23 – 26 .
- Mashaly , AMA , Ahmed , AM , Al-Abdula , MA and Al-Khalifa , MS. 2011 . The trail pheromone of the venomous samsum ant, Pachycondyla sennaarensis . Journal of Insect Science , 11 : 1 – 12 .
- Mashaly , AMA , Ali , AS and Ali , MF. 2010 . Source, optimal dose concentration and longevity of trail pheromone in two Monomorium ants (Formicidae: Hymenoptera) . Journal of King Saud University (Science) , 22 : 57 – 60 .
- Morgan , ED. 1984 . “ Chemical words and phrases in the language of pheromones for foraging and recruitment ” . In Insect communication , Edited by: Lewis , T . 169 – 194 . London : Academic Press .
- Pasteels , JM and Bordereau , C. 1998 . “ Releaser pheromones in termites ” . In Pheromone communication in social insects: Ants, wasps, bees, and termites , Edited by: Vander Meer , RK , Breed , MD , Winston , ML and Espelie , K . 193 – 215 . Boulder, CO : Westview Press .
- Robinson , SW , Moser , JC , Blum , MS and Amante , E. 1974 . Laboratory investigations of the trail-following responses of four species of leaf-cutting ants with notes on the specificity of a trail pheromone of Atta texana (Buckley) . Insectes Sociaux , 21 : 87 – 94 .
- Sudd , JH. 1959 . Interaction between ants on a scent trail . Nature , 183 : 1588
- Torgerson , RL and Akre , RD. 1970 . The persistence of army ant chemical trails and their significance for the Ecitonine ecitophile association (Formicidae: Ecitonine) . Melanderia , 5 : 1 – 28 .
- Traniello , JFA. 1989 . Foraging strategies of ants . Annual Review of Entomology , 34 : 191 – 210 .
- Traniello , JFA and Robson , SK. 1995 . “ Trail and territorial communication in insects ” . In Chemical ecology of insects , 2nd , Edited by: Cardé , RT and Bell , WJ . 241 – 286 . London : Chapman and Hall .
- Van Vorhis , Key SE , Gaston , LK and Baker , TC. 1981 . “ Effect of gaster extract trail concentration on the trail following behavior of the Argentine ant Iridomyrmex humilis (Mayr) ” . In Journal of Insect Physiology Vol. 27 , 363 – 370 .
- Vander Meer , RK and Alonso , LE. 1998 . “ Pheromone directed behavior in ants ” . In Pheromone communication in social insects: Ants, wasps, bees and termites , Edited by: Vander Meer , RK , Breed , MD , Espelie , KE and Winston , ML . 159 – 192 . Boulder, CO : Westview Press .
- Wilson , EO. 1962 . Chemical communication among workers of fire ant Solenopsis saevissima (Fr. Smith). I – The organization of massforaging; II – An information analysis of the odour trail; III – The experimental induction of social responses . Animal Behaviour , 10 : 134 – 164 .
- Wilson , EO and Pavan , M. 1959 . Glandular sources and specificity of some chemical releasers of social behaviour in dolichoderine ants . Psyche , 66 : 70 – 76 .