Abstract
Seasonal changes of abundance and composition of planktonic polychaete larvae were investigated monthly from January 2007 to December 2009 in Onagawa Bay, northeastern Japan. Larvae belonging to 18 families were identified: Spionidae, Serpulidae, Nephtyidae, Magelonidae, Phyllodocidae, Polynoidae, Syllidae, Capitellidae, Nereididae, Terebellidae, Arenicolidae, Chaetopteridae, Oweniidae, Pectinariidae, Glyceridae, Dorvilleidae, Sabellidae, and Lumbrineridae. The density of polychaete larvae varied from 18 to 6901 ind m−3. Spionid larvae occurred throughout the year, being the dominant family throughout the year, comprising 88.7% of the total, with those belonging to genus Polydora dominant during winter to spring and Pseudopolydora during summer to autumn. Larvae belonging to the Serpulidae, Magelonidae, Nephtyidae, Phyllodocidae, and Polynoidae tended to be frequent in summer and autumn. The close timing between phytoplankton blooms and the production of planktonic polychaete larvae is discussed, and it is noted that most planktonic polychaete larvae tended to synchronize with summer phytoplankton increases and autumn blooms in the near-surface water, but not with spring blooms. One possible explanation is the diversity of food during summer to autumn in Onagawa Bay. Only larvae belonging to the genus Polydora were synchronized with spring phytoplankton blooms, perhaps reflecting their northern biogeographic origin.
Introduction
Many marine invertebrates have a planktonic larval phase in their early life history. In most sessile and benthic organisms having little mobility, except some migratory species, the planktonic larval phase represents an important opportunity to acquire new habitats and to cross with other populations. Variability in recruitment is potentially important for the dynamics and structure of populations and communities (Caley et al. Citation1996; Fraschetti et al. Citation2002). However, little is known about the seasonal and spatial change in abundance of most invertebrate larvae, as abundance depends on the reproductive timing of the adults, the duration and behavior of the larvae, and ocean circulation patterns.
Some polychaetes also spend a temporary phase as planktonic larvae during their development until they finally settle on the bottom (Thorson Citation1946). The planktonic larvae of polychaetes comprise one of the most numerous and diverse groups of coastal zooplankton (Omel'yanenko & Kulikova Citation2002). Despite the great importance of the group in the composition and functioning of marine benthic populations, relatively little is known about the planktonic larval stages of polychaetes.
In Arctic or subarctic waters, the planktotrophic larvae of benthic polychaetes occur during the spring phytoplankton bloom and feed mainly on phytoplankton, while nonfeeding lecithotrophic larvae exist throughout the year (Curtis Citation1977; Lacalli Citation1981). In temperate seas, planktotrophic polychaete larvae feed on diatoms, dinoflagellates and bivalve veligers during spring to autumn (Daro & Polk Citation1973; Kuhl Citation1974; Turner & Anderson Citation1983). In Amurskii Bay, Sea of Japan, Omel'yanenko and Kulikova (Citation2002) showed that the larvae of Harmothoe imbricata, the most numerous species among planktonic polychaete larvae in the surveyed area, agrees in time with the first peak of the phytoplankton bloom in March or April. In Japan, Yokouchi (Citation1984, 1991) studied the seasonal and horizontal distribution of planktonic polychaete larvae and their food habitat in the subarctic coastal waters of southern Hokkaido, and showed that the abundance of polychaete larvae coincided with spring phytoplankton blooms from February to April.
In Onagawa Bay, northeastern Japan, as expected in temperate waters, a large spring phytoplankton bloom and a smaller autumn bloom are apparent (e.g. Tanaka et al. Citation1997; Tanaka & Taniguchi Citation1999). Additionally, chlorophyll a (chl. a) peaks are usually observed in summer (e.g. Tanaka et al. Citation1997; Tanaka & Taniguchi Citation1999). Phytoplankton composition of these tri-modal phytoplankton increases differs from each other, and it is one of the distinct features of Onagawa Bay.
The aim of the present study is to investigate seasonal changes in the abundance and composition of planktonic polychaete larvae and chl. a concentration, and to discuss the relationship between seasonal changes of chl. a concentration and planktonic polychaete larvae in Onagawa Bay.
Materials and methods
Sampling was carried out from January 2007 to December 2009 at a station (St. 1: 38°26.14'N, 141°27.83'E) in the innermost part of Onagawa Bay (); station depth 21–23 m. Zooplankton samples were collected once a month by one vertical haul from the bottom to the surface using a Norpac net (Motoda Citation1957) with a mesh size of 110 μm. The volume of water filtered was calculated from the flowmeter reading. Plankton samples were fixed in 5% neutralized formaldehyde solution. After splitting the samples into 1/8 aliquots by using a Motoda plankton splitter (Motoda Citation1959), planktonic polychaete larvae were identified and counted under a stereo microscope (Leica, WILD MZ8). To ensure accurate identification, not only fixed samples but also live samples were observed. Except for dominant spionids, identification was only to family, because generic morphological characters cannot be fully distinguished at the larval phase. For taxonomic identification of polychaete larvae, following resources were used as reference: Thorson (Citation1946), Hannerz (Citation1956), Yamaji (Citation1966), Blake (Citation1969, Citation1975), Blake and Woodwick (Citation1975), Srikrishnadhas and Ramamoorthi (Citation1977), Radashevsky (Citation1983 Citation1985), Bhaud and Cazaux (Citation1987), Plate and Husemann (Citation1994), Chihara and Murano (Citation1997), Shanks (Citation2001), and Rodriguez-Valencia (Citation2003).
Vertical profiles of temperature and salinity were determined by STD. Total chl. a concentration was determined bimonthly or monthly, after pre-filtering through a 200 μm mesh onto Whatman GF/F filter (average pore size of 0.7 μm); and size fractionated chl. a concentration was determined once a month. Water samples were collected from the surface down to 20 m depth at 5 m intervals with a 5-liter Van Dorn water sampler. Subsamples of 128 ml were taken at each depth and filtered onto a GF/F filter; or filtered through a 20 or 200 μm mesh and fractionated to size at 0.2–20 μm, 2–20 μm and 20–200 μm, using filters with a pore size of 0.2 μm, 2 μm and 20 μm. Chlorophyll a concentration of 0.2–2 μm fraction was calculated from chl. a concentration of 0.2–20 μm and 2–20 μm fractions. After filtration, each filter was immediately enveloped by a quantitative filter and aluminum foil to protect from light. Chlorophyll a was extracted for 24 h in 90% acetone, and fluorescence was determined with a Turner Designs fluorometer by the method of Yentsch and Menzel (Citation1963). (b) was plotted using the Matlab routines in EasyKrig 3.0 software (Chu Citation2004).
Results
Environmental factors
The seasonal changes of temperature and salinity at the surface and near the bottom of the water column in Onagawa Bay over three years are shown in . Water temperature ranged from 5.5 to 22.7°C, with lowest temperatures in March or April (minimum recorded near the bottom in April 2008), and highest in July or August (maximum at the surface in August 2009). Both the minima and maxima varied only by 1 to 2°C over the period of investigation. Thermal stratification in the water column began in March or April and lasted until August. In other seasons, the water column was well mixed vertically.
Figure 2. Seasonal changes of temperature (°C) and salinity (PSU) at the surface and near bottom of water column at St. 1 in Onagawa Bay during the period from 2007 to 2009.
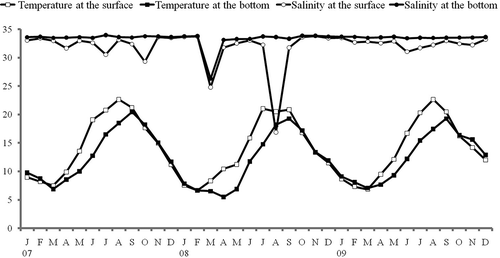
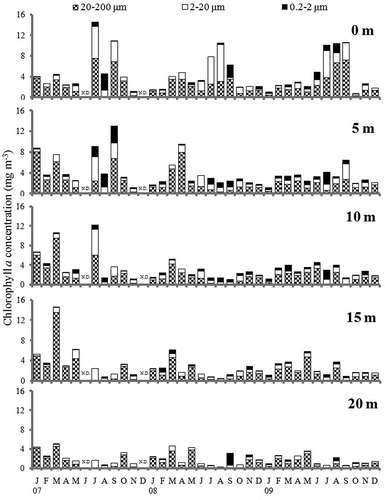
Figure 3. Seasonal changes of GF/F-filtered chlorophyll a concentration in the water column at St. 1 in Onagawa Bay from 2007 to 2009. (a) Chlorophyll a concentration at 0, 5, and 15 m in the water column; (b) vertical profile of chlorophyll a concentration.
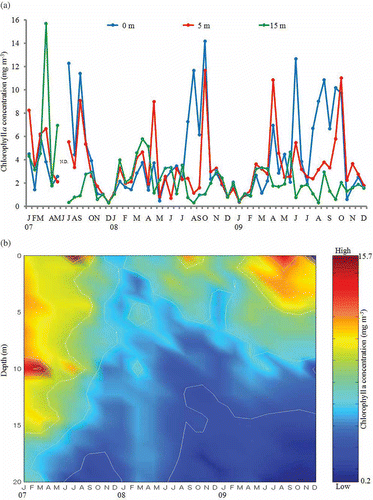
Salinity ranged from 16.9 to 34.0 PSU but was generally stable around 33 PSU, except at the surface in July and October 2007, August 2008 and all layers in March 2008. Episodic decreases in surface salinity are a consequence of heavy rainfall. The salinity decrease in all layers in March 2008 was probably due to the inflow of melt water from the spring thaw.
Seasonal changes of GF/F-filtered chl. a concentration at 0, 5, and 15 m depth in the water column are shown in . GF/F-filtered chl. a concentration varied in the range 0.16–15.68 mg m−3 during the three years of the study, with markedly seasonal and vertical variations. The chl. a concentration was typically low during winter. Typical of the annual pattern in temperate waters, there was a large spring phytoplankton bloom at all depths of the water column in 2007 (). However, in 2008 and 2009, phytoplankton increased only moderately, with a small spring bloom observed at 5 m depth in April. A second annual peak was observed at the surface water in summer, and a third peak at 0 and 5 m in autumn in each year. Exceptionally, chl. a concentration greatly increased at the surface in the first half of June 2009.
Seasonal changes of size-fractionated chl. a concentration at 5 m depth intervals in the water column are shown in . The microphytoplankton fraction (20–200 μm) of chl. a concentration accounted for 60.4% of the total chl. a concentration. It increased and dominated in spring, and was the major contribution to the annual spring bloom. The nanophytoplankton (2–20 μm) and picophytoplankton (0.2–2 μm) fractions accounted for 27.6% and 12.0% of the total, respectively. These fractions increased in the near-surface water during summer and autumn, contributing to the summer and autumn peaks ().
Seasonal change of planktonic polychaete larvae
Polychaete larvae occurred in the plankton throughout the year, density of 18 to 6902 ind m−3, with a seasonal abundance as shown in . Larvae belonging to 18 families were identified: Spionidae, Serpulidae, Nephtyidae, Magelonidae, Phyllodocidae, Polynoidae, Syllidae, Capitellidae, Nereididae, Terebellidae, Arenicolidae, Chaetopteridae, Oweniidae, Pectinariidae, Glyceridae, Dorvilleidae, Sabellidae, and Lumbrineridae (). The lowest density was recorded in January 2009, and the highest in January 2007. Generally, polychaete larvae showed relatively high density in spring and summer, and low density in winter with the exception of January 2007 when density of polychaete larvae was exceptionally large.
Table I. Seasonal density dynamics of planktonic polychaete larvae in Onagawa Bay from 2007 to 2009. Each number shows the minimum and maximum densities (ind m−3) for each month, and total shows the total individual numbers counted (ind m−3) for each group over three years
Figure 5. Seasonal change of abundance of polychaete larvae at St. 1 in Onagawa Bay from 2007 to 2009. (a) Abundance of spionid polychaete larvae; (b) abundance of other polychaete larvae except Spionidae.
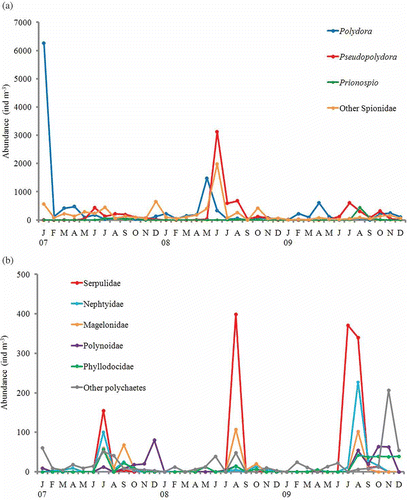
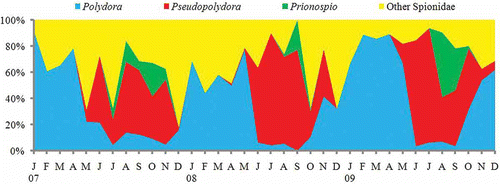
As shown in , in terms of polychaete larvae abundance, the Spionidae were the overwhelmingly dominant family throughout the year (comprising 88.7% of the total), followed by the Serpulidae (4.1%), Nephtyidae (1.5%), Magelonidae (1.2%), Polynoidae (1.2%) and Phyllodocidae (1.0%). The remaining 12 families comprised less than 1% of the total. Among the Spionidae, larvae belonging to six genera (Polydora, Pseudopolydora, Prionospio, Dipolydora, Scolelepis, and Boccardiella) were identified, although only three specimens belonging to Scolelepis and one of Boccardiella were identified during the entire study. Many spionids could not be identified to genus level. Larvae belonging to the genera Polydora and Pseudopolydora were dominant, comprising 39.2 and 23.5%, respectively, of all spionids. Polydora larvae appeared throughout the year, except in September 2008. Their density was particularly high in winter and spring (), with a maximum recorded in January 2007 (6267 ind m−3). Pseudopolydora larvae appeared from April–May to November–December, and were most abundant in summer, with its highest density in June 2008 (3121 ind m−3) (). Prionospio larvae were observed only during summer and autumn. The larvae belonging to Serpulidae and Magelonidae, also, appeared only during summer and autumn, and those of Nephtyidae, Phyllodocidae, and Polynoidae were most abundant during these periods (). Larvae of the Syllidae showed a sudden increase, up to 195 ind m−3, in November 2009 ().
The density of planktonic polychaete larvae varied somewhat each year. However, their seasonal composition showed a qualitatively similar pattern. From January through May, Polydora larvae were dominant, and the diversity of polychaete larvae was relatively low (). In June, the proportion of Polydora larvae showed a sharp decline, and Pseudopolydora larvae started to increase, becoming dominant from June through October–November (). Starting around July, larvae belonging to other polychaete families (particularly the Serpulidae, Magelonidae, Nephtyidae, Phyllodocidae, and Polynoidae) began to occur, and polychaete larvae showed a rich diversity in summer and autumn (). Subsequently, the proportion of Pseudopolydora larvae, and the diversity of polychaete larvae, declined gradually, and both were very low in December. In winter, Polydora larvae began to increase to return to its dominant position ().
Discussion
Chlorophyll a concentration
The bi-modal winter/spring and autumn blooms typical in the phytoplankton annual cycle in temperate latitudes and the two maxima (winter/spring and autumn) and one minimum (in the summer) are generally recognized as a typical pattern of the phytoplankton annual cycle in temperate latitudes (Longhurst Citation1995; Sathyendranath et al. Citation1995). However, the results of this study showed a tri-modal phytoplankton annual cycle. In addition to the winter/spring and autumn blooms, the chl. a concentration increased only at the surface in July or August, shortly before the autumn bloom in Onagawa Bay.
A spring bloom usually lasts approximately one month during January to March/April. Spring bloom is characterized by uniformly high chl. a concentration in the water column due to phytoplankton movement throughout the water column by vertical mixing. The microphytoplankton fraction (20–200 μm) of chl. a concentration dominated during the spring bloom, indicating an increase in the population of diatoms. It is known that diatoms, especially Thalassiosira nordenskioeldii and Chaetoceros debilis, are major components of spring phytoplankton blooms in Onagawa Bay (Inoue Citation1995; Sato Citation1996; Nakamura Citation2007). At the end of April, spring bloom terminated probably due to depletion of the nitrate stock and/or increasing role of the grazing pressure imposed by the growing zooplankton community.
A second annual peak of the chl. a concentration was observed only at the surface in July or August. During the warm period, although the light conditions are favorable for photosynthesis, nutrient is limited because the water column stratification acts as a barrier to prevent the supply of the additional nutrients from the bottom layers to the surface. Generally, pico- and nanophytoplankton species are abundant under oligotrophic conditions in warm waters, but few under eutrophic conditions in cold waters. In the present study, although the percentage of total chlorophyll a concentration accounted for by pico- and nanophytoplankton increased near surface in summer, the same was also true for microphytoplankton. Chaetoceros socialis, Skeletonema costatum, and Pseudo-nitzschia spp. are the major components of diatoms in summer surface waters in Onagawa Bay (Sato Citation1996; Nakamura Citation2007).
The surface chl. a concentration began to increase again in September or October and an autumn bloom occurs. The autumn blooms were restricted to the upper 5–10 m. As with the winter/spring bloom, the autumn bloom is mainly driven by mixing processes. As vertical mixing intensifies in early autumn, the seasonal thermocline starts to erode and favors the vertical transport of nutrients from deep to surface waters. It is known from previous results that the autumn bloom lasts only a short time and Skeletonema costatum is a major component of diatoms in autumn blooms in Onagawa Bay (Sato Citation1996; Nakamura Citation2007; Masuda Citation2008).
Seasonal change of planktonic polychaete larvae
The results of this study show that the abundance of meroplanktonic polychaete larvae in Onagawa Bay fluctuates considerably among seasons and years. On the other hand, their qualitative composition did not vary greatly. It is known that the annual variations in the numbers and composition of planktonic larvae of benthic invertebrates are determined by the spawning terms of respective species (Omel'yanenko et al. Citation2004). In the shelf waters of temperate and high-latitude zones, these terms are mainly affected by the yearly dynamics of the water temperature.
A large number of planktonic larvae belonging to the Serpulidae, Nephtyidae, Magelonidae, Phyllodocidae, and Polynoidae occurred during summer and autumn, at water column temperatures above 15°C. In 2008 and 2009, larvae belonging to these families started to occur in August, as the water column temperature reached 18°C. However, in 2007, when water temperature rose earlier, these larvae started to occur earlier in July. In the Spionidae, Pseudopolydora larvae occurred largely in summer at temperatures ranging from 6.5 to 21.0°C, with the majority present at temperatures above 13°C. Polydora larvae occurred throughout the year except in September 2009, but particularly during winter and spring. Prionospio larvae occurred only at temperatures above 16°C.
Three types of planktonic larvae of marine invertebrates are known. That is lecithotrophic larvae, planktotrophic larvae with a long pelagic life, and planktotrophic larvae with a short pelagic life. Most invertebrate species belong to the group having planktotrophic larvae with a long pelagic life (Thorson Citation1950). In Arctic or subarctic waters, the planktotrophic larvae of benthic polychaetes occur during the spring bloom of the phytoplankton upon which they feed, while non-feeding lecithotrophic larvae exist throughout the year (Curtis Citation1977; Lacalli Citation1981). In temperate waters, planktotrophic polychaete larvae feed on diatoms, dinoflagellates, and bivalve veligers during spring to autumn (Daro & Polk Citation1973; Kuhl Citation1974; Turner & Anderson Citation1983). In the present study, larvae belonging to the genus Polydora showed a substantial increase in January 2007 when the large-scale phytoplankton bloom started (density of polychaete larvae in December 2006 was about 300 ind m−3), and a small increase in April 2009 coinciding with the smaller spring phytoplankton bloom, such that occurrence of the planktonic larvae belonging to the genus Polydora coincides with optimal feeding conditions. In 2008, however, the increase of the larvae belonging to Polydora took place in May, a month after the phytoplankton bloom. This delay might be explained by the strong decrease in salinity in all layers of the water column recorded in March 2008. No other planktonic polychaete larvae coincided with the spring blooms.
It is reported that larvae belonging to the Spionidae feed primarily on diatoms, mainly Thalassiosira and Coscinodiscus, during the spring phytoplankton blooms in Volcano Bay (Yokouchi Citation1991). Anger et al. (Citation1986) fed 11 species of phytoplankton (including diatom, dinoflagellates, Haptophyceae and Chlorophyceae) to the three spionid polychaete species, and revealed that T. rotula led to a relatively high growth rate of spionid larvae. Diatoms are a major component of spring phytoplankton blooms and it is reported that T. nordenskioeldii and Chaetoceros debilis are major components of spring phytoplankton blooms in Onagawa Bay (Inoue Citation1995; Sato Citation1996; Nakamura Citation2007). However, due to their long setae, C. debilis is considered not to be consumed by polychaete larvae, which are filter feeders: Yokouchi (Citation1991) found no Chaetoceros in the guts of polychaete larvae, although Chaetoceros is a major component of the spring bloom in Volcano Bay. Therefore, diatoms, particularly T. nordenskioeldii, may be the most important food item for the larvae belonging to Polydora in Onagawa Bay.
Many planktonic polychaete larvae belonging to the genera Pseudopolydora and Prionospio, and the families Serpulidae, Nephtyidae, Magelonidae, Phyllodocidae, and Polynoidae occurred during the summer and autumn. It is known from the previous reports that the pico- and nanophytoplankton increase near the surface in summer and autumn, and at the same time the chl. a concentration drastically increases near the surface (e.g. Tanaka & Taniguchi Citation1996, 1999; Tanaka et al. Citation1997; Miyauchi Citation2004; Masuda Citation2008) in Onagawa Bay. Adding to these previous reports, increases of not only pico- and nanoplankton but also microplankton near the surface in summer and autumn was observed in the present study. Thorson (Citation1964) and Forward (Citation1976) have pointed out that the larvae of intertidal species are photopositive throughout their planktonic period, to facilitate encounter with shallow habitats at settlement, and, in contrast, larvae of subtidal species were initially photopositive, which is useful for dispersal, but then became photonegative before settlement, to enhance their chances of encountering subtidal adult habitats. The photopositive responses also bring the larvae up toward the phytoplankton-rich surface waters where they can graze on the phytoplankton (Thorson Citation1946). The planktonic polychaete larvae belonging to the genera Pseudopolydora and Prionospio, and the families Serpulidae, Nephtyidae, Magelonidae, Phyllodocidae, and Polynoidae would spend their larval period in food-rich surface summer waters due to their photopositive behavior.
Larvae belonging to the Nephtyidae, Magelonidae, Phyllodocidae, and Polynoidae are said to be mixed feeders, feeding on both phytoplankton and zooplankton (Yokouchi Citation1991). Microzooplankton such as ciliates and non-photosynthetic (heterotrophic) dinoflagellates increase near the surface during summer and autumn in Onagawa Bay (Masuda Citation2008). Moreover, in the present study, microscopic observations revealed the presence of bivalve larvae in the gut of polychaete larvae belonging to three families: the Magelonidae, Phyllodocidae, and Polynoidae. Thorson (Citation1946) and Kuhl (Citation1974) reported that magelonid larvae feed exclusively on bivalve veligers in the North Sea. In addition, Wilson (Citation1982) observed Pectinaria larvae, planktonic eggs, and tintinnids in the guts in addition to bivalve veligers. Yokouchi (Citation1991) observed only unidentified particles and tintinnids in the gut of magelonid larvae, although bivalve larvae usually existed in the surrounding waters. On the other hand, he observed bivalve larvae in the gut of larvae belonging to the Polynoidae, Phyllodocidae, and Nephtyidae. During the present study, bivalve larvae were observed to increase considerably in summer, so it is also possible to speculate that these zooplankton members, too, may be an important food source for polychaete larvae during summer and autumn.
The close timing between the spring phytoplankton blooms and the production of planktonic larvae by benthic invertebrates has been widely reported since Thorson (Citation1946), and was observed in this study as well. However, one noteworthy characteristic of the present study in Onagawa Bay is that most planktonic polychaete larvae tended to synchronize with summer phytoplankton increases and autumn blooms, rather than with spring blooms. This may be a temperature-related phenomenon and/or a manifestation of a higher diversity of available food organisms.
It has been demonstrated that increasing phytoplankton abundance provides a chemical cue for the spawning of certain invertebrates (Miyazaki Citation1938; Crisp Citation1956; Crisp & Spencer Citation1958; Starr et al. Citation1990, Citation1991, Citation1992, Citation1993, Citation1994). A large number of planktonic larvae belonging to Serpulidae, Nephtyidae, Magelonidae, Phyllodocidae, and Polynoidae in Onagawa Bay occurred during summer and autumn. While benthic polychaetes which belong to Serpulidae, Phyllodocidae, and Polynoidae were abundant on hard substrates in water column, such as buoys and ropes which used for hanging culture of scallops and wall surface of revetments, benthic polychaetes which belong to Magelonidae and Nephtyidae occurred abundantly just in soft bottom substrates in Onagawa Bay. Spawning of members belonging to Serpulidae, Phyllodocidae, and Polynoidae, which are observed to relate to hard substrate epibenthic community near the surface water in Onagawa Bay, might be accelerated by increase of phytoplankton abundance near the surface in summer and autumn. However, spawning of members belonging to Magelonidae and Nephtyidae, which are observed to relate to soft bottom substrate in Onagawa Bay, may not be accelerated by increase of phytoplankton because the chl. a concentration near the bottom was very low in summer and autumn. Spawning of these soft bottom-related polychaetes may be induced by physical environmental factors such as water temperature and salinity which control both phytoplankton increase and gamete or larval release (Forward Citation1987; Giese & Kanatani Citation1987).
It was interesting to observe the almost exclusive dominance by Polydora larvae during winter. There were a large number of Polydora cf. neocaeca which burrows into the shells of scallop cultured in Onagawa Bay, and this species spawn mainly in winter in Onagawa Bay (unpublished observations). Therefore, there is a possibility that the Polydora larvae included a large number of Polydora cf. neocaeca larvae. This species is thought to have been introduced continuously from west coast of Hokkaido, northern Japan, by commercial transport of young scallops to Onagawa Bay for the purpose of mariculture. The occurrence of the larvae of this species is probably affected not only by phytoplankton dynamics but also by the temperature range of prespawning development and spawning partially related to their origin.
In the present study, planktonic polychaete larvae were mainly identified to family level except dominant Spionidae. It was difficult to identify planktonic polychaete larvae in more detail because of the lack of both a complete list of polychaete species living in Onagawa Bay and literature on the morphology of Japanese planktonic polychaete larvae. To identify planktonic polychaete larvae accurately to species level, better knowledge of the larval development of polychaetes is needed, and molecular biological techniques for identification could also be effective in streamlining identification. To understand the detailed dynamics of planktonic polychaete larvae in Onagawa Bay requires further research on the horizontal and vertical distribution of the larvae, as well as larval dispersal and recruitment.
Acknowledgements
We thank Captain T. Hiratsuka and the staff of the Field Science Center, Graduate School of Agricultural Science, Tohoku University, for their help with collecting the samples in Onagawa Bay; and the students and staff of the Laboratory of Biological Oceangraphy of Tohoku University, for their cooperation. Sincere thanks are due to Dr I. G. Gleadall for his critical reading of the manuscript. We are also grateful to Dr V. I. Radashevsky, an anonymous referee, and the editor Dr A. Giangrande for their valuable comments during the review process.
References
- Anger , K , Anger , V and Hagmeier , E. 1986 . Laboratory studies on larval growth of Polydora ligni, Polydora ciliata, and Pygospio elegans (Polychaeta, Spionidae) . Helgolander Meeresuntersuchungen , 40 : 377 – 395 .
- Bhaud , M and Cazaux , C. 1987 . Description and identification of polychaete larvae; their implications in current biological problems . Oceanis , 13 : 596 – 753 .
- Blake , JA. 1969 . Reproduction and larval development of Polydora from northern New England . Ophelia , 7 : 1 – 63 .
- Blake , JA. 1975 . The larval development of polychaeta from the northern California coast. III. Eighteen species of errantia . Ophelia , 14 : 23 – 84 .
- Blake , JA and Woodwick , KH. 1975 . Reproduction and larval development of Pseudopolydora paucibranchiata (Okuda) and Pseudopolydora kempi (Southern) (Polychaeta: Spionidae) . Biological Bulletin , 149 : 109 – 127 .
- Caley , MJ , Carr , MH , Hixon , MA , Hughes , TP , Jones , GP and Menge , BA. 1996 . Recruitment and the local dynamics of open marine populations . Annual Review of Ecology and Systematics , 27 : 477 – 500 .
- Chihara , M. and Murano , M , eds. 1997 . An illustrated guide to marine plankton in Japan , 1571 Tokyo : Tokai University Press . [in Japanese]
- Chu D. 2004. The GLOBEC kriging software package EasyKrig3.0. http://globec.whoi.edu/software/kriging/easy_krig/easy_krig.html (http://globec.whoi.edu/software/kriging/easy_krig/easy_krig.html) (Accessed: 15 July 2004 ).
- Crisp , DJ. 1956 . A substance promoting hatching and liberation of young in cirripedes . Nature, London , 178 : 263
- Crisp , DJ and Spencer , CP. 1958 . The control of the hatching process in barnacles . Proceedings of the Royal Society, Series B , 148 : 278 – 299 .
- Curtis , MA. 1977 . Life cycles and population dynamics of marine benthic polychaetes from the Disko Bay area of West Greenland . Ophelia , 16 : 9 – 58 .
- Daro , MH and Polk , P. 1973 . The autecology of Polydora ciliata along the Belgian coast . Netherlands Journal of Sea Research , 6 : 130 – 140 .
- Forward , RB Jr. 1976 . “ Light and diurnal vertical migration: Photobehavior and photophysiology of plankton ” . In Photochemical and photobiological reviews , Edited by: Smith , KC . Vol. 1 , 157 – 209 . New York : Plenum Press .
- Forward , RB Jr. 1987 . Larval release rhythms of decapod crustaceans: An overview . Bulletin of Marine Science , 41 : 165 – 176 .
- Fraschetti , S , Giangrande , A , Terlizzi , A and Boero , F. 2002 . Pre- and post-settlement events in benthic community dynamics . Oceanologica Acta , 25 : 285 – 295 .
- Giese , AC and Kanatani , H. 1987 . “ Maturation and spawning ” . In Reproduction of marine invertebrates, Vol. IX. General aspects: Seeking unity in diversity , Edited by: Giese , AC , Pearse , JS and Pearse , VC . 251 – 329 . Palo Alto, CA : Blackwell Scientific Publications .
- Hannerz , L. 1956 . Larval development of the polychaete families Spionidae Sars, Disomidae Mensil, and Poecilochaetidae n. fam. in the Gullmar Fjord (Sweden) . Zoologiska Bidrag fran Uppsala , 31 : 1 – 204 .
- Inoue , T. 1995 . Population dynamics of the four diatom species which form resting spores in Onagawa Bay, Japan , Master thesis, Tohoku University, Sendai. 120 . [in Japanese]
- Kuhl , VH. 1974 . “ Uber vorkommen und nahrung der larven von Magelona papillicornis O. F. Muller (Polychaeta Sedentaria) im Mundungsgebiet von Elbe ” . In Weser und Ems. Berichte der Deutschen Wissen–schaftlichen Kommission för Meeresforschung Vol. 23 , 296 – 301 .
- Lacalli , TC. 1981 . Annual spawning cycles and planktonic larvae of benthic invertebrates from Passamaquoddy Bay, New Brunswick . Canadian Journal of Zoology , 59 : 433 – 440 .
- Longhurst , A. 1995 . Seasonal cycles of pelagic production and consumption . Progress in Oceanography , 36 : 77 – 167 .
- Masuda , Y. 2008 . Pelagic and benthic microbial food webs in Onagawa Bay , 183 Sendai : Doctor thesis, Tohoku University . [in Japanese]
- Miyauchi , Y. 2004 . Seasonal variation in standing stocks of major components of the microbial loop in Onagawa Bay, the Pacific coast of northern Japan , 102 Sendai : Master thesis, Tohoku University . [in Japanese]
- Miyazaki , I. 1938 . On a substance which is contained in green algae and induces spawning action of the male oyster (Preliminary note) . Bulletin of the Japanese Society of Scientific Fisheries , 7 : 137 – 138 .
- Motoda , S. 1957 . North Pacific standard plankton net . Information Bulletin on Planktology in Japan , 4 : 13 – 15 . [in Japanese]
- Motoda , S. 1959 . Device of simple plankton apparatus . Memoirs of the Faculty of Fisheries, Hokkaido University , 7 : 73 – 94 .
- Nakamura , S. 2007 . Seasonal change and sinking of diatom community in Onagawa Bay , 67 Sendai : Master thesis, Tohoku University . [in Japanese]
- Omel'yanenko , VA and Kulikova , VA. 2002 . Composition, seasonal dynamics, and long-term fluctuations in the density of pelagic polychaetes in Amurskii Bay, Sea of Japan . Biologiya Morya , 28 : 348 – 355 .
- Omel'yanenko , VA , Kulikova , VA and Pogodin , AG. 2004 . The meroplankton of Amurskii Bay (Peter the Great Bay, Sea of Japan) . Biologiya Morya , 30 : 159 – 174 .
- Plate , S and Husemann , E. 1994 . Identification guide to the planktonic polychaeta larvae around the island of Helgoland (German Bight) . Helgolander Meeresuntersuchungen , 48 : 1 – 58 .
- Radashevsky , VI. 1983 . Reproduction and larval development of the polychaete Pseudopolydora paucibranchiata (Spionidae) in Peter the Great Bay of the Sea of Japan . Marine Biology, Vladivostok , 2 : 38 – 46 . [in Russian, with English abstract]
- Radashevsky , VI. 1985 . The larval development of the polychaete Pseudopolydora kempi japonica (Spionidae) in Peter the Great Bay of the Sea of Japan . Marine Biology, Vladivostok , 2 : 39 – 46 . [in Russian, with English abstract]
- Rodriguez-Valencia , JA. 2003 . Composition and dynamics in space and time of polychaete larvae in coastal waters of the North Sea , PhD thesis, University of Kiel .
- Sathyendranath , S , Longhurst , A , Caverhill , CM and Platt , T. 1995 . Regionally and seasonally primary production in the North Atlantic. Deep-Sea Research: Part 1 . Oceanographic Research Papers , 42 : 1773 – 1802 .
- Sato , K. 1996 . Seasonal variation of phytoplankton community in Onagawa Bay , 86 Sendai : Master thesis, Tohoku University . [in Japanese]
- Shanks , AL. 2001 . An identification guide to the larval marine invertebrates of the Pacific Northwest , Corvallis, OR : Oregon State University Press .
- Srikrishnadhas , B and Ramamoorthi , K. 1977 . “ Development of Pseudopolydora kempi (Southern, 1921) in the laboratory ” . In Proceedings of the Symposium on Warm Water Zooplankton , 671 – 677 . Special Publication UNESCO/NIO .
- Starr , M , Himmelman , JH and Therriault , JC. 1990 . Direct coupling of marine invertebrate spawning with phytoplankton blooms . Science , 247 : 1071 – 1074 .
- Starr , M , Himmelman , JH and Therriault , JC. 1991 . Coupling of nauplii release in barnacles with phytoplankton blooms: A parallel strategy to that of spawning in the sea urchin Strongylocentrotus droebachiensis . Journal of Plankton Research , 13 : 561 – 571 .
- Starr , M , Himmelman , JH and Therriault , JC. 1992 . Isolation and properties of a substance from the diatom Phaeodactilum tricornutum which induces spawning in the sea urchin Strongylocentrotus droebachiensis . Marine Ecology Progress Series , 79 : 275 – 278 .
- Starr , M , Hinlmelman , JH and Therriault , JC. 1993 . Environmental control of green sea urchin, Strongylocentrotus droebachiensis, spawning in the St Lawrence Estuary . Canadian Journal of Fisheries and Aquatic Science , 50 : 894 – 901 .
- Starr , M , Therriault , JC , Conan , GY , Comeau , M and Robichaud , G. 1994 . Larval release in a sub-euphotic zone invertebrate triggered by sinking phytoplankton particles . Journal of Plankton Research , 16 : 1137
- Tanaka , T and Taniguchi , A. 1996 . Short-term variation in abundance of bacteria and heterotrophic nanoflagellates in summer observed in Onagawa Bay, Japan . Bulletin of the Plankton Society of Japan , 43 : 21 – 29 .
- Tanaka , T and Taniguchi , A. 1999 . Predator–prey eddy in heterotrophic nanoflagellate–bacteria relationships in a bay on the northeastern Pacific coast of Japan . Marine Ecology Progress Series , 179 : 123 – 134 .
- Tanaka , T , Fujita , N and Taniguchi , A. 1997 . Predator–prey eddy in heterotrophic nanoflagellate–bacteria relationships in a coastal marine environment: A new scheme for predator–prey associations . Aquatic and Microbial Ecology , 13 : 249 – 256 .
- Thorson , G. 1946 . Reproduction and larval development of Danish marine bottom invertebrates . with special reference to the planktonic larvae in the Sound (Oresund) Meddelelser fra kommissionen for Danmarks Fisker-og Havundersogelser. Serie Plankton , 4 : 1 – 523 .
- Thorson , G. 1950 . Reproductive and larval ecology of marine bottom invertebrates . Biological Reviews of Cambridge , 25 : 1 – 45 .
- Thorson , G. 1964 . Light as an ecological factor in the dispersal and settlement of larvae of marine bottom invertebrates . Ophelia , 1 : 167 – 208 .
- Turner , JT and Anderson , DM. 1983 . Zooplankton grazing during dinoflagellate blooms in a Cape Cod embayment, with observations of predation upon tintinnids by copepods . Marine Ecology , 4 : 359 – 374 .
- Wilson , DP. 1982 . The larval development of three species of Magelona (Polychaeta) from localities near Plymouth . Journal of the Marine Biological Association of the UK , 62 : 385 – 401 .
- Yamaji , I , ed. 1966 . The plankton of Japanese coastal waters , 538 Osaka : Hoikusha Publications . [in Japanese]
- Yentsch , CS and Menzel , DW. 1963 . A method for determination of phytoplankton chlorophyll and phaeophytin by fluorescence . Deep-Sea Research , 10 : 221 – 231 .
- Yokouchi , K. 1984 . Surface distribution of polychaete larvae in Volcano Bay, southern Hokkaido, during the vernal phytoplankton blooms of 1982 . Bulletin of the Plankton Society of Japan , 31 : 113 – 122 .
- Yokouchi , K. 1991 . Seasonal distribution and food habits of planktonic larvae of benthic polychaeta in Volcano Bay, southern Hokkaido, Japan . Ophelia (Suppl) , 5 : 401 – 410 .