Abstract
Benthic recovery following cessation of wastewater discharges in shallow soft-bottom environments off Barcelona was assessed by revisiting an old sampling site in 2008 that had been studied when the impacts of such discharges were more acute (1987–88). In 1987–88, sediments were highly polluted by organic matter and inorganic contaminants. Although the amount of silt–clay in the sediments diminished from 95 to 25% during the intervening 20-year period and significant improvements were observed in the content of polychlorinated biphenyls and polycyclic aromatic hydrocarbons, their metal content was still very high. However, the benthic community currently showed a clear increase in complexity and diversity. It changed from an assemblage that was 98%-dominated by the Capitella capitata complex to an assemblage dominated by Mediomastus fragilis, Capitella capitata and Ophryotrocha hartmanni. By 2008, more species were found and there was increased representation from different trophic groups, whereas the abundance and biomass values were clearly reduced by almost two and one order of magnitude, respectively. Mean annual density evolved from 385,261 ind. m–2 with a mean annual biomass of 12.75 g dry wt m–2 in 1987–88 to 8155 ind. m–2 and 0.94 g dry wt m–2 in 2008. Using a regression model that allowed comparability between both sets of data, secondary production of the community was reduced from 207.7 g dry wt m–2 year–1 in 1987–88 to 8.0 g dry wt m–2 year–1 in 2008. The organic input decreased, but the metal concentration present in the sediments may inhibit their full recovery to normal conditions.
Introduction
Human beings have significantly altered benthic habitats throughout the world's environments. From the many kinds of pollution and ecosystem alterations produced by humans, an excess of organic matter is probably the most universal and documented impact on marine benthic communities, which occurs principally as sewage but also includes other types of waste (Gray Citation1981). In addition, sewage discharges through pipelines and outfalls can also contain hazardous metals that can contribute to the pollution of the marine environment (Valiela Citation2006; Halpern et al. Citation2008). To follow the effects of such disturbances, as well as the recovery potential of local communities, the use of the marine benthos has been employed as an indicator of environmental changes due to its long life span, sensitive stages, and reduced motility (Pearson & Rosenberg Citation1978; Warwick & Clarke Citation1994).
Benthic infaunal communities are organized structurally, numerically and functionally in relation to organic enrichment gradients. Changes in soft-bottom communities due to organic enrichment were modelled by Pearson and Rosenberg (Citation1978) and Rhoads and Germano (Citation1986), and the model has been demonstrated elsewhere. This model established that spatial and temporal changes occurred when heavy or moderate inputs of organic enrichment were introduced into the marine environment. Organic discharges into confined bodies of water frequently lead to the well-known symptoms of eutrophication, in the most extreme cases resulting in a total lack of oxygen and the presence of hydrogen sulphide in the sediment, with a corresponding absence of fauna. With increasing distance from a point source discharge there is a corresponding recovery in sediment characteristics and benthic faunal communities. This model is consistent with benthic spatial distributions and temporal responses of the benthos to sewage discharges in open coastal waters (Swartz et al. Citation1986). These changes always show the same patterns that basically consist of decreases in diversity, dominance by opportunistic species, and reduction in the mean size of the dominant species. However, following such events, the communities typically undergo a period of change, often referred to as succession, which ends with a return to the same faunal composition as the pre-impacted state (Rosenberg et al. Citation2002). Pearson and Rosenberg (Citation1978) showed in parallel studies from Scotland, where environmental disturbance increased, and Sweden, where the conditions improved after pollution abatement, that the benthic community structure was similar under similar degrees of disturbance even when the succession of these communities changed in different directions.
The relationships between organic enrichment and benthic productivity have been well documented in the past (Heip Citation1992). As populations of pioneering species with high rates of growth are the basic responders to organic enrichment, these organisms may enhance secondary production of benthic habitats (Rhoads et al. Citation1978). Although secondary production can integrate information about dynamics in stressed ecosystems with high energy flux, secondary production has not been computed as a response to these sources of environmental disturbances, and few data are available (Steimle et al. Citation1990; Méndez et al. Citation1997).
The Mediterranean basin has particular oceanographic characteristics (relatively shallow, semi-enclosed and with limited natural water exchanges) and it is experiencing heavy demographic, urban and industrial pressures on its coastal areas (Tolosa et al. Citation1997; Bianchi & Morri Citation2000). The metropolitan area of Barcelona is one of the most active urban environments in the south of Europe, and is comprised of 33 municipalities. The flow of raw materials, water and energy that takes place every day in such a social-ecological system helps to maintain economical activities, but also produces a large volume of waste, such as sewage. A total of 3.2 million inhabitants live in this area and they produce large amounts of urban and industrial wastewaters that are primarily treated and discharged at high rates into the marine environment. During the last two decades, following the introduction of the European environmental policy on water quality (CIS-WFD Citation2005), wastewater discharges treated in the region have drastically improved in quality, and the amount of organic pollution has been highly reduced. This improvement may have also produced a change in the marine benthic communities inhabiting the coastal region following alleviation of the pressure that organic matter previously exerted on them.
In this article, the results obtained during two surveys carried out at the same sampling station off the city of Barcelona are presented. These two surveys were separated by 20 years. Additional data on the spatial effects of past wastewater discharges in the region can be seen in Ros and Cardell (Citation1992), Cardell (Citation1996) and Cardell et al. (Citation1999). Data presented in this article will be compared against seasonal data obtained from a reference station included in the MacroBen database (Vanden Berghe et al. Citation2009) representing the shallow soft-bottom environments along the Catalan coast. By using these results, the main goals of this article were: (a) to assess the changes observed in benthic communities during these years, and (b) to calculate the secondary production of this community with time, relating these values to sewage discharges, water quality and sediment improvement.
Material and methods
Study site
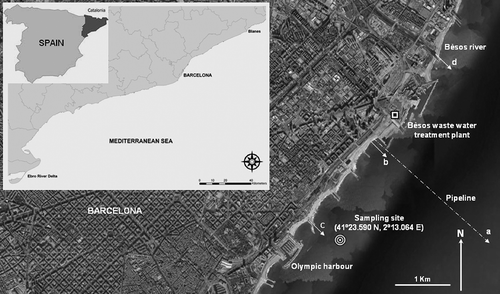
The article is based on samples collected from a single sampling site. This sampling station was located off the Barcelona municipal area (NE, Spain) in a soft-bottom environment at a 20 m depth (). Although at that depth, shallow soft-bottom non-vegetated areas from the Western Mediterranean are commonly inhabited by the medium to fine sand community represented by Spisula subtruncata (sensu Pérès & Picard Citation1964), in the case of Barcelona, these habitats have been receiving the pressures and effluents from the city for hundreds of years, which in turn have produced a change in their physical and chemical properties. The sampling station was first widely affected by the city's non-treated effluents, then, in the last century, by the wastewater treatment plant facilities installed during the 1970s, and, more recently, by the new updated and modernized Besòs wastewater treatment plant. A monthly sampling procedure was carried out at this station in 1987–88. After 20 years, the station was revisited and sampled again to detect any changes and to relate those changes with the improvement in the city's depuration of its wastewaters.
During the first period of the study (1987–88), sediments off Barcelona city received effluents from different sources. The Besòs treatment plant had primary sludge treatment (half of this was solid suspended material) of over 4.105 m3 day–1, which was sent through a 4 km pipeline from the shore to a depth of 56 m (), with wastewater discharged via an outlet pipe just 600 m long (Ros & Cardell Citation1992; Cardell Citation1996; López-Sánchez et al. Citation1996) (). In addition, another important untreated sewage discharge flowed mainly through the mouth of the Besòs River (3.105 m3 day–1) (), and the rest of the sewage inputs of the city were depurated in the Bogatell treatment plant (8.104 m3 day–1) that discharged directly on the shoreline (c). The sampling site was located to the south of these three inputs and received their inflow directly due to the prevailing NE–SW direction of the coastal currents in this geographical area (Font & Miralles Citation1978).
During the 1990s, the Besòs treatment plant was remodelled with new compact technologies (expanding the biological treatment process) to meet the standards required by the European Union to which Spain was incorporated in 1986. Today, the plant treats the sewage water for an area with a population of 1.6 million inhabitants, comprising the municipalities from Barcelona (75%), Sant Adrià de Besòs, Santa Coloma de Gramanet, Badalona, Montgat and Tiana. It is a very large plant (one of the biggest in the world), with a rated flow of over 6.105 m3 day–1 for an equivalent population of about 3 million inhabitants. The effluent from the new plant flows into the sea at a depth of 45 m through a subsea pipeline 2900 m long (2.1 m diameter). Previous outlets and the Bogatell plant have been closed and the water flowing through the mouth of the Besòs River has been highly depurated, so the organic enrichment impacts on the benthic habitats living in the area of the sampling station have been drastically reduced.
The variability of climatic and hydrographic conditions of the littoral is typical of temperate zones, and the surface seawater temperature has been shown to range between 11–12°C and 25–26°C (Amengual et al. Citation1988; Cebrián et al. Citation1996). The freshwater regime depends on the Besòs River increasing its flow during autumn and spring. It is combined with intermittent heavy discharges (pulses) after storm periods. Planktonic gross primary production peaks during late winter in this area (Estrada Citation1980; Satta et al. Citation1996). All these factors mentioned above were important to the understanding of the dynamics and regulation of the biological benthic secondary production.
Sampling and laboratory procedures
The station was sampled monthly from September 1987 to September 1988 during the course of the SPIO project, and then, after 20 years, the same station was sampled again in June and November 2008. These months were selected as sampling time points as it had been shown (Sardá et al. Citation1995, 1999) that abundance averages are comparable with the annual abundance average of the entire year when monthly sampling is carried out. Sediment samples were collected using 0.1 m2 and 0.06 m2 Van Veen grabs, respectively. Three grabs were taken for the analysis of benthic infauna and one for sedimentological analyses, although in 2008 only a 0.06 m2 Van Veen grab was used. The grabs were able to penetrate 30 cm into the sediments. No biogenic structures were seen on the bottom of the grab samples. Samples were immediately sieved on a 500-μm mesh and the fauna retained were fixed in 5% formaldehyde. Polychaete species were later identified to the lowest practical taxonomic level and counted. Individual species biomass was determined as dry weight.
During the first sampling (1987–88), selected individuals from representative size categories for the most important species were measured through a binocular microscope equipped with a camera lucida and digitizing tablet. They were then dried (24 h at 70°C) and their biomass was obtained as dry weight (g m–2). Using these data, regressions of width vs. dry weight were computed for each major species found and they were used to convert width to biomass (Sardá et al. Citation1999). In 2008, biomass was obtained as wet weight. For comparison purposes, all the data given in this article are expressed in dry weight using, where necessary, the dry weight (17.6%) from wet weight conversion factor calculated for polychaetes in Rumohr et al. (Citation1987). Organisms were classified into five trophic groups according to the classical literature (Fauchald & Jumars Citation1979): filter feeders; mixed (filter and surface-deposit feeders); surface-deposit feeders; subsurface-deposit feeders; and carnivores/omnivores.
Within different research projects, the structure and dynamics of a shallow soft-bottom macroinfaunal assemblage of the medium to fine sand sediment community of Spisula subtruncata was investigated (Sardá et al. Citation1999). This station has been incorporated into the MacroBen database (Vanden Berghe et al. Citation2009) as a reference point due to the five-year data series obtained there. In this current study, its observed dynamics and parameters were compared against those registered on the Barcelona coast at the sampling site.
During 1987–88, the samples obtained for sedimentological analysis were frozen immediately, stored until analysed, and then defrosted and dried in the laboratory. Metal content was analysed by flame atomic absorption using a Perkin-Elmer 460 spectrophotometer (Palanques & Diaz, Citation1994). The polychlorinated biphenyl (PCB) and polycyclic aromatic hydrocarbon (PAH) contents were obtained by gas chromatography. The samples collected in 2008 were sent to an accredited laboratory for metals analysis, whereas PCBs and PAHs were analysed using standard techniques.
Measurement methods and secondary production estimates
In 1987–88, secondary production of the two most important contributors for biomass in the assemblage, Capitella capitata and Malacoceros fuliginosus, was studied by conventional direct production estimates. The maximum thoracic width was used as a size estimate in order to measure growth and to identify generations in the populations of both species of polychaetes. Size measurements were carried out through a stereo microscope using a camera lucida and a digitalizing tablet. For the two species, a thoracic width (TW, mm) versus dry weight (DW, mg) regression was calculated to obtain the biomass of the different specimens:
Secondary production was estimated by the Hynes method (Hynes & Coleman Citation1968; Hamilton et al. Citation1969; Benke Citation1979) following the formula given by Menzie (Citation1980):
For the remainder of the macroinfaunal species, an approximate estimate of secondary production was obtained by compiling the average monthly standing stock. Increases in biomass which occurred from one sampling date to the next were added together. Production estimates compiled in this fashion yielded similar values to most of the values calculated from empirical models (Sardá Citation1997).
During samples taken in 2008, and with the purpose of comparing present data with the historical data obtained in 1987–88, secondary production of polychaetes was obtained following a regression model developed by Brey (Citation1990):
where P is the secondary production [g (dry wt) m–2 year–1], B is the mean biomass of dry weight (g m–2), and W the mean dry weight (g).
The P/B ratio was calculated because it could reflect the metabolism and life cycle of species in a community population. In this case, the following allometric equation was used:
where A is the abundance, B is the biomass, B/A is the mean body size and 0.73 is the average exponent of the regression of annual production on body size for macrobenthic invertebrates (Brey Citation1990; Warwick et al. Citation2010).
Computation of indices for the analysed benthic assemblages
Macroinfaunal data were used for the computation of different univariate indices: Species richness, Shannon–Wiener index (Hʹ), Margalef index (d), and Simpson index (1–λʹ). Together with ABC plots, these indices were computed using the Primer© 6 software package (version 6.1.6) (Warwick Citation1986).
Several indices based on ecological groups (measuring disturbance of the benthic community) were also computed: AMBI, M-AMBI (Borja et al. Citation2000, Citation2003; Borja & Muxika Citation2005; Borja & Mader Citation2008) and PAMBI, and all data were transformed using the square root, following Warwick et al. (Citation2010). All analyses were performed with the AMBI index software (version 4.1) (Borja & Mader Citation2008). AMBI accounts for the relative abundance of several ecological groups of species (corresponding to different levels of sensitivity/tolerance) in a sample, and ranges between 0 and 6. Low AMBI values are associated with the dominance of sensitive species and thus high-quality environments, whereas high AMBI values are associated with the dominance of tolerant species and thus low-quality environments. In this study, the PAMBI was also calculated as it has recently been shown to be more robust, besides being ecologically and functionally much more relevant (Warwick et al. Citation2010). The root-transformed species production data was employed for both study periods.
Results
Sedimentology
The sediment characteristics of the sampled site during the two periods analysed are shown in . The content of silt–clay in the sediments decreased from 95% (1987–88) to 25% (2008). As a consequence, the granulometrical typology of the sampling station varied from being representative of a clear muddy environment to characterizing a silty-sand enriched environment. In addition, the organic carbon content of the sediments was reduced by half. The cessation of wastewater discharges at shallow depths in the region was mainly responsible for this sedimentological change by the reduction of the flow of suspended solid materials onto the bottoms.
Table I. Sediment characteristics and pollutant indicators (na, data not available)
During 1987–88, the analysed sediments contained a high organic content and were heavily polluted by hazardous metals, PCBs and PAHs (). However, besides the higher rates of organic matter and inorganic pollutants, hypoxia was never observed in the overlying water during the study period. After spending a considerable amount of money on wastewater improvement, effluents were treated to a higher degree and the discharge was reduced drastically. However, the analysis carried out in 2008 showed that the sediments were still far from being completely clean and in a normal condition. Although significant improvements were observed in the content of PCBs and PAHs, the sediments were still highly polluted due to their metal content. Local sources, other than the wastewater discharge, were probably responsible for this metal contamination imprint.
Community structure and dynamics
The faunal composition of the benthic samples that were analysed in the period 1987–88 showed a poorly structured community with a relatively small number of species (). The species found at the sample site included 38 species of polychaetes as the major group of fauna, followed by 10 species of bivalves, and a few cumacean, amphipod and prosobranch individuals. No other faunal groups were observed. Polychaetes were by far the most important group, constituting 99% of the mean annual density (385,261 ind. m–2), and 71% of the mean annual biomass (12.75 g m–2). The mean annual diversity of this altered benthic community, calculated by the different diversity indices computed, was very low (Table III).
Table II. Species composition with mean annual macroinfaunal abundance (ind. m–2) and biomass (g dry wt m–2) for the two periods analysed
There was a seasonal pattern in total macroinfaunal abundance and biomass (). Abundance increased gradually from September to a maximum during July (750,000 ind. m–2). Following this early summer peak, there was a sharp decrease through the summer. A one-way ANOVA of the abundance data revealed significant seasonal differences (F statistic = 41.57, p < 0.01). The seasonality for biomass values was less pronounced than that of abundance (). Two main peaks were detected during the year, with an early initial peak during February, and another in June. These peaks were clearly attributed to the seasonal dynamics of the main contributor species.
Figure 2. Seasonal abundance (left-hand graphs) and biomass data (right-hand graphs) of key species at the studied station (September 1987 to September 1988).
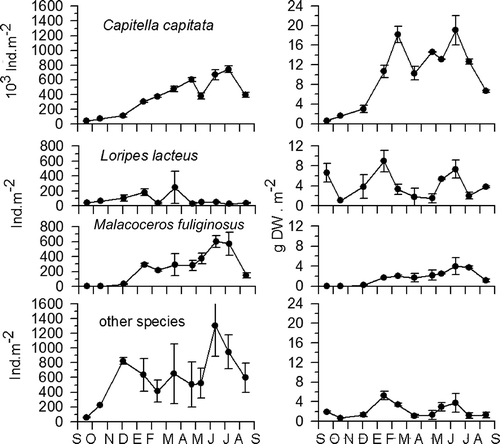
During this initial period, the community was clearly dominated by opportunistic species. The main contributor to the abundance (97%) and biomass (55%) was due to the Capitella capitata complex (), since the general dynamics of this altered community mirrored the dynamics of C. capitata. The average density of C. capitata ranged from 38,864 ind. m–2 in September 1987 to 742,653 ind. m–2 in July 1988. On the other hand, the average biomass of the species ranged from 0.59 g dry wt m–2 in September to 19.08 g dry wt m–2 in June. However, two clear peaks of biomass were observed during the year, which was the opposite of that observed for the abundance values. The bivalve Loripes lacteus contributed 4.1 g dry wt m–2 to the mean annual biomass of the community (23%), while the spionid polychaete Malacoceros fuliginosus added 1.7 g dry wt m–2. These two species, together with C. capitata, were clearly the three key species in this community at that time (). The dynamics of both species of polychaetes had similar increasing densities until a peak in late spring–early summer. On the other hand, L. lacteus showed a different behaviour, with annual peaks of density through the winter. As a consequence of the previously mentioned faunal composition, the biomass of the community was dominated by subsurface-deposit feeders (58%), followed by filter-feeders (25%), and surface-deposit feeders (12%). The rest of the trophic groups were clearly less important.
Twenty years after the cessation of the high inputs of wastewater discharges into these shallow soft-bottom environments, a clear increase was observed in the complexity of the studied assemblage. In 2008, more species (60 species of polychaete), higher diversity values and increased representation of different trophic groups were found (). During these 20 years, the assemblage that was 98%-dominated in abundance by a Capitella capitata complex changed to an assemblage dominated by Mediomastus fragilis (17.3%), Capitella capitata complex (13.4%), and Ophryotrocha hartmanni (10.4%) with a clear increase in diversity (). Concerning biomass values, other species, such as the capitellid Notomastus latericius (29.7%), the glycerid Glycera unicornis (14.2%) and the lumbrinerid Lumbrineris latreilli (6.8%), replaced the highly important C. capitata complex (78.7%) over the same period.
Table III. Mean annual main community parameters and biotic indices calculated
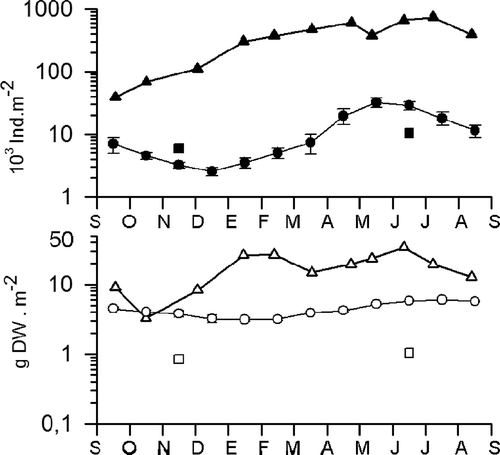
Abundance and biomass values were clearly reduced by almost two and one order of magnitude, respectively (Table II). Annual densities of 385,261 ind. m–2 with a mean annual biomass of 12.75 g m–2 in 1988 decreased to 8155 ind. m–2 in 2008 with an annual biomass of 0.94 g m–2. Mean abundance values obtained in 2008 were a little lower than the average value obtained for the reference station in the MacroBen database (10,623 ind. m–2; Sardá et al. Citation1999) (). In addition, the mean biomass values obtained in 2008 were also a little lower than the average value obtained for the same reference station (2.41 g dry wt m–2; Sardá et al. Citation1999) ().
Figure 3. Abundance data (upper graph) and biomass data (lower graph) of the studied series and comparison with the reference station from the MacroBen database. Triangles represent data for 1987–88 from the studied site; squares represent data for 2008 from the studied site and circles are data from the Blanes reference station – pooled data from 1992 to 1996.
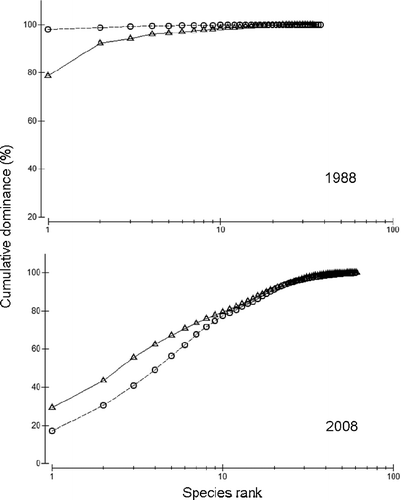
The differences observed between both periods can be seen by the use of k-dominance curves where the biomass and abundance of each species is ranked from highest to lowest and plotted against the cumulative percentage biomass or percentage abundance (). In 1987–88, the plots showed how the abundance curve fell clearly above the biomass curve, which is the normal pattern when abundant species are small and rapidly growing, typical of disturbed and enriched communities. By 2008, the plots changed their positions indicating that the previous stress had been reduced.
Figure 4. k-dominance curves for abundance (open circles) and biomass (open triangles) of macrofauna inhabiting the studied site during the two periods analysed.
AMBI values have been recognized as an efficient tool for detecting changes in benthic communities receiving impacts derived from human activities. AMBI values changed the status of the studied sample station from heavily disturbed to slightly disturbed (Table III). However, the AMBI values were still indicating a major presence of tolerant species rather than sensitive ones. When the PAMBI index was used, the poor status observed in 1987–88 changed to a good status in 2008.
Secondary production
Using the Hynes methodology, in 1987–88, the estimated production for Capitella capitata was calculated as 77.8 g dry wt m–2 for the average annual cohort, and with a mean annual biomass of 10.03 g dry wt m–2 this gave the production to mean biomass ratio (P/B) a value of 7.75. The analysis of the evolution of the C. capitata size classes allowed three main generations to be recognized throughout the year (). The main recruitment peaks, observed in as the increased percentage presence of recruiters in the populations, were observed in January, March, and July. Following these three peaks of recruitment, the biomass of C. capitata increased to a maximum of approximately 20 g dry wt m–2. Using these shifts in size distribution during the year, the CPI for the C. capitata population (4 months) was estimated as 121.6 days. Consequently, the annual secondary production for the C. capitata population in this community would be 233.5 g dry wt m–2 year–1.
Figure 5. Main recruitment peaks and biomass values for the two main populations during September 1987 and September 1988.
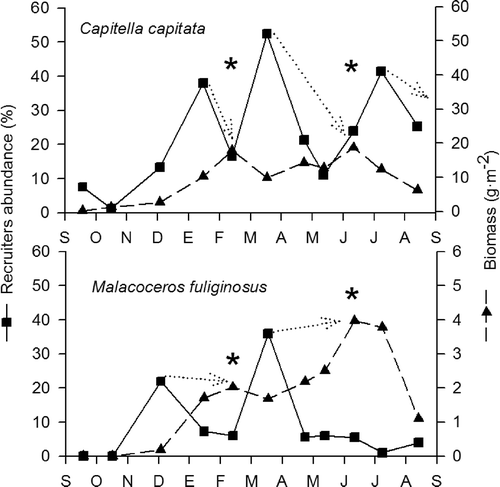
For Malacoceros fuliginosus, the production was estimated as 6.7 g dry wt m–2, with a mean annual biomass of 1.74 g dry wt m–2, which resulted in a production to mean biomass ratio (P/B) of 3.85. The analysis of the variation in the size-structure of the population during the year showed two generations with recruitment peaks in December and March. Following these two peaks of recruitment, the biomass increased to approximately 2 and 4 g dry wt m–2, respectively. Using these shifts in size distribution, a CPI of 182.5 days (6 months) was estimated for M. fuliginosus. This calculation yielded an annual secondary production value for the species of 13.4 g dry wt m–2 year–1. In the case of Loripes lacteus, the population cohorts were able to be identified. Following the production of the cohorts through the year, a secondary production value was obtained for L. lacteus of 21.5 g dry wt m–2 year–1, with a mean annual biomass value of 4.11 g dry wt m–2. The P/B ratio obtained in this case would be 5.23.
By adding together the increases in biomass that occurred from one sample date to the next, the secondary production of the rest of the macroinfaunal component of the community was estimated as 7.95 g dry wt m–2 year–1. The total secondary production from September 1987 to September 1988 in the entire community was then calculated as 276.3 g dry wt m–2 year–1 (85% from the Capitella capitata complex).
When we compared the productivity using the regression model (Brey Citation1990; Warwick et al. Citation2010) to compare data from 1987–88 and 2008, the productivity obtained for the period 1987–88 yielded an annual secondary production of 207.7 g dry wt m–2 year–1, which was a little lower than that obtained by conventional methodologies, whereas, in 2008, this regression model yielded an annual secondary production of only 8.0 g dry wt m–2 year–1.
Discussion
Wastewater from the Barcelona metropolitan area was discharged for decades through near-shore outfalls off Barcelona itself. Together with the direct effluents discharged by the Besòs River, they were responsible for a profound alteration of the physical and biological composition of the shallow soft-bottom environments. In 1987–88, sediment properties of the benthic assemblage were heavily influenced by the sewage discharges, and high values of organic carbon content and other inorganic pollutants were registered (Amengual et al. Citation1988). These values were essential for the interpretation of the faunal data. In contrast to the earlier conditions, 20 years after cessation of wastewater discharge at shallow depths, muddy sediments were partially washed by the currents, and the recent sediments showed no PCB and PAH pollutants, although metal contamination was still important. The presence of high values of lead, zinc, copper, and nickel suggested that metals were also delivered from other sources rather than only wastewater, such as atmospheric deposition and/or water runoff from the city, as certain metals have become broadly used in several industries and activities, and their concentrations increased accordingly in waters receiving inputs from the land. Therefore, currently, shallow soft-bottom sediments off Barcelona contain less organic carbon, but they are still polluted by dangerous concentrations of hazardous metals.
The effects of organic enrichment on benthic dynamics have been widely accepted since the model of Pearson and Rosenberg (Citation1978). At early stages of enrichment, benthic communities tend to increase abundance, biomass and, even, the number of species. However, if enrichment continues, although abundance still increases, biomass and species richness decline (transitional phase) until, finally, at the peak of enrichment, opportunistic species dominate the community, at which point the abundance is very high, biomass increases again and species richness remains very low (polluted phase). In the most extreme cases, with lack of oxygen and the presence of hydrogen sulphide, macrofauna is lost completely. The observations made at the Barcelona station sampled showed a clear move (recovery) from a highly polluted phase to a transitional phase. Nevertheless, 20 years after the removal of the high inputs of wastewater discharges into these shallow soft-bottom environments, there were still signs of disturbance, such as the high abundance of some opportunistic species and low biomass values.
The effects of the organic enrichment did not alter the foreseen seasonal cycle of density expected for this area. In terms of abundance, as already described for Northwestern Mediterranean soft-bottom shallow habitats (Sardá et al. Citation1995, 1999), macroinfaunal density increased to a peak in May–June as new recruiters settled into the sediments. Then, a sharp decrease occurred during summer and lower density values were observed through the autumn and winter. In this area, it is generally accepted that autotrophic (mainly phytoplankton) production is the largest organic carbon source for the benthos under normal conditions (Satta et al. Citation1996). In non-enriched conditions, these shallow sublittoral habitats tend to be structurally complex in species composition, showing sporadic appearances of many species and large recruitments of others that are concentrated in shorter periods of the year. As has been observed in the reference station, spring peaks of density normally ranged from 15,000 to 50,000 ind. m–2 (Sardá et al. Citation1995). The ecological change due to the sewage outfall led to the sediment being colonized by a very low diversity assemblage mainly comprised of three species, where density values were more than one order of magnitude higher than those calculated for non-enriched shallow communities. However, the seasonal cycle of this modified community was similar to the one observed in a non-impacted community, with the exception that, in this case, the largest organic carbon source originated from man-made activities.
The biomass of the infaunal community affected by the sewage outfall responded differently to that observed for the abundance. In non-enriched communities of the Western Mediterranean, the seasonal pattern of biomass followed roughly that of abundance and only one very predictable biomass peak was observed (Sardá et al. Citation1995, 1999). The annual pattern of biomass in this enriched community showed two main peaks of biomass throughout the year, with the Capitella capitata complex being almost entirely responsible for such a seasonal pattern. Mean biomass values of the macrobenthic community decreased by an order of magnitude during these 20 years. In the Northwestern Mediterranean region, shallow, non-vegetated, soft-bottom habitats are, in general, poor in biomass and productivity. Except in the vicinity of large rivers where values up to 18.4 g dry wt m–2 have been recorded (the Gulf of Fos, France: Massé Citation1972), in enclosed areas or coastal lagoons (157.2 g dry wt m–2 for the Camargue area: Massé Citation1972; 66.0 g dry wt m–2 in the Alfacs Bay, Ebro Delta: Martin et al. Citation2000) or large cities, as in our case study, where biomass and productivity tend to increase, the rest of the values are, in general, small.
Laboratory experiments have shown that, even if food and other environmental conditions are maintained constant, the populations of Capitella capitata can have large temporal oscillations (Chesney & Tenore Citation1985; Grémare et al. Citation1988). It has been proposed that these oscillations would be due to the achievement of the population carrying capacity by over-exploitation of food and spatial resources. Several authors (Chesney & Tenore Citation1985; Grémare et al. Citation1988, Citation1989) suggested a density-dependent effect for the decrease in the reproductive output of the species due to the lower availability of food resources by unit of biomass. In our sampling site, it seems that the population of C. capitata grew until it reached a biomass of approximately 20 g dry wt m–2, when it suddenly crashed. After the crash, the population recovered rapidly, except during the summer time. In summer, even if recruitment of new individuals was still very high, the survival was clearly reduced and the population declined abruptly. There are at least three possible explanations for this: (1) the decline was due to the development of reducing conditions in the sediments (Tsutsumi Citation1990); (2) the absence of sufficient food in quantity and quality to maintain an increased metabolic rate in the species (Charles & Amouroux Citation1995; Grémare et al. Citation1997, Citation1998); and (3) other physical disturbances over the sediment. However, no anoxic conditions were observed in the analysed sediment during the sampled season (Amengual et al. Citation1988); therefore, the depletion of infauna by the development of reducing conditions in the sediments cannot be an explanation. On the other hand, although the quality of the organic input could be lower in summer its quantity could not be, as shown by the specific conditions of the studied site. Therefore, the crash in summer could probably be due to a combination of physical conditions, carrying capacity and food, since mixing processes in the water column are greatly reduced when the temperature is high.
Secondary production of the benthic community inhabiting the studied station was drastically reduced by the cessation of wastewater discharges. In 1987–88, direct measurements used to calculate this production yielded accurate results (276.3 g dry wt m–2 year–1), and these values were in agreement with those calculated by Méndez et al. (Citation1997) at a similar station (1991–92). In 2008, the data obtained did not allow us to use such conventional methodologies, although the use of multiple regressions based on pooled biological data has been shown to be useful (Sardá Citation1997). Using the methodology developed by Brey (Citation1990), we were able to obtain a production number in 1987–88 (207.7 g dry wt m–2 year–1) not far from that obtained by conventional methodologies, and, moreover, we could compare this data with the one obtained for 2008 (8.0 g dry wt m–2 year–1), demonstrating the drastic reduction in secondary production of this community.
During these 20 years, a drastic reduction in wastewater discharges into the shallow soft-bottom environments off Barcelona was reported. However, although pulses of organic matter were highly reduced, sediments were still polluted by metals as a consequence of the multiplicity of human activities that leads to metal loading in the marine environment. The recovery process of the benthic community inhabiting these soft-bottom environments is still under debate. Although the organic input decreased, the metal concentration present in the sediments may inhibit its full recovery to normal conditions. Mirroring the decrease in the organic input, secondary production of the macrofaunal community was also sharply reduced. It is a fact that wastewater treatment plants today can greatly reduce the organic outputs of these installations. Nevertheless, we should understand that the marine environment can assimilate a certain quantity of domestic wastes without large adverse changes and, as a result, if metal contamination can also be avoided, some increase in coastal productivity by a bioenhancement factor due to lower-level wastewater discharges could even be positive.
Acknowledgements
The authors wish to thank the original project SPIO (1987-88), funded by the former Ministry of Public Works and the ‘Corporación Metropolitana de Barcelona’. Data obtained in 2008 were obtained within the framework of the MeVaPlaya-II project, funded by the Spanish Ministry of Education and Science under contract CSO2009-14589-C03. The first author was also supported by IFARHU-SENACYT research grants.
References
- Amengual , P , Borrás , G , Julía , A and Navarro , R. 1988 . “ Proyecto integrado para el estudio del depósito submarino de lodos de la zona del río Besós sobre la zona costera de Barcelona ” . In Technical report 4 – 5 . Hidrografía
- Benke , AC. 1979 . A modification of the Hynes method for estimating secondary production with particular significance for multivoltine populations . Limnology and Oceanography , 24 : 168 – 171 .
- Benke , AC. 1993 . Concepts and patterns of invertebrate production in running waters . Verhandlungen der Internationalen Vereinigung für theoretische und angewandte Limnologie , 25 : 15 – 38 .
- Bianchi , CN and Morri , C. 2000 . Marine biodiversity of the Mediterranean Sea: Situation problems and prospects for future research . Marine Pollution Bulletin , 40 : 367 – 376 .
- Borja , A and Mader , J. 2008 . Instructions for the use of the AMBI index software version 4.1 . AZTI-Tecnalia , : 13
- Borja , A and Muxika , I. 2005 . Guidelines for the use of AMBI AZTI's Marine Biotic Index in the assessment of the benthic ecological quality . Marine Pollution Bulletin , 50 : 787 – 789 .
- Borja , A , Muxika , I and Franco , J. 2003 . The application of a Marine Biotic Index to different impact sources affecting soft-bottom benthic communities along European coasts . Marine Pollution Bulletin , 46 : 835 – 845 .
- Borja , AJ , Franco , J and Pérez , V. 2000 . A Marine Biotic Index to establish the ecological quality of soft-bottom benthos within European estuarine and coastal environments . Marine Pollution Bulletin , 40 : 1100 – 1114 .
- Brey , T. 1990 . Estimating productivity of macrobenthic invertebrates from biomass and mean individual weight . Archive of Fishery and Marine Research , 32 : 329 – 343 .
- Cardell , MJ. 1996 . Estructura y dinámica de la macrofauna bentónica en sedimentos marinos sometidos a vertidos domésticos e industriales: efecto de las aguas y lodos residuales de la planta depuradora de Sant Adrià de Besòs Barcelonès , 450 Tesis doctoral, Barcelona : España, Universidad de Barcelona .
- Cardell , MJ , Sardá , R and Romero , J. 1999 . Spatial changes in sublittoral soft-bottom polychaete assemblages due to river inputs and sewage discharges . Acta Oecologica , 204 : 343 – 351 .
- Cebrián , J , Duarte , CM and Marbà , N. 1996 . Herbivory on the seagrass Cymodocea nodosa (Ucria) Ascherson in contrasting Spanish Mediterranean habitats . Journal of Experimental Marine Biology and Ecology , 204 : 103 – 111 .
- Charles , F and Amouroux , JM. 1995 . A bioassay approach to temporal variation in the nutritional value of sediment trap material . Journal of Experimental Marine Biology and Ecology , 191 : 65 – 81 .
- Chesney , EJ and Tenore , KR. 1985 . Oscillations of laboratory populations of the polychaete Capitella capitata Type I: Their cause and implications for natural populations . Marine Ecology Progress Series , 20 : 289 – 296 .
- CIS-WFD . Common Implementation Strategy for the Water Framework Directive (2000/60/EC) . Guidance document no. 14. Guidance of the intercalibration process 2004–2006 . 2005 . Office of the official publications of the European communities, Luxemburg: 1–31. ISBN 92-894-9471–9
- Day , JH and Wilson , DP. 1934 . On the relation of the substratum to the metamorphosis of Scolecolepis fuliginosa Claparede . Journal of the Marine Biological Association of the United Kingdom , 192 : 655 – 662 .
- Estrada , M. 1980 . Composición taxonómica del fitoplancton en una zona próxima a la desembocadura del río Besós Barcelona de octubre de 1978 a marzo de 1979 . Investigación Pesquera , 44 : 275 – 289 .
- Fauchald , K and Jumars , PA. 1979 . The diet of worms: A study of polychaete feeding guilds . Oceanography and Marine Biology Annual Review , 17 : 193 – 284 .
- Font , J. and Miralles , L. 1978 . Circulación geostrófica en el mar Catalán . Resultados de la Expedición Científica del Buque Oceanográfico Cornide de Saavedra , 7 : 155 – 162 .
- Gray , JS. 1981 . The ecology of marine sediments: An introduction to the structure and function of benthic communities , Cambridge : Cambridge University Press .
- Grémare , A , Amouroux , JM , Charles , F , Dinet , A , Riaux-Gobin , C , Baudart , J , Medernach , L , Bodiou , JY , Vétion , G , Colomines , JC and Albert , P. 1997 . Temporal changes in the biochemical composition and nutritional value of the particulate organic matter available to surface deposit-feeders: A two year study . Marine Ecology Progress Series , 150 : 195 – 206 .
- Grémare , A , Amouroux , JM , Charles , F , Medernach , L , Jordana , E , Nozais , C , Vetion , G and Colomines , JC. 1998 . Temporal changes in the biochemical composition of particulate organic matter sedimentation in the bay of Banyuls-sur-mer . Oceanologica Acta , 21 : 783 – 792 .
- Grémare , A , Marsh , AG and Tenore , KR. 1988 . Short-term reproductive responses of Capitella sp. I (Annelida: Polychaeta) fed on different diets . Journal of Experimental Marine Biology and Ecology , 123 : 147 – 162 .
- Grémare , A , Marsh , AG and Tenore , KR. 1989 . Secondary production and reproduction of Capitella capitata Type I (Annelida: Polychaeta) during a population cycle . Marine Ecology Progress Series , 51 : 99 – 105 .
- Guérin , JP. 1975 . Redescription of adults and comparison of different ontogenic stages between Mediterranean and Atlantic populations of Scolelepis fuliginosa Claparede (Annelida: Polychaeta) . Cahiers de Biologie Marine , 16 : 21 – 37 .
- Halpern , BS , Walbridge , S , Selkoe , KA , Kappel , CV , Micheli , F , D'Agrosa , C , Bruno , JF , Casey , KS , Ebert , C Fox , HE . 2008 . A global map of human impact on marine ecosystems . Science , 319 : 948 – 952 .
- Hamilton , AL , Andrew , L and Hynes , H. 1969 . On estimating annual production . Limnology and Oceanography , 145 : 771 – 782 .
- Heip , C. 1992 . Benthic studies: Summary and conclusions . Marine Ecology Progress Series , 91 : 265 – 268 .
- Hynes , H and Coleman , MJ. 1968 . A simple method of assessing the annual production of stream benthos . Limnology and Oceanography , 134 : 569 – 573 .
- López-Sánchez , JF , Rubio , R , Samitier , C and Rauret , G. 1996 . Trace metal partitioning in marine sediments and sludges deposited off the coast of Barcelona Spain . Water Research , 301 : 153 – 159 .
- Martin , D , Pinedo , S and Sardá , R. 2000 . Distribution patterns and trophic structure of soft-bottom polychaete assemblages in a North-Western Mediterranean Shallow-Water bay . Ophelia , 53 : 1 – 17 .
- Massé , H. 1972 . Contribution a l’étude de la macrofaune de peuplements des sables fin infralittoraux des côtes de Provence . VII Discussion comparison et interpretation des données quantitative. Tethys , 4 : 397 – 422 .
- Méndez , N , Romero , J and Flos , J. 1997 . Population dynamics and production of the polychaete Capitella capitata in the littoral zone of Barcelona Spain NW Mediterranean . Journal of Experimental Marine Biology and Ecology , 218 : 263 – 284 .
- Menzie , CA. 1980 . A note on the Hynes method of estimating secondary production . Limnology and Oceanography , 254 : 770 – 773 .
- Palanques , A and Diaz , JI. 1994 . Anthropogenic heavy metal pollution in the sediments of the Barcelona continental shelf Northwestern Mediterranean . Marine Environmental Research , 38 : 17 – 31 .
- Pearson , TH and Rosenberg , R. 1978 . Macrobenthic succession in relation to organic enrichment and pollution of the marine environment . Oceanography and Marine Biology. An Annual Review , 16 : 229 – 311 .
- Pérès , JM and Picard , J. 1964 . Nouveau manuel de bionomie benthique de la mer Méditerranée. Recueil des Travaux de la Station Marine d'Endoume . Bulletin , 31–47 : 1 – 138 .
- Rhoads , DC and Germano , JD. 1986 . Interpreting long-term changes in benthic community structure: a new protocol . Hydrobiologia , 142 : 291 – 308 .
- Rhoads , DC , McCall , PL and Yingst , JY. 1978 . Disturbance and production on the estuarine seafloor . American Scientist , 66 : 577 – 586 .
- Ros , JD and Cardell , MJ. 1992 . “ Seasonal distribution patterns of polychaetes from a heavily polluted coastal area Barcelona NE Spain NW Mediterranean ” . In Marine eutrophication and population dynamics , Edited by: Colombo , G. 101 – 110 . Fredensborg : Olsen & Olsen .
- Rosenberg , RS , Agrenius , S , Hellman , B , Nilsson , HC and Norling , K. 2002 . Recovery of marine benthic habitats and fauna in a Swedish fjord following improved oxygen conditions . Marine Ecology Progress Series , 234 : 43 – 53 .
- Rumohr , H , Karakassis , I and Jensen , JN. 1987 . A compilation of biometric conversion factors for benthic invertebrates of the Baltic Sea . Baltic Marine Biologists Publication , 9 : 1 – 56 .
- Sardá , R. 1997 . The use of general relationships to estimate secondary production in coastal habitats: A revision based on a case study . Publicaciones Especiales. Instituto Español de Oceanografía , 23 : 11 – 22 .
- Sardá , R , Martin , D , Pinedo , S , Dueso , A and Cardell , M J. Seasonal dynamics of shallow soft-bottom communities in Western Mediterranean . Biology and Ecology on Shallow Coastal Waters. Proceedings of the 28th European Marine Biology Symposium, Creta 1993 . Fredensborg. pp. 191 – 198 . Olsen & Olsen .
- Sardá , R , Pinedo , S and Martín , D. 1999 . Seasonal dynamics of macroinfaunal key species inhabiting shallow soft-bottoms in the Bay of Blanes NW Mediterranean . Acta Oecológica , 204 : 315 – 326 .
- Satta , MP , Agustí , S , Mura , MP , Vaqué , D and Duarte , CM. 1996 . Microplankton respiration and net community metabolism in a bay on the NW Mediterranean coast . Aquatic Microbial Ecology , 10 : 165 – 172 .
- Steimle , FW , Kinner , P , Howe , S and Leathem , W. 1990 . Polychaete population dynamics and production in the New York Bight associated with variable levels of sediment contamination . Ophelia , 31 : 105 – 123 .
- Swartz , RC , Cole , FA , Schults , D and DeBen , W. 1986 . Ecological changes in the Southern California Bight near a large sewage outfall: Benthic conditions in 1980 and 1983 . Marine Ecology Progress Series , 31 : 1 – 13 .
- Tolosa , I , Readman , JW , Fowler , SW , Villeneuve , JP , Dachs , J , Bayona , JM and Albaiges , J. 1997 . PCBs in the western Mediterranean. Temporal trends and mass balance assessment . Deep Sea Research Part II: Topical Studies in Oceanography 44 , : 907 – 928 .
- Tsutsumi , H. 1990 . Population persistence of Capitella sp . Polychaeta; Capitellidae on a mud flat subject to environmental disturbance by organic enrichment. Marine Ecology Progress Series , 63 : 147 – 156 .
- Tsutsumi , H , Fukunaga , S , Fujita , N and Sumida , M. 1990 . Relationship between growth of Capitella sp . and organic enrichment of the sediment. Marine Ecology Progress Series , 63 : 157 – 162 .
- Valiela , I. 2006 . Global coastal change . Malden, USA: Blackwell Publishing Ltd , : 368
- Vanden , Berghe E , Claus , S , Appeltans , W , Faulwetter , S , Arvanitidis , C , Somerfield , PJ , Aleffi , IF , Amouroux , JM , Anisimova , N Bachelet , G . 2009 . MacroBen integrated database on benthic invertebrates of European continental shelves: A tool for large-scale analysis across Europe . Marine Ecology Progress Series , 382 : 225 – 238 .
- Warwick , RM. 1986 . A new method for detecting pollution effects on marine macrobenthic communities . Marine Biology , 924 : 557 – 562 .
- Warwick , RM and Clarke , KR. 1994 . Relearning the ABC: Taxonomic changes and abundance/biomass relationships in disturbed benthic communities . Marine Biology , 1184 : 739 – 744 .
- Warwick , RM , Clarke , KR and Somerfield , PJ. 2010 . Exploring the marine biotic index AMBI: Variations on a theme by Ángel Borja . Marine Pollution Bulletin , 604 : 554 – 559 .