Abstract
Data from several research and monitoring projects carried out between 1999 and 2009, for a total of 26 study areas located along the Italian continental shelf (Mediterranean Sea), were extracted from ISPRA's data set, to revise and update the existing information on the ecology and spatial distribution of 20 selected soft-sediment polychaete species. The species were selected taking into account their spatial distribution and ecological role in the benthic assemblages and the existence of voucher specimens deposited in ISPRA's reference collection. Samplings were taken at 872 stations on soft sediments, at depths ranging from 1 to 155 m. Surface sediment composition data were available at each site. The number of specimens from the selected species was extracted at each site, and relative abundance (%)calculated. The spatial distribution of each species was investigated according to the biogeographical zones identified in the Italian Seas. The distribution of five species (Aponuphis bilineata, A. brementi, A. fauveli, Nothria conchylega, and Onuphis eremita) was updated. Several species that were previously considered to be characteristic of a specific biocenosis, sensu Pérès & Picard (Citation1964), e.g. Diopatra neapolitana, Ditrupa arietina, Notria conchylega and Sternaspis scutata, were found to be distributed over a wider bathymetric and granulometric range of surface sediments. Indicator Species Analysis highlights that the distribution of 17 selected species depends on definite granulometric characteristics of the sediment. This new relevant information outlines the important contribution of environmental monitoring programmes to scientific knowledge.
Introduction
Polychaetes are amongst the most frequent and abundant organisms characterizing marine benthic communities, accounting for up to more than a third of the total number of macrobenthic species in soft substrata (Day Citation1967; Knox Citation1977). Polychaetes have proven to be excellent indicators of environmental conditions due to their distribution over a broad range of environments and their ample display of ecological requirements. Because of their ecological variability, they are widely used in applied environmental research (Giangrande et al. Citation2005). Although there have been numerous studies investigating the systematics, taxonomy, morphology and ecology of Mediterranean polychaetes, there are still significant gaps in the understanding of their ecology. Information on species distribution is incomplete, and many species are frequently recorded outside their known bathymetric and granulometric range and of their characteristic ecological boundaries, which define specific biocoenosis sensu Pérès & Picard (Citation1964). There is only a general account, dating back to Bellan (Citation1964), on the ecology of Mediterranean polychaetes. Further studies only analysed the distribution of polychaetes at local scales (e.g. Gravina Citation1986; Castelli et al. Citation1992; Bianchi et al. Citation1993a,Citationb,Citationc; Crema et al. Citation1993; Simboura et al. Citation2000; Çinar Citation2005; Moreira et al. Citation2006; Cosentino & Giacobbe Citation2008; Zaâbi et al. Citation2009). The information on the ecological and spatial distribution of species should therefore be revised and updated on the base on newly collected information.
Environmental monitoring programmes can be a source of valuable scientific data to increase the scientific knowledge of benthic communities and species, as these programmes allow the integration of multidisciplinary information. Moreover, frequent monitoring surveys and common sampling methodologies allow the collection of numerous and comparable data from different study areas.
This study proves the relevance of multidisciplinary environmental characterization and monitoring programmes for scientific research collating data extracted from a number of surveys (e.g. sand dredging, offshore platforms developments, aquaculture, beach nourishments) carried out in Italy over the last 10 years by the Italian National Institute for Environmental Protection and Research (ISPRA). These projects have provided data on sediment composition, associated benthic communities and depth range of a number of species in different areas of the Italian continental shelf.
In this study we present a first update on the spatial distribution and ecology (related to sediment composition and depth range) of 20 soft-sediment polychaete species along the Italian continental shelf. These species were selected taking into account their spatial distribution and their ecological role in benthic assemblages. For each species, voucher specimens were available in ISPRA's reference collection, which is based on the material collected in numerous monitoring surveys.
Material and methods
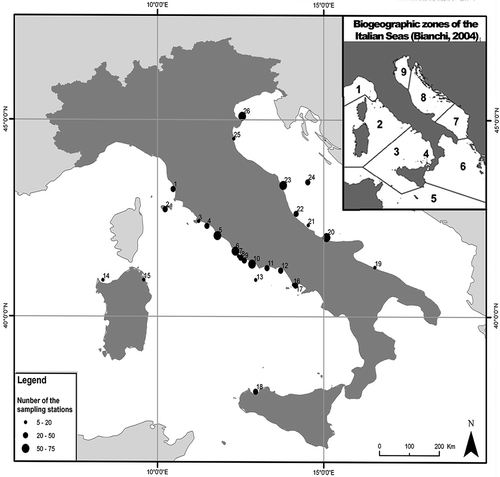
Data from 26 study areas located along the Italian continental shelf were extracted from ISPRA's data set (). These locations were selected with base on the common procedures of data collection and analyses at each sampling period from 1999 to 2009. Samples had been taken in two replicates at 872 stations along transects perpendicular to the coast, at depths ranging from 1 to 155 m (), using a Van Veen grab (0.1 m2 covering area). The sediments sampled were sieved through a 1-mm mesh and the retained material was preserved in seawater adding 4% CaCO3-buffered formalin. Macrozoobenthic samples were further sorted into major taxonomical groups and the collected polychaetes counted and classified to the lowest possible taxonomic level. Surface sediments were collected at each station with a box-corer, analysed and classified according to sand percentage, following Nota (Citation1958): sand (S) (>95% content of sand), muddy sand (mS) (95–70%), very sandy mud (vsM) (70–30%), sandy mud (sM) (30–5%) and mud (M) (<5%).
Table I. The 26 study areas located in the nine biogeographical zones identified in the Italian Seas (Bianchi Citation2004), the number of sampling stations for each area, divided into depth ranges ( C= Central; S= South; N=North)
Figure 1. Location of the 26 study areas from which data was extracted. Stations are numbered from the Ligurian Sea to the northern Adriatic Sea. The dimension of the circle is proportional to the number of stations investigated in each area. 1, Rosignano; 2, Elba; 3, Porto Ercole; 4, Montalto; 5, Civitavecchia; 6, Ostia; 7, Torpaterno; 8, Torvaianica; 9, Anzio; 10, Sabaudia; 11, Terracina; 12, Gaeta; 13, Ponza; 14, Porto Torres; 15, Olbia; 16, Baia; 17, Bagnoli; 18, Castellammare; 19, Bisceglie; 20, Molise; 21, Ortona; 22, Giulianova; 23, Marche; 24, Civitanova Marche; 25, Ravenna; 26, Chioggia. The nine biogeographical zones described in the Italian Seas are as according to Bianchi (Citation2004) and followed in the checklist of flora and fauna of the Italian Seas (Relini Citation2008)
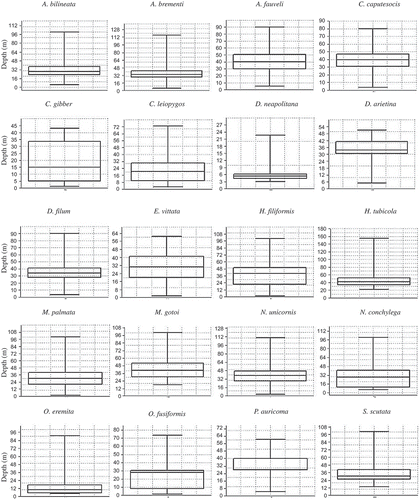
Twenty species, listed in , were selected according to their spatial distribution, their ecological role and the existence of voucher specimens deposited in ISPRA's reference collection. For each species number of specimens and relative abundance (%) were calculated. Box-and-whisker plots (Tukey Citation1977) were performed to analyse the depth distribution of species. Depths were grouped in five ranges (1–10 m; 10.1–20 m; 20.1–30 m; 30.1–50 m; >50 m).
Table II. Distribution of the 20 selected species by the nine biogeographical zones of the Italian Seas. In white, the distribution of the selected species as reported in the Checklist of Flora and Fauna of the Italian Seas (Relini Citation2008); in grey, records confirming the species distribution; in black, new records distribution of five species (Aponuphis bilineata, A. brementi, A. fauveli, Nothria conchylega, and Onuphis eremita)
The spatial distribution of each species was further analysed according to the division of the Italian Seas into nine biogeographical zones, proposed by Bianchi (Citation2004) () and applied in the Italian Checklist of marine flora and fauna (Relini Citation2008). No study areas were available for this study from biogeographical zones 4, 5 and 6.
Where available, information on the selected species on biocoenosis affiliation (Bellan Citation1964; Pérès & Picard Citation1964; Picard Citation1965; Augier Citation1982), preferred sediment typologies and depth range distribution was also extracted from existing literature.
Indicator Species Analysis (ISA) (Dufréne & Legendre Citation1997) has been performed in order to identify the species associated with or indicative of five groups of stations, derived from Nota (Citation1958) classification. This analysis combines the relative abundance of the species with their relative frequency of occurrence in the various groups and provides an indicator value subsequently tested by randomization.
Results and discussion
A total of 55,000 individuals from 200 species of polychaetes distributed across the Italian biogeographical zones 1, 2, 3, 7, 8, and 9 () were identified.
The allocation of the 20 selected species by biogeographical zones, as reported in the Checklist of flora and fauna of the Italian seas (Relini Citation2008), is presented in white in . The results confirmed the presence of these species in some biogegraphical zones (in grey) and updated the distribution of five of these species (in black). Aponuphis bilineata, A. brementi and A. fauveli, were recorded along the northern coast of the Island of Elba (Tuscan Archipelagos) in zone 1, which is considered to be a climate transitional area between zones 1 and 2 (Relini Citation2008). Nothria conchylega was found in zone 2, in the southern coast of the Island of Elba and along the coast of Latium (Central Tyrrhenian Sea) and Onuphis eremita was collected in zone 7, along the eastern coast of Apulia (Southern Adriatic Sea).
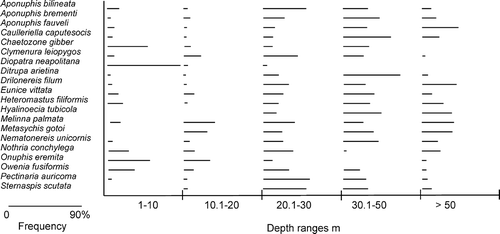
In , box-and-whisker plots describe the depths distribution of the selected species. The analysis of the distribution revealed that most of the 20 species occurred at all depth ranges (). Amongst the exceptions, Chaetozone gibber occurred exclusively from shallow waters to 50 m depth, Diopatra neapolitana was mostly distributed in shallow areas (1–10 m depth) and absent below 30 m, Hyalinoecia tubicola was sampled deeper than 20 m, Metasychis gotoi and Sternaspis scutata were only found deeper than 10 m, and Onuphis eremita, was absent in the 30–50 m depth range.
Figure 3. Distribution of the 20 selected species along the different depth ranges (in meters), taking into account the percentage of analysed stations, for each depth range, where the species were found. The maximum found percentage was 90%.
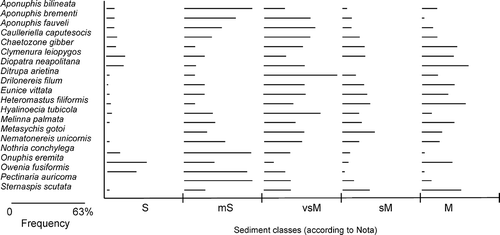
The species distributions show that all species were found in all sediment classes (Nota's sediment classification) except Diopatra neapolitana, which was not found in sandy-muds (sM) and Metasychis gotoi and Pectinaria auricoma, which were absent from sands (S) ().
Figure 4. Distribution of the 20 selected species amongst different sediment classes according to Nota's classification (1958): sand (S) (>95% content of sand), muddy sand (mS) (70–95% content of sand), very sandy mud (vsM) (70–30% content of sand), sandy mud (sM) (30–5% content of sand) and mud (M) (<5% content of sand). The distributions take into account the percentage of analysed stations, for each sediment class, where the species were found. The maximum found percentage was 63%.
The species selected were present in the range of 0–20% relative abundance within the associated polychaete assemblages in all sediment typologies; higher values of relative abundance were recorded only for some species and in some classes of sediment ().
Table III. Frequency of station in each Nota's sediment class where each species was found. Species occurrence is also clustered into classes of relative abundances (%) of each species within the whole polychaete assemblages (S = sand; mS = muddy sand; vsM = very sandy mud; sM = sandy mud; M = mud)
The species-group contributions obtained from ISA are reported in . Results demonstrate that the species distribution depends on definite granulometric characteristics of the sediment and the indicator values of ISA statistic specify the groups of stations that are associated with the species with different levels of significance. It is interesting to highlight that 17 of the selected species are associated with specific groups with a high level of significance (p≤0.01 and p≤0.001) while Hyalinoecia tubicola, Melinna palmata and Nothria conchylega are associated with a low level of significance (p≤0.05). Chaetozone gibber, Clymenura leiopygos and Diopatra neapolitana have no significant relationship with the groups identified, suggesting a wide ecological tolerance against the sediment fractions. Moreover, Drilonereis filum and Eunice vittata have values of ISA that suggest a high tolerance to mixed sediment (vsM and sM) and Sternaspis scutata show a significant preference for muddy sediment (sM and M).
Table IV. The species-group contributions obtained from Indicator Species Analysis (ISA). The indicator values of ISA specify the groups of stations that are associated with the species with different levels of significance
For each species, the information on biocoenoses affiliation, ecological significance, sediment typologies and depth ranges, as emerging from previous literature and from the results of this study, is summarized in . These results are also listed below, in alphabetic order.
Table V. For each species, information on biocoenoses affiliation, ecological significance, sediment typologies and depth ranges, as emerging from previous literature and from the results of this study. Results are listed in alphabetic order. Abbreviations: C = coralligenous biocoenoses, DC = biocoenoses of coastal detritic, DE = biocoenoses of muddy detritic, DL = biocoenoses of the shelf-edge detritic, SFBC = biocoenoses of well-sorted fine sand, SVMC = biocoenoses of surface muddy sands in sheltered waters, VTC = biocoenoses of coastal terrigenous mud, MI = Communities of unstable soft seabeds, excl. exclusive species, pref. = preferential species, sand tol. = species living on sands and tolerant to other fractions, mud tol. = species living on muds and tolerant to other fractions, Grav. = species living on gravely sediments, Lre = species with wide ecological distribution, Mixt. = species living on mixed sediments
Aponuphis bilineata (Onuphidae) was found from 4 to 100 m depth () although it was mostly frequent between 20 and 30 m depth (). The species mainly prefers muddy sand (mS) and very sandy mud (vsM) ( and ), where gravels are present, as along the Island of Elba and western Sardinia coasts. Moreover, this species seems to tolerate a high percentage of mud occurring in both sandy mud and muddy stations (sM and M). Our results partially agree with the existing information that signalled A. bilineata in detritic and sandy substrata and in mixed sediment ().
Aponuphis brementi and Aponuphis fauveli (Onuphidae) displayed similar sediment preferences to the congeneric species A. bilineata showing a significant association with very sandy mud (vsM) ( and ). A. brementi was more frequent below 20 m depth and up to 50 m while A. fauveli occurred below 30 m and up to 90 m depth ( and ). Our results add information to the existing literature where these species were described as ‘misticolous’ and A. fauveli in particular was affiliated to the muddy detritic biocoenoses ().
Caulleriella caputesocis (Cirratulidae) was found from 3.5 to 80 m depth () and more frequently in deeper stations, below 30 m depth (). It occurred in stations with high percentages of mud (vsM, sM and M) ( and ), in accordance with the existing information (), but also in few stations where the sandy fraction was ≥95–70% of the sediment (S and mS).
Chaetozone gibber (Cirratulidae) was distributed in all sediment typologies, with higher frequency in muddy stations (vsM, sM and M) () as indicated in the literature (). The species was mainly found in shallow water and never below 43 m depth ( and ).
Clymenura leiopygos (Maldanidae) was collected from 2.5 to 73 m () especially in stations located between 20 and 30 m depth (). The presence of this species was signalled in sandy stations, as well as in all the other sediment typologies, with comparable frequencies (). Our data show that C. leiopygos has a higher tolerance to different fractions of mud than what expressed in the existing literature ().
Diopatra neapolitana (Onuphidae) was mostly found in shallow stations (above 10 m depth) but is present up to about 23 m depth ( and ). It was mainly collected in sediments with high percentages of mud (vsM and M) and only in a few sandy stations (S and mS) (). These results suggest that this species has a good tolerance for mud, in partial agreement with previous literature that considered D. neapolitana a species typical of fine well-sorted sand assemblages (Picard Citation1965) and characteristic of sandy sediments, but tolerant to small amounts of mud ().
Ditrupa arietina (Serpulidae) was mostly collected between 30 and 50 m depth, although its range spans from 5 to 51 m depth ( and ). According to our results, this species seems to tolerate different fractions of mud in the sediment, since it was mostly recorded on very sandy mud (vsM) and in other types of sediment, ranging from sand to mud ( and ). It is also interesting to underline that the gravel fraction was very scarce in all the stations where D. arietina was recorded, although this is a species normally affiliated to detritic coastal assemblages and it has been signalled on mixed sediments ().
Drilonereis filum (Oenonidae) was found from 3 to 90 m depth, mostly below 20 m ( and ), in all sediment types even if it showed a high tolerance to mixed sediment (vsM and sM) ( and ); moreover, it was very rare in sandy (S) and shallow stations and up to 20 m depth. Our results, on the whole, confirm the information existing on this species ().
Eunice vittata (Eunicidae) was found from 1.5 to 60 m depth (). It is mostly frequent on muddy substrata, preferably in very sandy mud (vsM), sandy mud (sM) () and mud (M) (depth range 30–50m), rather than on sandy sediments (S and mS) ( and ). The percentage of gravel (mainly composed of organic detritic matter) was more abundant in vsM sediments which characterized stations located around Island of Elba and few stations of Baia (Gulf of Naples). Our results partially agree with previous scientific literature ().
Heteromastus filiformis (Capitellidae) was recorded between 1.5 and 99 m depth ( and ) in sediments characterized by a high percentages of mud (vsM, sM and M) and only in few sandy (S) and muddy sand (mS) stations ( and ). These results agree with the existing information, which defined the species as an exclusive species of upper muddy-sand assemblages living also in mud-rich sediments ().
Hyalinoaecia tubicola (Onuphidae) was sampled exclusively below 20 m depth, frequently between 30 and 50 m ( and ). This species inhabited primarily very sandy mud (vsM), sandy mud (sM) and muddy sand (mS) sediments and was found in other sediment typologies in only a few stations (S and M) ( and ). In some of the stations where H. tubicola was recorded (mainly vsM and mS stations located in the Tyrrhenian Sea along the Island of Elba and the coast of Terracina and Sabaudia), the percentage of gravel suggested the presence of detritic habitats where, according to the scientific literature, this species is most common ().
Melinna palmata (Ampharetidae) was collected from 1 to 99 m depth ( and ), and results show that the species is strongly linked to the muddy fraction of the sediment ( and ), in accordance with several authors (). In fact, the stations where the species mostly occurred were characterized by an increasing percentages of mud (from mS to M classes).
Metasychis gotoi has been defined as an alien species for the Mediterranean Sea (Cantone et al. Citation2004; Occhipinti-Ambrogi et al. Citation2011). The species was sampled, in comparable frequencies, in sediments with a variable fraction of mud, with a significant preference for sandy mud (sM), and at depths ranging from about 17 to 100 m (Figures and ). This species was absent in shallow waters with sand percentages >95%. The data that we collected on this species integrate the information on the substrata inhabited by M. gotoi ().
Nematonereis unicornis distribution (Eunicidae) highlights the wide bathymetric range of this species, recorded from 3 to 110 m depth with highest frequencies between 30 and 50 m depth ( and ). N. unicornis inhabits sediments with different percentages of mud (from mS to M) (, ) and – in some locations – with abundant fractions of gravel (Elba Island, Baia and Anzio, Tyrrhenian Sea). These results confirmed the information about this species, which normally has been affiliated to muddy-detritic assemblages () and classified as one of the few taxa able to burrow inside Posidonia oceanica sheaths (Gambi Citation2002).
Nothria conchylega (Onuphidae) was recorded from 5 to 100 m depth with variable frequencies at different depth ranges ( and ). It was mostly found in sediments with 70–95% sand (mS) and it was less frequent either at stations characterized exclusively by sand (S) or by lower percentages of sand (vsM, sM and M) ( and ). These results add information to previous literature, which described N. conchylega as a species typical of muddy sediment, often found in shelf terrigenous mud and in shelf edge detritic assemblages ().
Onuphis eremita (Onuphidae) was collected up to 90 m depth and mostly between 4 and 10 m; no individuals were found between 30 and 50 m depth ( and ). O. eremita was frequent in sandy stations (S and mS) ( and ), in accordance with the literature that affiliated this species to the biocoenoses of well-sorted fine sand (). Nevertheless, we signal that this species was also frequent in mud (M).
Owenia fusiformis (Oweniidae) was present in the depth range 1.5–73 m with variable frequencies ( and ). This species was mainly found in stations characterized by a high percentage of sand (S and mS) ( and ), in accordance with authors who described it as typical of fine well-sorted sand assemblages (). Moreover, a significant percentage of gravel characterized some of the stations were the species was found at the Island of Elba, Porto Torres (Western Sardinia), Montalto (Central Tyrrhenian Sea) and Marche (Central Adriatic Sea) indicating that O. fusiformis inhabits also detritic substrata, as stated by Pérès & Picard (Citation1964) ().
Pectinaria auricoma (Pectinariidae) was mostly recorded between 20 and 30 m depth, although it was collected in the range of 4.5–60.5 m ( and ), on sandy substrata with low fractions of mud (mS) ( and ). We noticed that the increase of mud in the sediment led to the decrease of P. auricoma, and the species was absent in exclusively sandy sediment (S). Our results are partially in accordance with the common description of this species as affiliated to the muddy-detritic assemblages (Picard Citation1965) and as a mud tolerant species ().
Sternaspis scutata (Sternaspidae) occurred exclusively below 13 m depth, mainly between 20 and 30 m ( and ). Our results indicate that this species inhabits primarily muddy sediments (sM and M), even if it was also found in several stations with very sandy mud (vsM) or muddy sand (mS) ( and ). These results partially confirm the previous information, which described this species as typical of the terrigenous mud-shelf assemblages and inhabiting sandy mud ().
Conclusions
New relevant information emerged from the comparison of the results of this study with pre-existing knowledge. In fact, the data analysed allow us to update the distribution in the Italian Biogeographical Zones of five species (Aponuphis bilineata, A. brementi and A. fauveli, Nothria conchylega, and Onuphis eremita).
These results contribute to update the ecological characteristics of the investigated species in relation to depth range and sediment typologies, highlighting significant associations of a number of species with definite sediment classes. Moreover, this results outline that multidisciplinary data sets, as ISPRA's, are essential in increasing the scientific knowledge on the benthic communities and species that populates soft seabed substrata.
This study furthermore outlines the importance of environmental monitoring programmes as a source of important scientific data such as relevant information on marine invertebrate ecology. This work is meant to be a first contribution in the framework of a more extensive revision of our understanding of the ecological requirements and the distribution of polychaete species in soft sediments, improving our knowledge on the functioning and evolution of marine ecosystems.
Acknowledgements
We are grateful to the editor and the two referees for their invaluable comments and contribution. Many thanks to Dr Maria Cristina Gambi (Stazione Zoologica Anton Dohrn of Naples) and Prof. Michele Scardi (University of Rome ‘Torvergata’), for their invaluable support on data analysis and for the suggestions that improved this work considerably. Thanks to the researchers and the students for their assistance in field activities and for their time and invaluable dedication to taxonomic identification.
We want to express our fond memory and gratitude also to Prof. Eugenio Fresi (University of Rome ‘Tor Vergata’) for the precious contribution he has made to marine biology and to the study of benthic communities.
Part of this study was supported by the local authorities of the Regione Lazio and the Regione Marche. Bioservice s.c.r.l. (Napoli) also contributed with a set of data.
References
- Abbiati , M , Bianchi , CN and Castelli , A. 1987 . Polychate vertical zonation along a littoral cliff in the western Mediterranean . P.S.Z.N.: Marine Ecology , 8 : 33 – 48 .
- Augier , H. 1982 . “ Inventaire et classification des biocénoses marines benthiques de la Méditerranée ” . In Publication du Conseil de l'Europe, Collection Sauvegarde de la Nature 25 – 59 .
- Bellan , G. 1961 . Résultats scientifiques des campagnes de la ‘Calypso’. Annélides Polychètes. Campagne de la Calypso (Septembre–Octobre 1955) . Annales de l'Institut Océanographique , 39 : 161 – 178 .
- Bellan , G. 1963 . Nouvelle contribution à l’étude de la microfaune annélidienne de la region de Marseille . Recueil des Travaux de la Station Marine d'Endoume , 29 : 43 – 56 .
- Bellan , G. 1964 . Contribution il l’étude systématique . bionomique et écologique des Annelides Polychètes de la Méditerranée. Thèse Doct. Sci. Marseille. , 135
- Bellan , G. 1985 . “ Effects of pollution and man-made modifications on marine benthic communities in the Mediterranean: A review ” . In Mediterranean Marine Ecosystems, NATO Conf. Ser. 1, Ecology , Edited by: Moraitou-Apostolopoulou , M and Kiortsis , V . 163 – 194 . New York, NY : Plenum Press .
- Bellan , G and Bellan-Santini , D. 1991 . Polychaetous annelids (excluding Serpulidae) from artificial reefs in the Marseille area (French Mediterranean coast) . Ophelia , 5 : 433 – 442 .
- Ben-Eliahu , MN and Fiege , D. 1995 . Polychaeta from the continental shelf and slope of Israel collected by the ’Meteor’ 5 expedition (1987) . Senckenbergiana Maritima , 25 : 85 – 105 .
- Bianchi , CN. 2004 . Proposta di suddivisione dei mari italiani in settori biogeografici . Notiziario SIBM , 46 : 57 – 59 .
- Bianchi , CN , Ceppodomo , I , Galli , C , Sgorbini , S , Dell'Amico , F and Morri , C. 1993a . “ Benthos dei mari toscani. I: Livorno – Isola d'Elba (crociera Enea 1985) ” . In Arcipelago Toscano. Studio oceanografico, sedimentologico, geochimico e biologico , Edited by: Ferretti , O , Immordino , F and Damiani , V . 263 – 290 . Rome : ENEA .
- Bianchi , CN , Ceppodomo , I , Niccolai , I , Aliani , S , De Ranieri , S , Abbiati , M , Dell'Amico , F and Morri , C. 1993b . “ Benthos dei mari toscani. II: Isola d'Elba – Montecristo (crociera Enea 1986) ” . In Arcipelago Toscano. Studio oceanografico, sedimentologico, geochimico e biologico , Edited by: Ferretti , O , Immordino , F and Damiani , V . 291 – 316 . Rome : ENEA .
- Bianchi , CN , Ceppodomo , I , Cocito , S , Aliani , S , Cattaneo Vietti , R and Morri , C. 1993c . “ Benthos dei mari toscani. III: La Spezia – Livorno (crociera Enea 1987) ” . In Arcipelago Toscano. Studio oceanografico, sedimentologico, geochimico e biologico , Edited by: Ferretti , O , Immordino , F and Damiani , V . 317 – 337 . Rome : ENEA .
- Cacabelos , E , Moreira , J and Troncoso , JS. 2008 . Distribution of Polychaeta in soft-bottoms of a Galician Ria (NW Spain) . Scientia Marina , 72 : 655 – 667 .
- Cantone , G , Castelli , A , Gambi , MC , Giangrande , A , Catalano , D , Cigliano , M , Como , S and Musco , L. 2004 . Relazione finale nell'ambito del programma di ricerca ‘Aspim’, area scientifica della tassonomia dei policheti , Roma : ICRAM .
- Carvalho , S , Moura , A , Miguel , BG , Pereira , P , Cancela da Fonseca , L , Falcão , M , Drago , T , Leitão , F and Regala , J. 2005 . Spatial and inter-annual variability of the macrobenthic communities within a coastal lagoon (Óbidos lagoon) and its relationship with environmental parameters . Acta Oecologica , 27 : 143 – 159 .
- Castelli , A , Crema , R , Prevedelli , D and Zunarelli Vandini , R . 1992 . Caratterizzazione bionomica di fondi molli infralitorali dell’ Alto Tirreno Toscano Atti della Società Toscana di Scienze Naturali, Memorie Serie B , 99 : 39 – 60 .
- Chimenz Gusso , C , Gravina , MF and Maggiore , FR. 2001 . Temporal variations in soft bottom benthic communities in Central Tyrrhenian Sea (Italy) . Archivio di Oceanografia e Limnologia , 22 : 175 – 182 .
- Çinar , ME. 2005 . Polychaetes from the coast of northern Cyprus (eastern Mediterranean Sea) with two new records for the Mediterranean Sea . Cahiers de Biologie Marine , 46 : 143 – 159 .
- Cosentino , A and Giacobbe , S. 2008 . Distribution and functional response of sublittoral soft bottom assemblages to sedimentary constraints . Estuarine, Coastal and Shelf Science , 79 : 263 – 276 .
- Crema , R , Castelli , A , Bonvicini Pagliai , AM , Zunarelli Vandini , E , Prevedelli , D and Alboni , L. 1993 . “ Studio delle comunità bentoniche di fondi molli infralitorali dell'Alto Tirreno Toscano ” . In Progetto Mare. Ricerca sullo stato biologico chimico e fisico dell'Alto Tirreno Toscano. Relazioni conclusive delle ricerche 445 – 488 .
- Dauvin , JC. 2000 . The muddy fine sand Abra alba-Melinna palmata community of the Bay of Morlaix twenty years after the Amoco Cadiz Oil Spill . Marine Pollution Bulletin , 40 : 528 – 536 .
- Day , JH. 1967 . “ Monograph on the Polychaeta of southern Africa. Vol. Part 1 Errantia ” . In British Museum (Natural History) 458 London
- De Gaillande , D. 1968 . Monographie des peuplements benthiques d'une calanque des côtes de Provence: Port-Miou . Recueil des Travaux de la Station Marine d'Endoume , 44 : 358 – 401 .
- Desbruyères , D , Guille , A and Ramos , JM. 1972–73 . Bionomie benthique du plateau continental de la côte catalane espagnole . Vie et Milieu , 23 : 335 – 363 .
- Dewarumez , JM , Davoult , D and Glacon , R. 1992 . Nouvelles signalisations d'espèces macrobenthiques sur les côtes françaises de la Manche orientale et de la Mer du Nord . I. Annélides. Cahiers de Biologie Marine , 33 : 83 – 93 .
- Diaz-Castañeda , V and Safran , P. 1988 . Dynamique de la colonization par les annélides polychètes de sédiments défaunés par la pollution dans des enceintes expérimentales en rade de Toulon (France) . Oceanologica Acta , 11 : 285 – 297 .
- Dos Santos Brasil , AC and Gonçalves da Silva , SH . 2000 . Spatial distribution of Polychaeta in a soft-bottom community at Saco do Céu, Ilha Grande, Rio de Janeiro, Brazil . Bulletin of Marine Science , 67 : 103 – 112 .
- Drago , N and Albertelli , G. 1978 . Étude faunistique et bionomique du littoral de Cogoleto (golfe de Genes) . Téthys , 8 : 203 – 212 .
- Dufrêne , M and Legendre , P. 1997 . Species assemblages and indicator species: The need for a flexible asymmetrical approach . Ecological Monographs , 67 : 345 – 366 .
- Duineveld , GCA , Kunitzer , A , Niermann , U , De Wilde , PAWJ and Gray , JS. 1991 . The macrobenthos of the North Sea . Netherlands Journal of Sea Research , 28 : 53 – 65 .
- FAO/UNEP . 1986 . Report of the FAO/UNEP meeting on the effects of pollution on marine ecosystems (Blanes, Spain, 7–11 October 1985) . FAO fisheries report. , 352 : 20
- Gambi , MC. 2002 . Spatio-temporal distribution and ecological role of polychaete borers of Posidonia oceanica (L.) Delile scales . Bulletin of Marine Science 71 , : 1323 – 1331 .
- Gambi , MC and Giangrande , A. 1986 . Distribution of soft-bottom polychaetes in two coastal areas of the Tyrrhenian Sea (Italy): structural analysis . Estuarine, Coastal and Shelf Science , 23 : 847 – 862 .
- Giangrande , A and Gambi , MC. 1985 . Distribution of soft bottom Polychaetes in the Gulf of Salerno (Tyrrhenian Sea) . Rapport de la Commission Internationale pour l'Exploration Scientifique de la Mer Méditerranée , 29 : 233 – 235 .
- Giangrande , A , Licciano , M and Musco , L. 2005 . Polychaetes as environmental indicators revisited . Marine Pollution Bulletin , 50 : 1153 – 1162 .
- Gravina , MF. 1986 . Analisi della distribuzione dei policheti nei fondi mobili costieri delle Cinque Terre (Liguria) . Bollettino dei Musei e degli Istituti Biologici dell'Università di Genova , 52 ( Suppl ) : 197 – 212 .
- Gravina , MF and Somaschini , A. 1990 . Censimento dei policheti dei mari italiani: Capitellidae Grube, 1862 . Atti della Società Toscana di Scienze Naturali. Memorie. Serie B , 97 : 259 – 285 .
- Grémare , A , Amouroux , JM and Vétion , G. 1998 . Long-term comparison of macrobenthos within the soft bottoms of the Bay of Banyuls-sur-Mer (Northwestern Mediterranean Sea) . Journal of Sea Research , 40 : 281 – 302 .
- Hily , C. 1987 . Spatio-temporal variability of Chaetozone setosa (Malmgren) populations on an organic gradient in the Bay of Brest, France . Journal of Experimental Marine Biology and Ecology , 112 : 201 – 216 .
- Intès , A and Le Loeuff , P . 1986 . Les Annélides Polychètes de Côtes d'Ivoire . V. Mise en evidence et description des peuplements. Tropical Oceanography , 21 : 117 – 142 .
- Knox , GA. 1977 . “ The role of polychaetes in benthic soft-bottom communities ” . In Essays on Polychaetous Annelids in Memory of Dr. Olga Hartman , Edited by: Reish , DJ and Fauchald , K . 547 – 604 . Los Angeles, CA : Allan Hancock Foundation .
- Massé , H. 1972 . Contribution à l’étude de la macrofaune de peuplements des sables fins infralittoraux des côtes de Provence . VI. Données sur la biologie des espèces. Téthys , 4 : 63 – 84 .
- Moreira , J , Quintas , P and Troncoso , JS. 2006 . Spatial distribution of soft-bottom polychaete annelids in the Ensenada de Baiona (Ría de Vigo, Galicia, noth-west Spain) . Scientia Marina , 70S3 : 217 – 224 .
- Nota , DJG. 1958 . Sediments of the western Guiana shelf. Reports of the Orinoco Shelf Expedition, 2 . Mendedelingen van de Landbouwhogeschool te Wageningen , 58: 98
- Occhipinti-Ambrogi , A , Marchini , A , Cantone , G , Castelli , A , Chimenz , C , Cormaci , M , Froglia , C , Furnari , G , Gambi , MC Giaccone , G . 2011 . Alien species along the Italian coasts: An overview 2011 . Biological Invasions , 13 : 215 – 237 .
- Pérès , JM. 1954 . Contribution à l’étude des Annélides Polychètes de la Méditerranée occidentale . Recueil des Travaux de la Station Marine d'Endoume , 13 : 83 – 155 .
- Pérès , JM. 1982 . “ Zonations. General features of organismic assemblages in pelagial and benthal. Structure and dynamics of assemblages. Major benthic assemblages. Specific benthic assemblages ” . In Marine Ecology , Edited by: Kinne , O . 9 – 66 . 119 – 186 . 375 – 581 . London : John Wiley & Sons .
- Pérès , JM and Picard , J. 1964 . Nouveau manuel de bionomie benthique de la Mer Méditérranée . Recueil des travaux de la Station Marine d'Endoume , 31 : 137
- Picard , C . 1972 . Les peuplements de vase au large du Golfe de Fos . Téthys , 3 : 603 – 607 .
- Picard , J. 1965 . Recherches qualitatives sur les biocoenoses marines des substrats meubles dragables de la région marseillaise . Recueil des Travaux de la Station Marine d'Endoume , 36 : 1 – 160 .
- Relini , G. 2008 . Checklist of flora and fauna of the Italian seas. 2008 . Biologia Marina Mediterranea , 15 ( Suppl. 1 ) : 385
- Sardà , R. 1986 . Fauna de Anélidos Poliquetos de la Región del Estrecho de Gibraltar, III – Eunicida, Orbiniida, Spionida, Magelonida, Chaetopterida, Ctenodrilida, Flabelligerida, Opheliida, Oweniida, Capitellida, Terebellida, Sabellida y Nerillida . Miscellània Zoològica , 10 : 71 – 85 .
- Simboura , N , Nicolaidou , A and Thessalou-Legaki , M. 2000 . Polychaete community of Greece: An ecological overview . Marine Ecology , 21 : 129 – 144 .
- Tukey , JW . 1977 . Box-and-whisker plots . Exploratory Data Analysis , : 39 – 43 .
- Zaâbi , S , Gillet , P , Afli , A and Mboumaiza , M. 2009 . Biodiversity of polychaetous annelids from the peninsula of Cap Bon, northeast coast of Tunisia . Zoosymposia , 2 : 587 – 600 .
- Zavodnik , D , Vidakovic , J and Amoureux , L. 1985 . Contribution to sediment macrofauna in the area of Rovinj (North Adriatic Sea) . Cahiers de Biologie Marine , 26 : 431 – 44 .