Abstract
The colonization dynamic of Syllidae (Polychaeta) is described by means of a manipulative experiment carried out into the mesotrophic marine Faro Lake (NE Sicily). Small modules of artificial expanded fire-clay granules (EFCG-module) have been positioned in a shallow water experimental field from May to July 2008; they were plunged into the lake bottom or suspended in the water column. Three sampling times (15, 30, 60 days) and three treatments (heavy and moderate organic supply, plus a control) constituted the crossed factors. The natural sandy bottom was equally sampled. Both univariate and multivariate statistics have been employed to test the significance of distribution patterns at small spatio-temporal scale. Twenty-three species were identified. Since the early stage of the fouling process, buried and suspended modules were colonized by two different syllid assemblages that showed different temporal patterns, due to the relative proximity to the source habitat of adult immigrants. Organic contamination did not seem to affect the overall colonization process, although few species showed a moderate decrease from references to enriched modules. Moreover, a remarkable population of the new record Syllis hyllebergi (Licher, Citation1999) has been found. The population was rather patchy, mainly distributed in the upper coarser sediment with a greater content of shell debris. Morphometric assessment of most characters indicated an increase in size with respect to the holotype. The species has an actual bipolar distribution, being recorded also in the southern Brazil. According to the present record, S. hyllebergi might spread from the Levantine Sea to the central Mediterranean, step by step throughout similar confined transitional environments.
Introduction
Shallow transitional waters are environments characterized by a relevant variability in terms of abiotic and biotic factors (Guelorget & Perthuisot Citation1992). Sources of natural and anthropogenic variability interact in a complex way and are emphasized by the geomorphologic confinement of these habitats. The faunistic contingent is likely adapted to cope with unpredictable changes and potential stressors such as high temperature and salinity excursions or relevant organic load (Cognetti & Maltagliati Citation2000). At the local scale, lagoons and coastal lakes, in particular, may mimic some ecological processes, which also occur at a wider geographical and longer temporal scale. These confined environments are therefore important observatories for the multifactorial environmental change, and observational as well as manipulative investigations allow to detect processes at the beginning stage and to forecast their possible outcomes.
Disturbance caused by the organic enrichment of the substratum constitutes one of the most investigated processes. Its dynamic has been widely studied with regard to both established communities of natural bottoms and fouling assemblages of defaunated or artificial substrata. Relevant indications concerning with the spatial and temporal extent of the biotic alteration, the intrinsic resistance/resilience to the level of organic disturbance, and the timing of recovery as a function of the physical properties of the stressed substratum and of the neighbouring undisturbed area, are well documented (Pearson & Rosenberg Citation1978; Gray et al. Citation2002; Ditzel Faraco & da Cunha Lana Citation2003; Hughes et al. Citation2005). Moreover, the northward spreading of warm-water species with the diffusion of non-indigenous species as a consequence of the global sea warming (Streftaris et al. Citation2005; Galil Citation2008) represents another biotic process of compelling importance for scientist and policies. This phenomenon representing a worldwide natural process, also favoured by anthropogenic activities such as aquatic farming, ballast wash, ship transport, trade of exotic species (Naylor et al. Citation2001; Minchin Citation2007; Rilov & Crooks Citation2009), may be favoured by the refuge effect and by the lower extent of interspecific competition occurring in transitional aquatic systems.
On account of their wide representativeness in almost all marine habitats, their variety of feeding modes and life cycles, their sensitivity and their rapid response to environmental changes, Annelida Polychaeta are one of the most indicative taxa of natural or induced ecological change, utilized in the assessment of the ecosystem status (Grassle & Grassle Citation1974; Fauchald & Jumars Citation1979; Bellan et al. Citation1988; Salen-Picard et al, Citation1994; Ditzel Faraco & da Cunha Lana Citation2003; Giangrande et al. Citation2005; Dean Citation2008). More particularly, Syllidae is one of the best diversified polychaete families (Nygren Citation1999; Aguado & San Martín Citation2009), and its ecological sensitivity makes this taxon a useful bioindicator of environmental quality and coastal habitat change (Giangrande et al. Citation2005).
In the present study, which is part of a wider on-field experimental investigation on the fouling benthic community dynamic under increasing level of organic treatment within the studied biotope, two aspects are discussed: (i) the different syllid assemblages that colonized the artificial substratum from the neighbouring natural sediments, in two different media (bottom or water column) and at increasing levels of organic supply; and (ii) the occurrence in the central Mediterranean of Syllis hyllebergi (Licher Citation1999), a species recently described in the shallow lake waters of the Gulf of Aqaba (Red Sea). For this taxon, morphometric assessment with updated body-size ranges, and current world biogeographic distribution, with notes on habitat preference, are also provided.
Material and methods
Study area
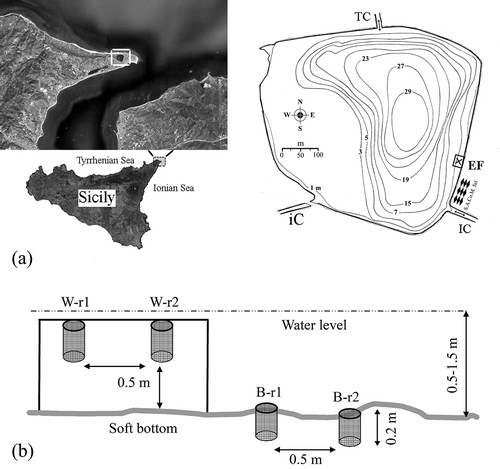
The Faro Lake (38°16ʹ07ʹʹN, 15°38ʹ13ʹʹE) is a temperate deep marine confined basin which extends 0.26 km2, with a sub-circular shape, a major axis of 660 m (a) and a maximum depth of about 29 m. Two channels connect the basin with the sea and are responsible for a limited hydrodynamic exchange, driven by the tidal regime of the Strait of Messina. Water body and sediments tend to a condition of mesotrophy and a high microbial productivity has been ascertained (Maugeri et al. Citation2000; Leonardi et al. Citation2009). Anthropogenic impact is moderate due to the urbanization of the area and the existence of commercial facilities for trade and stabling of edible bivalves (mussels, clams, oysters). The experimental field was 7 × 4 m, at 1.2 m average depth, extended over a broadly homogeneous bottom, which consisted of coarse sand mixed with bioclastic fragments (cobbles 11.96%; gravel 21.93%; coarse sand 52.88%; medium sand 11.16%; fine sand 2.03%; silt/clay 0.04%), poorly to scarcely sorted (mean σφ 1.28), with a less conspicuous mud fraction (mean Skφ 0.02). Sediments were highly reduced even from the first few centimetres (mean pH 7.76; mean Eh –315.49); biogenic carbonates ranged from 1 to 14%; the organic matter content ranged from 2.8 to 4.3% of total organic carbon (TOC).
Figure 1. (a) The coastal marine Lake of Faro (NE Sicily, Central Mediterranean Sea) and location of the experimental field (EF) on the left. The adjacent mollusc farm and connections to the Ionian Sea (IC), Tyrrhenian Sea (TC), and a third internal canal (iC), are shown. (b) Scheme of position of the net cylinders (modules) into the experimental field: W, water column; B, sedimentary bottom; r1/r2, replicates.
Experimental design
Artificial substratum consisted in expanded fire-clay granules (EFCG in acronym), from 4.0 to 8.0 mm diameter (mode of 5.6 mm) and density of 0.31 g/cm3. Washed granules were packaged into cylindrical modules of 20 × 15 cm green plastic net (5 mm mesh). Groups of 12 modules were subjected to three different experimental treatments: (i) high organic supply (P), in which the EFCG was preliminarily mingled with 50 g of solid granulate fish-feed pellet (Advance Marine-3P, Hendrix-Skretting SpA); (ii) moderate organic supply (Y), in which the EFCG was mixed with 100 g of soluble yeast Saccharomyces cerevisiae; and (iii) control condition (C), with untreated EFCG. Ten grams of hydrated pellet were weekly dispersed over the top of each P and Y module surface in order to simulate a periodical organic supply. In order to prevent cross-contamination by diffusion and resuspension in the shallow water, interspersion between controls and treatment groups was not employed (Hulbert Citation1984). The reduced dimensions of the sheltered field and the homogeneity of bottom features assessed before and during the experiment execution, warranted for the substantial independence of sample units from unexpected location effects.
Thirty-six modules were tagged (n = 12 P, 12 Y, 12 C). Eighteen modules were almost entirely buried into the soft bottom using a plastic core (EFCG-B), whilst the other ones were suspended into the water column (EFCG-W), on 16 May 2008 (b). At 15, 30 and 60 days, quantitative samples were obtained by randomly drawing two cylinders (replicates) from each treatment, together with two 3.5-litre random cores of natural sediment inside the field (also sampled before the positioning of modules). Natural or artificial hard bottoms were relatively far-off the experimental field and were not sampled in this study. The cylinders and sediment cores were removed from the water and from the bottom, preventing the escape of highly motile fauna by enclosing each cylinder into a plastic bag. The cylinder top surface was quickly cut; the content was fixed with 10% buffered isotonic formaldehyde solution stained with Rose Bengal. In the laboratory, the artificial substratum was washed on a steel ASTM-USA sieve series with 5, 1 and 0.33 mm meshes. The syllid fauna, stored in ethanol 70°, was determined up to the lowest possible taxonomic level, according to San Martín (Citation2003) and Licher (Citation1999). Morphometry of 17 representative adult and juvenile specimens of the species S. hyllebergi was carried out by means of Zeiss stereo- and optical light microscope, equipped with a micrometric ocular. Microphotographs were performed by a camera AxioCam ICc 3 (3.3 Mpxl resolution) from 16× to 630× magnification power and also by SE microscope without metallization (FEI Inspect-S at variable pressure).
Data analysis
Density (ind.1−1) was arranged in square sample × species matrix for both univariate and multivariate statistical computations. Since the lack of homoscedasticity in abundance estimations, non-parametric analysis of variance (Kruskal–Wallis ANOVA) was employed to test for significance of species distribution, with respect to: (i) type of substratum (natural sands or artificial EFCG); (ii) location of the EFCG-module (bottom or water column); (iii) organic treatment (controls or organically enriched modules). The dissimilarity percentage (Bray–Curtis δ coefficient, group-average linkage) was also employed to corroborate the weight of single species contribution (not transformed data); the significance of this statistic has been evaluated in terms of δ to SD ratio, not as absolute value. Triangular resemblance matrix of similarities was calculated on square-root transformed data; sample classification (cluster analysis) and spatial arrangement on a 2D plane (non-metric multidimensional scaling, NMDS) were performed. Significance of sample clusters was assessed by the similarity profile permutation test (SIMPROF, π statistic). Significance of experimental factors in the EFCG samples (time, three levels; organic treatment, three levels) was also tested by two-way crossed analysis of similarity (ANOSIM test, Clarke Citation1993). Computational and analytical routines were executed by means of STATISTICA v8 (StatSoft Italia) and PRIMER-E v6.1 (Plymouth Marine Laboratory, UK) (Sokal & Rholf, Citation1995; Clarke & Warwick Citation2001).
Results
Local syllid assemblage and microhabitat colonization
During two months of experimentation, the whole syllid assemblage was constituted by 23 species, with a total of 4446 individuals, of which 71% were found in the sandy bottom, 20% in the buried artificial EFCG modules and 9% in the suspended EFCG modules. Exogoninae was the prevalent subfamily in terms of abundance (60%), followed by Eusyllinae (25%) and by Syllinae (15%). In the natural sediment, syllid average density varied from 2.3 to 8.4 ind.1−1, whilst in the artificial granular substratum it ranged from 0.1 to 2.2 ind.1−1 and from 0.02 to 1.0 ind.1−1 for the bottom and the water modules, respectively. Colonization of the introduced substratum was mostly promoted by adults for all the observed macrobenthic Syllinae, but adult specimens of Exogoninae and Eusyllinae also colonized it since the initial 15 days. Among the Exogoninae, Brania arminii, Erinaceusyllis cf. belizensis, Exogone naidina, Parapionosyllis minuta, Salvatoria clavata, Salvatoria limbata and the eusyllin Syllides edentatus showed ripe and/or brooding individuals in most EFCG modules during the whole period of experiment. Adults of the eusyllin Perkinsyllis anophthalma readily colonized bottom modules, but recruits appeared only in the last sampling time. Among Syllinae, Syllis armillaris was able to rapidly colonize the bottom modules, showing a juvenile to adult mean ratio of 0.36. In half of the samples some individuals were regenerating the anterior or hind body parts, although its stolons were never collected. By contrast, stolons of Trypanosyllis zebra, a late colonizer of the water modules with a mean ratio of 3.43 between new recruits and adults, were present inside the modules.
A total of 53 adult specimens of the species Syllis hyllebergi were sampled during the experimentation, 92.5% of which were found in the soft bottom. This species represents a new record for central Mediterranean and was mainly distributed within the coarser upper 10 cm of the sediment (mean of individuals 5.38, SD 5.24) with respect to the lower and finer sediment layer (mean 0.75, SD 0.89). The reduced mean to SD ratio suggested the spatial distribution was patchy and no significant temporal trend was observed (mean abundance 13.25, SD 1.71). Ovigerous females were found throughout the experimental study, whereas no schizogamic individuals were noted.
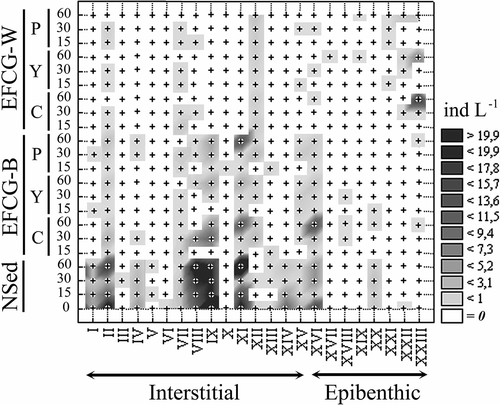
The spatio-temporal distribution of the whole assemblage is shown in . In general, the interstitial species mainly colonized the bottom modules from the neighbouring sediments, but never reaching values comparable to the source habitat. Differently, epibenthic syllids colonized the suspended modules preferentially. Within this general array, different species showed different distributional patterns. Among the most occurring species, Anoplosyllis edentula, E. cf. belizensis, S. limbata, Syllides convolutus and S. hyllebergi were almost restricted to the sandy sediments; Parapionosyllis elegans, P. minuta, P. anophthalma, S. edentatus, S. armillaris were prevalently distributed into both the natural and EFCG-B substrata; B. arminii, E. naidina and S. clavata were found in all the three matrices; Syllis prolifera, Syllis vittata and T. zebra were confined in the suspended EFCG-W modules, whereas Syllis gracilis was present only in the buried EFCG modules. The statistics of density distribution confirmed the high significance of quantitative differences for all the cited species between the suspended and bottom typologies of substratum (). Dissimilarity percentages were also in accordance to most of the H statistics, except for Exogone meridionalis and S. hyllebergi, due to their low and scattered occurrence into one of the two substrata. H and δ% were in agreement between the two location of artificial modules (), except for S. limbata, S. edentatus, S. prolifera, S. vittata and T. zebra, whose lower occurrence determined a lower δ to SD ratio. Differently, the significance of S. clavata was tested only in terms of δ%, due to its slight average prevalence in EFCG-W (δ/SD = 1.00). With regard to the organically conditioned microhabitats, it is worth noting that the distribution of almost all of the colonizing species was not directly related to the artificially induced contamination (, fifth and sixth columns). Neither the H statistic nor δ% contributions showed differences between the treated (Y–P samples pooled) and the untreated cylindrical modules. Most of the exogonins colonized controls as well as heavily polluted modules even at 15 days; likewise, S. armillaris for the bottom modules. Exceptions were B. arminii (δ/SD = 0.95) and S. clavata (δ/SD = 0.94), whose average density was mildly higher in the untreated modules. Moreover among syllinae, only the density of T. zebra was clearly decreasing according to the direction C–Y–P at 60 days of colonization, showing the highest δ% contribution although with low δ/SD of 0.57. It is meaningful that the populations of the natural sediment did not differ significantly between the two field locations (sectors) of control and treated modules. This invariance was confirmed in terms of both single-species density (np-ANOVA H statistic from 0.02 to 2.11, all P > 0.10) and total syllid density per sample within sector (Fisher F1;6 = 0.0015, P = 0.97). Therefore, the source area was statistically homogeneous, and no spatial (micro)gradients or bottom heterogeneities seemed to affect the resident syllid fauna.
Table I. Non-parametric analysis of variance (Kruskal–Wallis H test) and intergroup percentage dissimilarity analysis (Bray–Curtis coefficient, δ%) of individual density (ind.1−1) of the whole syllid assemblage sampled in the Faro Lake. Asterisk indicates H significance (*0.05; **0.01; ***0.001). Underlined values indicate the most significant species contribution in terms of δ to SD ratio
Figure 2. Trivariate diagram of the syllid densities (N1−1; shaded areas) with respect to the substratum (NSed, natural sediment; EFCG, artificial substratum), medium of module location (B, bottom; W, water), time of sampling (days) and level of organic treatment (see Methods section for details). Symbol ‘+’ indicates true value; contour areas are obtained by interpolation between two adjacent points of x-variables (species coded as Roman numbers in the ).
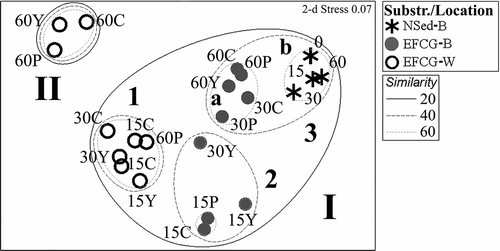
The multivariate arrangement of samples individuated two main clusters at 14.84 similarity (), which grouped all the ‘bottom’ samples plus 15- and 30-days EFCG-W samples (cluster I) and the remaining 60-days EFCG-W samples (cluster II), at π = 6.25, P < 0.001. The cluster I comprised other three significant clusters. The group I.1+I.2 was different from I.3. at 25.0 similarity (π = 5.75, P < 0.001), whilst the cluster I.1. was not significantly different from the cluster I.2. at 38.0 similarity (π = 2.21, P = 19.3). These two latter clusters grouped EFCG-modules in water or in bottom location, respectively, at the first and the second colonization times. The third cluster comprised two other significant sub-clusters at 48.3 similarity level (π = 4.98, P < 0.001): the cluster I.3a grouped all the remaining EFCG-B samples at 30- and 60-days; the cluster I.3b comprised all the samples from the lake sediments. During the first 30 days of colonization, the artificial modules suspended into the water column were still not distinguishable from the early 15-day bottom modules. The suspended modules constituted a clearly separate assemblage from the bottom environment at least after 60 days of colonization, whereas the bottom modules reached more similarity with respect to the confining habitat already from 30 days of colonization. Notwithstanding, these two latter natural and artificial microhabitats still remained different. Moreover, and similarly to what tested by the np-ANOVA, the organic matter enrichment did not affect the species composition significantly, even at the beginning of experimentation between the highest P-treatment and reference modules. Two-way crossed ANOSIM confirmed these results. Time factor, across all OM treatments, was moderately significant, providing a Global R of 0.34 (P = 0.0003); 15- and 60-days were the best discriminated groups (R statistic = 0.58, P = 0.0001), whilst 15- vs. 30-days were different at a lower extent (R statistic = 0.19, P = 0.024) and 30- vs. 60-days were not significantly different (R = 0.21, P = 0.054). By contrast, the level of organic enrichment, across all time groups, was not meaningful, both in terms of Global R = 0.063 (P = 0.22) and of pairwise comparisons C/Y (R = 0.09, P = 0.21), C/P (R = –0.03, P = 0.55) and Y/P (R = 0.13, P = 0.15).
Figure 3. NMDS of the syllid assemblage found in the natural sediment and fouling in the fire-clay modules, located in two media (water/bottom), at different times and organic matter load. Bray–Curtis similarity coefficient (average linkage) applied to the averaged replicates after square-root transformation. Overlaid clusters are indicated by bold number and letters. See for abbreviations.
Syllis hyllebergi morphometry
Most of the collected specimens of S. hyllebergi are deposited at the Laboratorio del Benthos, Dipartimento di Biologia Animale e Ecologia Marina, Università di Messina, coded as BAEM-PRA07-POLY-Syhil001/053. About 10 of them have been sent to Dr Guillermo San Martín, Departamento de Biología, Facultad de Ciencias, Universidad Autónoma de Madrid, for confirmation of diagnosis.
Fifteen adults and two juveniles from different samples were examined (). In general, the main external morphological characters (prostomium, parapodia, joined and simple chaetae) as well as internal structures (pharynx and proventricule size, tooth position, aciculae) matched the holotype description, but the specimens of the Faro Lake were quite larger than specimens from Red Sea population (a,b). Two small anterior eyespots were present besides the four frontal eyes. Mean body length was up to 5 mm higher and mean width exceed 100 μm; all antennae, peristomial cirri and dorsal cirri showed a greater mode of the article number: median antenna +5 articles; lateral antennae +3; dorsal tentacular cirri +8; ventral tentacular cirri +5; dorsal cirri (mid body region) +7/8 (shortest cirri) or +9/10 (longest cirri) (d,f). In contrast, the total number of chaetae per bundle (falcigers and spiniger-like compound chaetae) in the anterior parapodia was three to five less than reported for the Red Sea specimens, whilst the chaetal number was comparable in the mid and posterior parapodia (e,g,h). In the Sicilian specimens, length of the distal articles of spinigers reached 2.0–2.2-fold those of the holotype, and falcigers were up to 1.5-fold, showing a more stressed size gradient from the uppermost to the lowermost blades (); the mean width was similar. By oil-immersion, most spinigers showed a fine tip and no distinguishable secondary tooth. Differently from the holotype, aciculae were two both in the anterior and most of the middle body parapodia; only one in the hind region. Anal cirri were notably long, with more than 20 articles on average (); the unpaired single-articulated pigidial appendage was visible in most of the preserved specimens (c).
Figure 4. Syllis hyllebergi, Faro Lake, Peloro Cape Lagoon, NE Sicily. (a) Entire worm, adult specimen (scale bar 2 mm) and juvenile specimen (bar 200 μm). (b) Particular of anterior part and pharyngeal apparatus (bar 2 mm). (c) Hind body with long pigidial cirri (bar 100 μm) and the unpaired appendage (top right; bar 40 μm). (d) 35th chaetiger (bar 100 μm). (e) Magnification of the same (bar 100 μm). (f) 95th chaetiger (bar 100 μm) and magnification of the upper falciger (10 μm). (g) Mid body chaetae (35th) (bar 50 μm). (h) Posterior chaetae (93rd) (bar 50 μm).
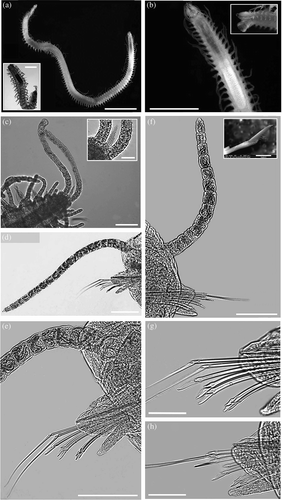
Table II. Syllis hyllebergi morphometry, coastal Lake of Faro, NE Sicily, central Mediterranean Sea
Syllis hyllebergi remarks and geographic distribution
Syllis hyllebergi has been recently described by Licher (Citation1999) for the North Red Sea as Typosyllis hyllebergi, in his accurate revision of the genus, wherein he analysed several morphological and anatomical features to better distinguish the closely related species. S. hyllebergi was included into the ‘cerina-Komplex’, mainly due to the morphology of anterior (both pointed and bent tips) and mid/posterior (thick, pointed flame-shaped tip) aciculae, the presence of spiniger-like chaetae with very fine spines, the presence of distinctly bidentate falcigers with large and often curved spines. The author referred this species was originally identified as S. bouvieri (in Licher Citation1999) and S. (Langerhansia) cornuta (Ben-Eliahu Citation1977); this latter identification was made on type materials from the Bitter Lake (Suez Canal) in 1969, Shavei Zion (Israeli coast) and also Cyprus Island in 1971 (). More recently, Nogueira and San Martín (Citation2002) have recorded the species in the south-eastern Brazilian coasts, associated to the shallow rocky bottom and coral reef. The actual world distribution of this syllid appears therefore to be peculiarly disjoined in two areals: the Red Sea–Indian population (up to the eastern and central Mediterranean); the south-western Atlantic population (, lower pane).
Discussion
Patterns of syllid colonization
Fouling studies focused on the syllid assemblages are not numerous in the scientific literature as well as experimental designs to test their own response to disturbance (see Shull Citation1997; Smith & Rule Citation2002). The present experimentation has attempted to test the small spatial and temporal response of the local syllid assemblage to the introduction of a new granular substratum, conditioned by two increasing levels of organic load. This study indicated that: (i) since the early stage of the fouling process two different assemblages of syllids were established in two different media, one into a ‘hard-bottom’ type substratum, suspended into the water column, and another one into a similar cavitary substratum, but buried into the bottom sediment; (ii) these assemblages showed different temporal patterns, due to the relative proximity of the source area of adult immigrants; (iii) the colonization process was therefore mainly promoted by adult immigration; and (iv) organic enrichment did not appear to affect the overall colonization substantially. The MDS arrangement showed the effect of different colonization sources for the artificial EFCG substratum and also highlighted the two related temporal patterns.
Members of exogonins and eusyllins are often related to incoherent and cavitary substrata, being typical component of the mesopsammon, whilst syllins and autolytins are mainly related to coherent substrata (rocks with algal coverage or living substrata as associates or symbionts) (Cognetti Citation1961; Gidholm Citation1962; Ben-Eliahu Citation1977; San Martín Citation1984; Giangrande Citation1988; Cardell & Gili Citation1988; Nacorda & Yap Citation1997; Martin & Britayev, Citation1998; López & Vièitez Citation1999). Virtually all subfamilies are abundant dwellers of seagrass beds (San Martín & Vièitez Citation1984; Gambi et al. Citation1989; Çinar Citation2003a; Bone & San Martín Citation2003). In this study, the colonization appeared to be promoted by adults migrating laterally from the nearest sedimentary habitat; however, according to what was observed by Shull (Citation1997), colonization was probably due to the bed-load transport of small-sized mesopsammic exogonins and of juveniles, similar to that discussed for the transport of adult specimens in the meiofauna contingent (e.g. Palmer Citation1988). This process explains the lower but significant similarity of the water-suspended modules and the bottom-modules at 15 and 30 days of colonization. The distance of about 0.5 m from the lake floor to the cylinder bottom was enough to allow the active immigration of strictly interstitial species, as also recently observed for a deep environment (Sardá et al, Citation2009). However, after 60 days the suspended modules hosted a fouling syllid assemblage more typical of a true rocky habitat, and their multispecies arrangement was far from the original source environment. Hard-bottom species could colonize late the free substratum as adult stage from more distant coherent substrata (artificial walls, scattered boulders on the lake floor) by crawling or swimming, whereas the early colonization by juveniles was of a lesser extent. On the other side, two months of colonization were not enough to make the artificial bottom-modules significantly similar to its neighbouring source area. This lower dissimilarity, at almost 52.0 of value, was more likely quantitative than qualitative. The low densities of strictly interstitial syllids during the whole experiment in the modules were probably due to the top-down control of the larger peracarid and polychaete predators or competitors that were found in the samples (Cosentino & Giacobbe Citation2011), which probably reduced the population size of the mesopsammon (Edgar Citation1994; Ólafsson Citation2003). The coarser artificial granules and the consequent wider interstitial space provided by the EFCG microhabitat with respect to the natural sediment facilitated such ecological relationships (Frid Citation1989).
The spatial turnover from interstitial to hard-bottom species as a function of grain size, substratum texture and hydrodynamism has been demonstrated by Somaschini and Gravina (Citation1994) for the more complex habitat of the Posidonia meadow. Similarly, Çinar (Citation2003b) stated the existence of several syllid groups with respect to the substratum typology and to its related structural complexity, mainly tied to the vegetal covering. Other studies have also demonstrated the patchiness and the peculiar zonation resulting from several stenotopic syllid species, as well as the wider distribution of other euritopic species in both tropical and temperate habitats (Cognetti Citation1961; Giangrande Citation1988; Gambi et al. Citation1989; Morrisey et al. Citation1992; Böggemann et al, Citation2003; Serrano et al. Citation2006).
The present data have tested on-field the difference of colonization potentialities and habitat choices of some syllids, from a common or different habitat sources, and confirmed the variable breadth of their ecological spectrum. For example, B. arminii, E. naidina, S. clavata and, to a lesser extent, S. armillaris had the widest plasticity to colonize different substrata in different media, whereas other small-sized interstitial species and the new record S. hyllebergi were confined to the natural sands or, as for P. anophthalma and S. gracilis, were good colonizers of the buried EFCG only. In contrast, S. prolifera, S. vittata and T. zebra behaved as explorers from not adjacent substrata, being able to selectively immigrate into the suspended hard-bottom type substratum.
The highest organic enrichment seemed to be almost irrelevant for the colonization process. Several measures of (in)organic nutrients as well as of microbial and microalgal productivity supported the natural mesotrophic condition of the sediments and of the overall water column in the confined Faro Lake (Maugeri et al. Citation2000; Saccà et al. Citation2008; Leonardi et al. Citation2009). The local syllid assemblage is likely to be therefore adapted to live into such a naturally enriched environment, and the chemically induced disturbance by the treatments at the very small scale did not substantially alter the succession stages between the polluted and the unpolluted artificial modules. In addition, the notable intergranular void space of the artificial EFCG substratum allowed for a rapid renewal of the interstitial water, thus favouring the rapid decomposition of the organic matter and a faster removal of toxic catabolites. Nevertheless, the local syllid assemblage clearly showed to be more tolerant of organic contamination with respect to true marine conspecific populations. This aspect deserves particular attention when considering the whole family of Syllidae as a sensitive or tolerant group within transitional water systems, as well as its relative scores in the univariate benthic indices (Borja et al. Citation2000; Simboura & Zenetos Citation2002) for the assessment of the ecological quality of the environment (Water Framework Directive 2000⁄60⁄EC; Borja et al. Citation2009). In this context, the related corrections already proposed by other authors are even more supported (Mistri & Munari Citation2008; Munari & Mistri Citation2008).
The biogeography of Syllis hyllebergi
Recent investigations have provided an updated checklist of the Mediterranean polychaetes and have highlighted the areal shift of some warm/temperate species from the lower to the higher Mediterranean latitudes (Cantone Citation2003; Musco & Giangrande Citation2005; Zenetos et al. Citation2005, Citation2008; Mikac & Musco Citation2010). New records of Lessepsian or warm Atlantic immigrants, together with the absence of recent findings of known cold boreal species among syllids and polychaetes in general, constitute a further evidence of a new warming period for the Mediterranean waters (Bianchi Citation2007).
At the present state of knowledge, if Ben-Eliahu's finding of S. hyllebergi for Cyprus and Israeli Mediterranean coasts is confirmed, its alien status as well as its endemic status for the Red Sea (Wehe & Fiege Citation2002) will be questionable. The living habitats where the species was found in the different locality are also different and, as for other polychaete species, its world distribution is clearly bipolar. On the base of morphological features, the Sicilian specimens are more similar to the Brazilian population in the presence of anterior eyespots and the small number of anterior falcigers. In contrast, the Sicilian population was comparable to the Red Sea population in the length of pharynx, the finely pointed spinigers and the larger size of distal articulation in the posterior small falcigers.
In general, the observations on the population of the Faro Lake widen the size ranges for most of the morphological structures. This fact may be related to the trophic state of the sediments and to the scarcity of sympatric competitors, S. armillaris being the only frequently occurring Syllinae of comparable size in the incoherent sediments. The confined environment agrees with the ecological requirements of this species in its type locality. To date, the distribution of this syllid in the Lake has to be considered as punctiform. If the status of this species will be confirmed as true introduction, its presence would demonstrate the current lati/longitudinal spreading of this warm-water polychaete within the Basin, although at low population density. Due to the confinement effect, the brackish environments may mimic some ecological dynamics (such as water warming, anthropogenic impact) that are going to occur at larger spatio-temporal scale all over the Mediterranean, thus making them the best receivers as well as potential hotspots of spreading for the southern non-native biota. Although the real vector for this species remains unknown, its presence in this marine lake may be tied to a nearby shell-farming facility, which trades and stables living bivalves from the North-East Atlantic (Netherlands, France, Portugal, Spain), North Tyrrhenian (France), North Adriatic (Venice Lagoon) and Aegean Sea (Greece, Turkey, but not from Israel). The experimental findings have demonstrated that S. hyllebergi is strictly related to the sand substratum, showing preference towards heterogeneous sediments with calcareous (shell) debris and a moderate content of organic matter. The sediment entrapped among the highly rough lamellate surface make oysters, more than other farmed bivalves, one of the best potential vectors for this species in the Faro Lake (Reise et al. Citation1998; Cohen & Zabin Citation2009), and they are under investigation for the recent introduction of other allochthonous polychaete worms into the Lake sediments (Cosentino & Giacobbe Citation2011).
Cosmopolitanism is a common feature among Annelid polychaetes (Bhaud Citation1984; Gillet & Dauvin Citation2000; Musco & Giangrande Citation2005). Allopatric populations with a bipolar, multipolar, amphioceanic or pantropical distribution are probably due to the ancient evolutionary course of several polychaete taxa during the Tethyan period and the successive fragmentation into different oceanic realms (Glasby Citation2005 and references therein). On the other hand, recent advances in molecular methodologies have best supported the existence of sibling species as well as of the cryptogenic diversity among sympatric populations inside the same morphologic-type species (e.g. Westheide & Schmidt Citation2003; Nygren et al. Citation2010). Moreover, during the last five centuries, the transport throughout human vectors has determined a sort of ‘secondary’ or ‘anthropogenic cosmopolitanism’. At the present state of knowledge, the hypothesis of an involuntary recent introduction of S. hyllebergi in the Faro Lake is highly supported, although its occurrence by natural processes or even its long time presence in the Lake may not be totally excluded. The observed morphologic variation of some characters among the different world populations deserves a more accurate taxonomic and genetic investigation of this recently recognized syllid.
Acknowledgements
The present research study was funded by PRA-2005 and PRA-2008/2009 projects of University of Messina. The author wishes to thank Dr Andrea Potoschi (BAEM) and Dr Giuseppe Sabatino (Dipartimento di Scienze della Terra, University of Messina) for the support on microphotography and SEM assistance, respectively; Dr Guillermo San Martín for the taxonomic confirmation of diagnosis; and also Professor Salvatore Giacobbe, Professor Adriana Giangrande, Dr Luigi Musco and the anonymous reviewer for their critical suggestion to the early version of the manuscript.
References
- Aguado , MT and San Martín , G. 2009 . Phylogeny of Syllidae (Polychaeta) based on morphological data . Zoologica Scripta , 38 : 379 – 402 .
- Bhaud , M. 1984 . Biologie, morphologie et systématique des annélides polychètes cosmopolites . Oceanis , 10 : 707 – 721 .
- Bellan , G , Desrosierz , G and Willsie , A. 1988 . Use of an annelid pollution index monitoring a moderately polluted litoral zone . Marine Pollution Bulletin , 19 : 662 – 666 .
- Ben-Eliahu , MN. 1977 . Polychaete cryptofauna from rims of similar intertidal vermetid reefs on the Mediterranean coast of Israel and in the Gulf of Elat: Syllinae and Eusyllinae (Polycheata Errantia: Syllidae) . Israel Journal of Zoology , 26 : 1 – 58 .
- Bianchi , CN. 2007 . Biodiversity issues for the forthcoming tropical Mediterranean Sea . Hydrobiologia , 580 : 7 – 21 .
- Böggemann , M , Hessling , R and Westheide , W. 2003 . Horizontal distribution pattern of the syllid fauna (Annelida: Polychaeta) in the fringing reef lagoon of Anse Forbans (Seychelles, Mahé) and redescription of the abundant Streptosyllis aequiseta . Hydrobiologia , 496 : 17 – 26 .
- Bone , D and San Martín , G. 2003 . Ecological aspects of syllids (Annelida: Polychaeta: Syllidae) on Thalassia testudinum beds in Venezuela . Hydrobiologia , 496 : 289 – 298 .
- Borja , A , Franco , J and Pérez , V. 2000 . A marine biotic index to establish the ecological quality of soft-bottom benthos within European estuarine and coastal environments . Marine Pollution Bulletin , 40 : 1100 – 1114 .
- Borja , A , Miles , A , Occhipinti-Ambrogi , A and Berg , T. 2009 . Current status of macroinvertebrate methods used for assessing the quality of European marine waters: Implementing the Water Framework Directive . Hydrobiologia , 633 : 181 – 196 .
- Cantone , G. 2003 . Distribution of benthic polychaetous annelids in the Adriatic Sea with zoogeographic considerations . Biogeographia , 24 : 169 – 193 .
- Cardell , MJ and Gili , JM. 1988 . Distribution of a population of annelid polychaetes in the ‘trottoir’ of the midlittoral zone on the coast of North-East Spain, Western Mediterranean . Marine Biology , 99 : 83v92
- Çinar , ME. 2003a . Ecology of Syllidae (Annelida: Polychaeta) from northern Cyprus (eastern Mediterranean Sea) . Bulletin of Marine Science , 72 : 795 – 811 .
- Çinar , ME. 2003b . Ecological features of Syllidae (Polychaeta) from shallow-water benthic environments of the Aegean Sea, eastern Mediterranean . Journal of the Marine Biological Association of the UK , 83 : 737 – 745 .
- Clarke , KR. 1993 . Non-parametric multivariate analyses of changes in community structure . Australian Journal of Ecology , 18 : 117 – 143 .
- Clarke , KR and Warwick , MR. 2001 . Change in marine communities: an approach to statistical analysis and interpretation , 2nd Plymouth, , UK PRIMER-E Ltd
- Cognetti , G. 1961 . Les Syllidiens des côtes de Bretagne . Cahiers de Biologie Marine , 2 : 291 – 312 .
- Cognetti , G and Maltagliati , F. 2000 . Biodiversity and adaptive mechanisms in brackish water fauna . Marine Pollution Bulletin , 40 : 7 – 14 .
- Cohen , AN and Zabin , CJ. 2009 . Oyster shells as vectors for exotic organisms . Journal of Shellfish Research , 28 : 163 – 167 .
- Cosentino , A and Giacobbe , S. 2011 . The new potential invader Linopherus canariensis (Polychaeta: Amphinomidae) in a Mediterranean coastal lake: Colonization dynamics and morphological remarks . Marine Pollution Bulletin , 62 : 236 – 245 .
- Dean , HK. 2008 . The use of polychaetes (Annelida) as indicator species of marine pollution: A review . International Journal of Tropical Biology , 56 : 11 – 38 .
- Ditzel Faraco , LF and da Cunha Lana , P. 2003 . Response of polychaetes to oil spills in natural and defaunated subtropical mangrove sediments from Paranaguá bay (SE Brazil) . Hydrobiologia , 496 : 321 – 328 .
- Edgar , GJ. 1994 . Observations on the size-structure of macrofaunal assemblages . Journal of Experimental Marine Biology and Ecology , 176 : 227 – 243 .
- Fauchald , K and Jumars , PA. 1979 . The diet of worms: A study of the polychaete feeding guilds . Oceanography and Marine Biology: an Annual Review , 17 : 193 – 284 .
- Frid , CLJ. 1989 . The role of recolonization processes in benthic communities, with special reference to the interpretation of predator-induced effects . Journal of Experimental Marine Biology and Ecology , 126 : 163 – 171 .
- Galil , BS. 2008 . Alien species in the Mediterranean Sea – Which, when, where, why? . Hydrobiologia , 606 : 105 – 116 .
- Gambi , MC , Giangrande , A , Chessa , L , Manconi , R and Scardi , M. 1989 . “ Distribution and ecology of Polychaetes in the foliar stratum of a P. oceanica bed in the bay of Porto Conte ” . In International Workshop on Posidonia oceanica Beds. GIS Posidonie 2 Edited by: Boudouresque , CF , Meinesz , A , Fresi , E and Gravez , V . 175 – 188 .
- Giangrande , A. 1988 . Polychaete zonation and its relation to algal distribution down a vertical cliff in the western Mediterranean (Italy): A structural analysis . Journal of Experimental Marine Biology and Ecology , 120 : 263 – 276 .
- Giangrande , A , Licciano , M and Musco , L. 2005 . Polychaetes as environmental indicators revisited . Marine Pollution Bulletin , 50 : 1153 – 1162 .
- Gidholm , L. 1962 . Sur quelques polychètes syllidiens des sables de la région de Roscoff avec description de deux nouvelles espèces . Cahiers de Biologie Marine , 3 : 249 – 260 .
- Gillet , P and Dauvin , J-C. 2000 . Polychaetes from the Atlantic seamounts of the southern Azores: biogeographical distribution and reproductive patterns . Journal of the Marine Biological Association of the UK , 80 : 1019 – 1029 .
- Glasby , CJ. 2005 . Polychaete distribution patterns revisited: An historical explanation . Marine Ecology , 26 : 235 – 245 .
- Grassle , JF and Grassle , JP. 1974 . Opportunistic life histories and genetic systems in marine benthic Polychaetes . Journal of Marine Research , 32 : 253 – 284 .
- Gray , JS , Wu , RS and Or , YY. 2002 . Effects of hypoxia and organic enrichment on the coastal marine environment . Marine Ecology Progress Series , 238 : 249 – 279 .
- Guelorget , O and Perthuisot , JP. 1992 . Paralic ecosystems: Biological organization and functioning . Vie et Milieu , 42 : 215 – 251 .
- Hughes , DJ , Cook , EJ and Sayer , MDJ. 2005 . Biofiltration and biofouling on artificial structure in Europe: The potential for mitigating organic impacts . Oceanography and Marine Biology: an Annual Review , 43 : 123 – 172 .
- Hulbert , SH. 1984 . Pseudoreplication and the design of ecological field experiments . Ecological Monographs , 54 : 187 – 211 .
- Leonardi , M , Azzaro , F , Azzaro , M , Caruso , G , Mancuso , M , Monticelli , L S , Maimone , G , La Ferla , R , Raffa , F and Zaccone , R. 2009 . A multidisciplinary study of the Cape Peloro brackish area (Messina, Italy): Characterization of trophic conditions, microbial abundances and activities . Marine Ecology , 30 : 32 – 42 .
- Licher , F. 1999 . Revision der Gattung Typosyllis Langerhans, 1879 (Polychaeta: Syllidae) . Morphologie, Taxonomie und Phylogenie. Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft , 551 : 76 – 78 .
- López , E and Vièitez , JM. 1999 . Polychaete assemblages on non-encrusting infralittoral algae from the Chafarinas Islands (SW Mediterranean) . Cahiers de Biologie Marine , 40 : 375 – 384 .
- Martin , D and Britayev , TA. 1998 . Symbiotic polychaetes: Review of known species . Oceanography and Marine Biology: An Annual Review , 36 : 217 – 340 .
- Maugeri , TL , Gugliandolo , C , Caccamo , D and La Rosa , T. 2000 . Biomass and productivity of microbial communities in the brackish environments of Ganzirri and Faro (Messina) . Biologia Marina Mediterranea , 8 : 316 – 321 .
- Mikac , B and Musco , L. 2010 . Faunal and biogeographic analysis of Syllidae (Polychaeta) from Rovinj (Croatia, northern Adriatic Sea) . Scientia Marina , 74 : 353 – 370 .
- Minchin , D. 2007 . Aquaculture and transport in a changing environment: Overlap and links in the spread of alien biota . Marine Pollution Bulletin , 55 : 302 – 313 .
- Mistri , M and Munari , C. 2008 . BITS: A SMART indicator for soft-bottom, non-tidal lagoons . Marine Pollution Bulletin , 56 : 580 – 606 .
- Morrisey , DJ , Howitt , L , Underwood , AJ and Stark , JS. 1992 . Spatial variation in soft-sediment benthos . Marine Ecology Progress Series , 81 : 197 – 204 .
- Munari , C. and Mistri , M. 2008 . The performance of benthic indicators of ecological change in Adriatic coastal lagoons . Throwing the baby with the water? Marine Pollution Bulletin , 56 : 95 – 105 .
- Musco , L and Giangrande , A. 2005 . Mediterranean Syllidae (Annelida: Polychaeta) revisited: biogeography, diversity and species fidelity to environmental features . Marine Ecology Progress Series , 304 : 143 – 153 .
- Nacorda , HME and Yap , HT. 1997 . Structure and temporal dynamics of macroinfaunal communities of a sandy reef flat in the northwestern Philippines . Hydrobiologia , 353 : 91 – 106 .
- Naylor , RL , Williams , SL and Strong , DR. 2001 . Aquaculture – A Gateway for Exotic Species . Science , 294 : 1655 – 1656 .
- Nogueira , JMM and San Martin , G. 2002 . Species of Syllis Lamarck, 1818 (Polychaeta: Syllidae) living in corals in the state of São Paulo, southeastern Brazil . Beaufortia , 52 : 57 – 93 .
- Nygren , A. 1999 . Phylogeny and reproduction in Syllidae (Polychaeta) . Zoological Journal of the Linnaean Society , 126 : 365 – 386 .
- Nygren , A , Eklöf , J and Pleijel , F. 2010 . Cryptic species of Notophyllum (Polychaeta: Phyllodocidae) in Scandinavian waters . Organisms Diversity and Evolution , 10 : 193 – 204 .
- Ólafsson , E. 2003 . Do macrofauna structure meiofauna assemblages in marine soft-bottoms? A review of experimental studies . Vie Milieu , 53 : 249 – 265 .
- Palmer , MA. 1988 . Dispersal of marine meiofauna: A review and conceptual model explaining passive transport and active emergence with implications for recruitment . Marine Ecology Progress Series , 48 : 81 – 91 .
- Pearson , TH and Rosenberg , R. 1978 . Macrobenthic succession in relation to organic enrichment and pollution of the marine environment . Oceanography and Marine Biology: An Annual Review , 16 : 229 – 311 .
- Reise , K , Gollasch , S and Wolff , WJ. 1998 . Introduced marine species of the North Sea coasts . Helgoland Marine Research , 52 : 219 – 234 .
- Rilov , G and Crooks , JA. 2009 . Biological invasions in marine ecosystems. Ecological, management and geographic perspectives. Ecological studies , Berlin : Springer .
- Saccà , A , Guglielmo , L and Bruni , V. 2008 . Vertical and temporal microbial community patterns in a meromictic coastal lake influenced by the Straits of Messina upwelling system . Hydrobiologia , 600 : 89 – 104 .
- Salen-Picard , C , Graham , C and Gérino , M. Éthologie alimentaire d'annélides polychètes endogée: détermination du niveau sédimentaire où s'effectue la collecte de la nourriture . Actes de la 4ème Conférence internationale des Polychètes . 1992 , Angers. pp. 527 – 533 .
- San Martín , G. Biogeography of the Syllids (Polychaeta: Syllidae) from the Spanish Mediterranean coasts . Proceedings of the First Polychaete Conference . 1983 , Sidney. pp. 303 – 322 .
- San Martin , G. 2003 . Annelida Polychaeta II. Syllidae. Fauna Iberica 21 , Madrid : Museo Nacional de Ciencias Naturales .
- San Martín , G and Vièitez , JM. 1984 . “ Anelidos poliquetos de los rizomas de Posidonia oceanica en las costas del Cabo de Palos (Murcia, España) ” . In Interational Workshop on Posidonia oceanica Beds Edited by: Boudouresque , CF , Jeudy de Grissac , A and Olivier , J . 149 – 157 . GIS Posidonie
- Sardá , R , Gil , J , Taboada , S and Gili , JM. 2009 . Polychaete species captured in sediment traps moored in northwestern Mediterranean submarine canyons . Zoological Journal of the Linnean Society , 155 : 1 – 21 .
- Serrano , A , San Martín , G and López , E. 2006 . Ecology of Syllidae (Annelida: Polychaeta) from shallow rocky environments in the Cantabrian Sea (South Bay of Biscay) . Scientia Marina , 70S3 : 225 – 235 .
- Shull , DH. 1997 . Mechanisms of infaunal polychaete dispersal and colonization in an intertidal sandflat . Journal of Marine Research , 55 : 153 – 179 .
- Simboura , N and Zenetos , A. 2002 . Benthic indicators to use in Ecological Quality classification of Mediterranean soft bottom marine ecosystems, including a new Biotic Index . Mediterranean Marine Science , 3 : 77 – 111 .
- Smith , SDA and Rule , MJ. 2002 . Artificial substrata in a shallow sublittoral habitat . Do they adequately represent natural habitats or the local species pool? Journal of Experimental Marine Biology and Ecology , 277 : 25 – 41 .
- Sokal , RR and Rohlf , FJ. 1995 . “ Biometry ” . In The principles and practise of statistics in biological research , 3rd , New York, NY : Freeman .
- Somaschini , A and Gravina , MF. Ecological analysis of some Syllidae (Annelida, Polychaeta) from the central Tyrrhenian Sea (Ponza Island) . 1992 . Actes de la 4ème Conférence internationale des Polychètes , pp. 567 – 573 . Angers
- Streftaris , N , Zenetos , A and Papathanassiou , E. 2005 . Globalization in marine ecosystems: The story of non-indigenous marine species across European Seas . Oceanography and Marine Biology: An Annual Review , 43 : 419 – 453 .
- Wehe , T and Fiege , D. 2002 . Annotated checklist of the polychaete species of the seas surrounding the Arabian Peninsula: Red Sea, Gulf of Aden, Arabian Sea, Gulf of Oman, Arabian Gulf . Fauna of Arabia , 19 : 7 – 238 .
- Westheide , W and Schmidt , H. 2003 . Cosmopolitan versus cryptic meiofaunal polychaete species: An approach to a molecular taxonomy . Helgoland Marine Research , 57 : 1 – 6 .
- Zenetos , A , Çinar , ME , Pancucci-Papadopoulou , MA , Harmelin , JG , Furnari , G , Andaloro , F , Bellou , N , Streftaris , N and Zibrowius , H. 2005 . Annotated list of marine alien species in the Mediterranean with records of the worst invasive species . Mediterranean Marine Science , 6 : 63 – 118 .
- Zenetos , A , Meriç , E , Verlaque , M , Galli , P , Boudouresque , CF , Giangrande , A , Çinar , ME and Bilecenoğlu , M. 2008 . Additions to the annotated list of marine alien biota in the Mediterranean with special emphasis on Foraminifera and Parasites . Mediterranean Marine Science , 9 : 119 – 165 .