Abstract
The larval development of Marphysa gravelyi (Polychaeta: Eunicidae) from Pulicat Lake, South India, was studied using light and scanning electron microscopy. The adults of M. gravelyi have an elongated, reddish-brown body and live inside burrows in the muddy bottom of the lake. They produce benthic jelly masses in which the embryos occupy the core and are protected until the completion of larval development. Four different larval stages were observed: prototrochophore, early metatrochophore, late metatrochophore and nectochaeta. These lecithotrophic larvae are found to be swimming inside the jelly mass. The larval development is completed in 8–10 days. Synchrony was observed in the development of larvae inside the jelly mass. Larval settlement begins after the nectochaeta stage.
Introduction
Polychaetes show diversity in reproductive traits that are unique amongst Metazoa (Wilson Citation1991; Giangrande Citation1997). Among polychaetes, Eunicidae are known to be polytelic and gonochoric, without sexual dimorphism. The reproductive habits studied for relatively few species of Eunicidae show free-spawning, brooding of the eggs, planktotrophism, lecithotrophism and direct development. Some forms even show schizogamy (Wilson Citation1991; Giangrande Citation1997; Rouse & Pleijel Citation2001; Gambi & Cigliano Citation2006).
Among the different species of Marphysa, the larval development of Marphysa sanguinea has been studied in detail (Hanjun et al. Citation1994; Pravedelli et al. Citation2007). In India, larval developmental studies on the genus Marphysa (species not mentioned) from Adyar Estuary was performed as early as the beginning of the twentieth century by Aiyar (Citation1931). Extensive work on various aspects Marphysa gravelyi from Adyar Estuary has been done (Aiyar Citation1933; Krishnamoorthi Citation1951, Citation1962, Citation1966). M. gravelyi of Pulicat Lake has been identified and recorded by Sunderraj and Sanjeevaraj (1984). This species is interesting because of the encapsulation of the eggs in a jelly mass and continuous breeding, which is indicated by the presence of jelly mass throughout the year. The present work is a detailed study of the larval development of M. gravelyi from Pulicat Lake.
Marphysa gravelyi is used as fish bait and also as a feed in aquaculture. The present study will enable understanding of the larval development. It will also help in the artificial culture of these organisms in large quantities to satisfy the feed requirement in the ever-expanding aquaculture industry.
Materials and methods
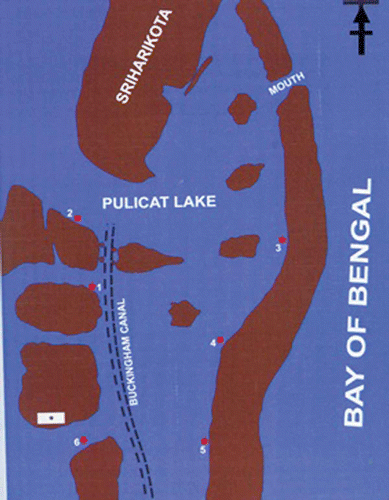
The Pulicat Lake, situated North East of Chennai between latitudes 13°1'–13°24'N and longitudes 80°2'–80°16'E, was selected as the collection site (). Six different locations were selected for collecting jelly masses periodically. The collection of jelly mass was done by scooping out a layer of mud at a depth of about 3 inches. Many jelly masses were found attached to the substratum in a given area of collection. Two to three jelly masses were collected and brought to the laboratory, each in separate containers, for observation. The larvae were separated from the jelly masses using pipettes with pore size larger than the diameter of the larvae. Larvae were observed under the microscope using cavity slides. Different stages of larval development were observed and photographed. The morphology of the larvae was studied using scanning electron microscopy (SEM). For SEM studies, different stages of the larvae were fixed in 2% glutaraldehyde buffered in sodium cacodylate buffer (0.1 M, pH 7.4) for 12 h at 4°C. The samples were then washed with distilled water and dehydrated sequentially in graded alcohol series. After dehydration, samples were critical point-dried and carefully mounted on a metal stub and gold coated. The gold-coated specimens were observed under JEOL-SEM 840 (JEOL Co., Japan). The photographs were taken using AGFA/PAN films. The results are based on observations of larval development in situ for a period of two years from October 2001 to September 2003.
Results
Marphysa gravelyi is a large sized, reddish-brown worm with no sexual dimorphism. The description corresponds to Southern (Citation1921). The fertilized eggs are enclosed inside a jelly mass wherein the indirect development takes place. The jelly masses were pear-shaped with a broad distal end and a narrower proximal end which continues as a stalk for attachment. M. gravelyi produces polylecithal eggs and hence the larvae are lecithotropic. There was accumulation of fertilized eggs towards the central core of the jelly mass where the jelly mass shows a thicker consistency. Peripheral to the central core, the gelatinous substance looks thinner, with an external thin sheath. In an ever-changing brackish environment with many micro- and macro-particles, the jelly mass becomes encrusted with the particles as it ages from the first day through the final settlement of the larvae ().
Based on microscopic observations of morphological characteristics there were four different stages of larval development.
Stage I
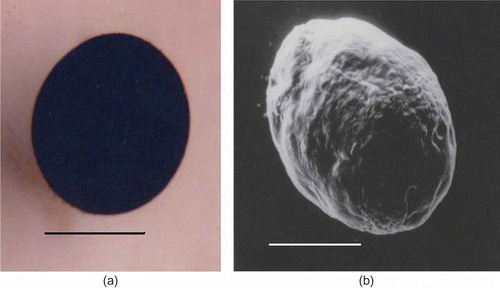
Prototrochophore: the larvae were spherical and uniformly ciliated, measuring 200–220 μm. They were found to revolve in the jelly with the help of cilia. The larvae appeared opaque due to the retention of yolk accumulated during vitellogenesis. The larva utilizes this yolk for its nutrition as it does not feed at this stage. The only observable feature at this stage is the appearance of cilia all over the surface. After 24 h, the spherical prototrochophore larva became oval (a,b, a).
Stage II
Early metatrochophore: the uniformly ciliated larva became oval and lost its ciliation in certain regions. At two-thirds the length of the body in a non-ciliated region, a pair of grooves appears. The first pair of parapodia develops in this region. A pair of eyes develops at the anterior dorso lateral region. This also helped in the identification of anterior and posterior regions of the larva. The anterior region of the larva was broader than the posterior end. The opacity of the larva reduces slightly. The parapodia are a simple structure having a single lobe – the notopodium. A single seta is formed which is unjointed pointed seta. This is followed by another unjointed seta. The movement of the larva inside the jelly mass is restricted. The length of the larva continues to increase and it enters into the next stage (a,b, b).
Stage III
Late metatrochophore: the larva at this stage is twice the length of the prototrochophore larva. Posterior to the first pair of parapodia, the second pair starts appearing. The first pair of parapodia becomes prominent. The larva shows a kind of screwing rotary motion. Occasionally, it contracts and expands its body. The posterior end of the larva becomes narrower.
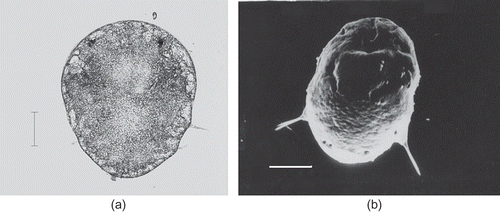
About 36 h after the appearance of the first pair of parapodia, the second pair of parapodia which bear a single jointed seta becomes prominently visible. The cilia continue to be the locomotor organ by which the larva moves in the jelly substance. The larva measures about 360–380 μm in length and 270–280 μm in width. The eyespots were well developed. This stage is called the late metatrochophore (a,b, c).
Figure 5. Marphysa gravelyi. Late metatrochophore. (a) Light microphotograph; (b) electron microphotograph. Scale bar: 100 μm.
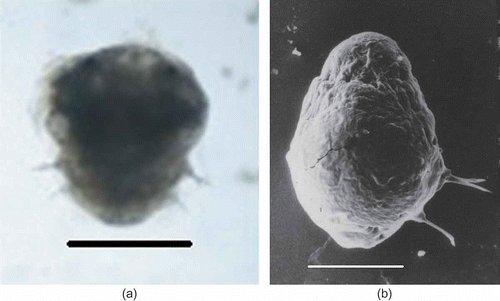
Nectochaeta: the third pair of parapodia develops and cilia disappear. A pair of lobular, fleshy, transparent anal styles is formed at the posterior end. This stage is marked by cephalization with the appearance of a pair of jaws. This stage corresponds to the nectochaeta stage (a,b, d). The body elongates and becomes twice as long as wide. The larva commences the creeping habit. The larva continues to be in this stage for another 24–48 h until the jelly mass disintegrates. Following this, the larva settles to the sediment. The entire larval development takes 8–10 days.
Figure 6. Marphysa gravelyi. Nectochaeta. (a) Light microphotograph; (b) electron microphotograph. Scale bars: (a) 500 μm, (b) 100 μm.
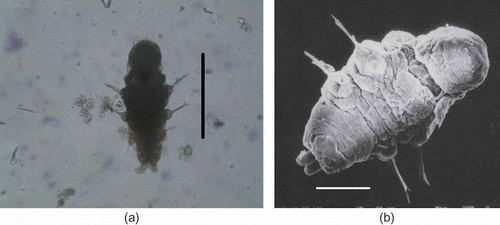
Figure 7. Marphysa gravelyi. Diagrammatic representation of the different developmental stages: (a) prototrochophore; (b) early metatrochophore; (c) late metatrochophore; (d) nectochaeta.
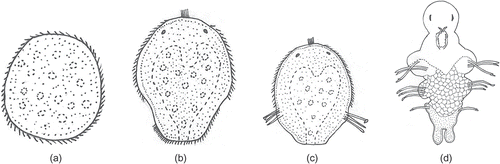
It is observed that there is a synchrony in the development of larvae inside the egg mass. There is also a synchrony in the development of larvae in all the jelly masses collected in a particular area at a particular time.
Discussion
Thorson (Citation1950) pointed out that the egg size in marine invertebrates is indicative of larval life and development pattern. This is shown by an empirical relationship in polychaetes. Small polychaete eggs (50–75 μm in diameter) develop into planktotrophic trochophores. Slightly larger eggs pass through a lecithotrophic, trochophore-like stage, but develop several segments and become metatrochophores or nectochaetae before feeding commences. Larvae from large eggs usually do not begin feeding until they become benthic. They are retained in some sort of protective brood structure and usually have a very short larval stage or no larval stage at all (Schroeder & Hermans Citation1975). Species which produce large eggs often encapsulate them in a jelly mass, which is probably an efficient system of risk avoidance (Pravedelli & Simonini Citation2003). The jelly mass has been attributed with various functions, such as promoting successful fertilization (Sato & Osanai Citation1996), protecting the embryos during early development (Vance Citation1973; Schroeder & Hermans Citation1975) supplying food for the developing embryos (Bookhout & Horn Citation1949; Sato et al. Citation1982) and limiting the dispersal of the juveniles to keep them in a suitable habitat (Chapman Citation1965; Gibbs Citation1968; Sato et al. Citation1982; Nishihira et al. Citation1984). Strathman (Citation1980, Citation1982) suggests that the presence of a jelly mass may be an adaptation to the estuarine condition. The jelly masses were found to tolerate even wide variations in salinity (Krishnamoorthi Citation1962; Chapman Citation1965).
The results showed that there was a synchrony in the development of larvae inside the jelly mass. This was also observed in the case of Nainereis laevigata (Giangrande & Petraroli Citation1991). The synchrony is common in their ribbon shaped gelatinous masses (Chaffee & Strathman Citation1984).
Aiyar (Citation1931) made a detailed description of the development of Marphysa from Adyar estuary, but the species name was not mentioned. He also states that it is different from M. gravelyi reported by Southern (Citation1921).
The apical tuft is a feature of many invertebrate larvae, including the trochophore larvae of polychaetes. Their function may be sensory in nature (Schroeder & Hermans Citation1975). The trochophore stage occurs in the development of most polychaetes, variously modified to size and yolkiness of the egg (Anderson Citation1959). A typical trochophore stage, characterized by having an opposed band method of feeding involving a prototroch and a metatroch (Rouse Citation1999) was not observed during the development of M. gravelyi. This may be due to the fact that there is no necessity for the lecithotrophic larvae inside the jelly mass to feed.
Acknowledgements
I thank the Head and Staff, Department of Zoology, University of Madras, for their help in taking photographs. My thanks are also due to the University Grants Commission, New Delhi for the travel assistance provided for presenting this paper at the International Polychaete Conference held at Lecce, Italy.
References
- Aiyar , RG. 1931 . An account of the development and breeding habits of a brackish water polychaete worm of the genus Marphysa . Journal of the Linnean Society London (Zoology) , 37 : 387 – 403 .
- Aiyar , RG. 1933 . “ On the anatomy of Marphysa gravelyi ” . In Southern Record of the Indian Museum 1933 Calcutta
- Anderson , DT. 1959 . The embryology of the Polychaete Scoloplos armiger . Quarterly Journal of Microscopical Science , 100 : 89 – 166 .
- Bookhout , CG and Horn , EC. 1949 . The development of Axiothella mucosa (Andrews) . Journal of Morphology , 84 : 145 – 185 .
- Chafee , C and Strathmann , RR. 1984 . Constraints on egg masses. I: Retarded development within thick egg masses . Journal of Experimental Marine Biology and Ecology , 84 : 73 – 84 .
- Chapman , G. 1965 . The egg cocoons of Scoloplos armiger . O. F. Muller. Biological Bulletin , 128 : 189 – 197 .
- Gambi , MC and Cigliano , M. 2006 . Observations on reproductive features of three species of Eunicidae (Polychaeta) associated with Posidonia oceanica seagrass meadows in the Mediterranean Sea . Scientia Marina , 70 ( Suppl 3 ) : 301 – 308 .
- Giangrande , A. 1997 . Polychaete reproductive patterns, life cycles and life histories: An over view . Oceanography and Marine Biology , 35 : 323 – 386 .
- Giangrande , A and Petraroli , A. 1991 . Reproduction, larval development and post-larval growth of Nainereis laevigata (Polychaeta, Orviniidae) in the Mediterranean Sea . Marine Biology , 111 : 129 – 137 .
- Gibbs , PE. 1968 . Observations on the population of Scoloplos armiger at Whitstable . Journal of the Marine Biological Association of the UK , 48 : 225 – 254 .
- Cai , Hanjun , Lin , Hou and Minghui , MA. 1994 . A study on the life history of Marphysa sanguinea (Montagu) . Journal of of Liaoning Normal Univrsity (Natural Science Edition) , 4
- Krishnamoorthi , B. 1951 . Studies on the osmotic properties of the eggs and larvae of a brackish water polychaete Marphysa gravelyi Southern . Proceedings of the Indian Academy of Science , 34 : 199 – 209 . sec B
- Krishnamoorthi , B. 1962 . Salinity tolerance and volume regulation in four species of Polychaeta . Proceedings of the Indian Academy of Science , 56 : 363 – 371 .
- Krishnamoorthi , B. 1966 . Physiological studies on Marphysa gravelyi Southern. III. Regulation of body fluid concentration . Proceedings: Plant Sciences , 63 : 117 – 125 .
- Nishihira , M , Tsuchiya , M and Sato , M. 1984 . Dispersal and recruitment of juveniles of the Polychaete Lumbrinereis latreili (Audouin Milne-Edwards) . Bulletin of the Marine Biology Station, Asamushi Tohoku University , 17 : 191 – 203 .
- Pravedelli , D and Simonini , R. 2003 . Life cycles in brackish habitats: Adaptive strategies of some polychaetes from the Venice lagoon . Oceanologica Acta , 26 : 77 – 84 .
- Rouse , GW . 1999 . “ Trochophore concepts: ciliary bands and the evolution of larvae in spiralian metazoa ” . In Biological Journal of the Linnean Society Vol. 66 , 411 – 454 .
- Pravedelli , D , Massamba N'siala , G , Ansaloni , I and Simonini , R. 2007 . Life cycle of Marphysa sanguinea (Polychaeta: Eunicidae) in the Venice lagoon (Italy) . Marine Ecology , 28 : 384 – 393 .
- Rouse , G and Pleijel , F. 2001 . “ Eunicidae Berthold, 1827 ” . In Polychaetes , Edited by: Rouse , GW and Pleijel , F . 155 – 157 . New York, NY : Oxford University Press .
- Sato , M and Osanai , K. 1996 . Role of jelly matrix of egg masses in fertilization of the Polychaete Lumbrinereis latreili . Invertebrate Reproduction and Development , 29 : 185 – 191 .
- Sato , M , Tsuchiya , M and Nishihira , M. 1982 . Ecological aspects of the development of the Polychaete, Lumbrinereis latreili (Audouin Milne-Edwards): Significance of direct development and non simultaneous emergence of the young from the jelly mass . Bulletin of the Marine Biology Station, Asamushi Tohoku University , 17 : 71 – 85 .
- Schroeder , PC and Hermans , CO. 1975 . “ Annelida: Polychaeta ” . In Reproduction of marine invertebrates , Edited by: Giese , AC and Pearse , JS . 1 – 213 . New York, NY : Academic Press .
- Southern , R. 1921 . Fauna of Chilka Lake – Polychaeta . Memoirs of the Indian Museum , 5 : 563
- Strathman , RR. 1980 . Why does a larva swim so long? . Paleobiology , 6 : 373 – 376 .
- Strathman , RR. 1982 . “ Selection for retention or export of larvae in estuaries ” . In Estuarine comparisons , Edited by: Kennedy , BS . 521 – 536 . New York, NY : Academic Press .
- Sunderraj , SK and Sanjeevaraj , PJ. 1987 . Polychaeta of the Pulicat Lake . Journal of the Bombay Natural History Society , 84 : 84 – 104 .
- Thorson , G. 1950 . Reproductive and larval ecology of marine bottom invertebrates . Biological Review , 25 : 1 – 45 .
- Vance , RR. 1973 . On reproductive strategies in marine benthic invertebrates . The American Naturalist , 107 : 339 – 352 .
- Wilson , WH. 1991 . Sexual reproductive modes in polychaetes: Classification and diversity . Bulletin of Marine Science , 48 : 500 – 516 .