Abstract
The chaetal structure of some species of Nephtyidae from the North Polar Ocean has been studied using both stereo compound light microscopy (LM) and scanning electron microscopy (SEM): Aglaophamus malmgreni, A. rubellus, Micronephthys hartmannschroederae, M. minuta, M. neotena, Nephtys assimilis, N. caeca, N. ciliata, N. cirrosa, N. hombergii, N. hystricis, N. kersivalensis, N. longosetosa, N. paradoxa, N. pente and N. rickettsi. All nephtyid chaetae are simple; noto- and neurochaetae are similar. There are four main chaetal types: capillary, barred, chaetae with spines and lyrate (the latter are absent in investigated species). Chaetae that appear smooth under LM usually have at least some small sporadically scattered spines under SEM, but truly smooth chaetae also exist. All barred chaetae are preacicular. Chaetae with spines, and capillary chaetae, are mainly postacicular. The basal regions of chaetae with spines are smooth, and spines appear in sporadically scattered arrangements, gradually becoming more dense but still in irregular rows. In the most ornate cases (spinose chaetae), the spines gradually join together basally, at first in irregular, then regular, rows and form ‘combs’. The number of spines in such combs can increase from 3–4 up to 20–25 along the chaetae. In old chaetae, the combs can become detached as a whole unit. The chaetal morphology is most developed in the middle of a spinose chaeta, and thereafter the pattern and form of the spines changes in reverse order (combs, irregular combs, sporadic spines) terminating in smooth tips. Spinose chaetae of different species and genera can differ in the number of spines forming the combs, the distance between combs and size of the spines. Some species have ‘combs’ with single spines (so-called serrate chaetae). Other species have no distinct combs at all. Geniculate chaetae are a form of serrate chaetae.
Keywords:
Introduction
The form and structure of polychaete chaetae are important in the taxonomy and systematics of the group. For the Nephtyidae, most authors have interpreted chaetae through observations made using stereo compound light microscopes (Ehlers Citation1868, Citation1887; McIntosh Citation1885, Citation1900; Hartman Citation1938, Citation1950, Citation1968; Banse Citation1959; Hartmann-Schröder Citation1959; Fauchald Citation1965, Citation1968; San Martin Citation1982; Imajima & Takeda Citation1985, Citation1987; Hilbig Citation1994, Citation1997; Mackie Citation2000; Dnestrovskaya & Jirkov Citation2001; and others).
However, the small size of chaetae makes complete resolution of structural detail difficult with stereo compound light microscopy (LM). In addition, light aberrations can influence the interpretation of features seen, and affect the accuracy of drawings made. Scanning electron microscopy (SEM) can reveal fine structure in much more detail, but such investigations are rare (e.g. Hausen Citation2005a; Martin et al. Citation2009; Nogueira et al. Citation2010). This study was undertaken to clarify nephtyid chaetal structure using LM and SEM.
Material and methods
This study is based on material collected by Russian and Soviet expeditions and deposited at the Department of Hydrobiology of Moscow State University (DHMSU), Zoological Museum of Moscow State University (ZMUM), and Zoological Institute of Russian Academy of Science (ZIN), St Petersburg (formerly Leningrad). In addition, Micronephthys hartmannschroederae was obtained from the Zoological Museum and Institute of the University of Hamburg (ZMH).
The sampling area covers most parts of the Arctic Ocean, from the Faroe Islands and Iceland to the Bering Strait, and from the upper shelf to abyssal depths ().
Table I. Sampling data
The Barents Sea and the White Sea are the best investigated areas, but samples from the Norwegian and Siberian Seas are also numerous. Specimens were fixed in a 4% formaldehyde seawater solution and transferred to 70% alcohol (ethanol or isopropanol).
Parapodia were cut from the worms and placed in 95% ethanol for 3–5 h, then placed in an ultrasonic generator for 5 min to clear the chaetae of mud particles. The parapodia were subsequently dried on filter paper and transferred to 100% ethanol. After several days, the parapodia were dried as before and glued to SEM stubs then slightly dried near a heater for two or three days and covered by 25 nm Au–Pd for examination at Camscan S-2 Cambridge Instruments 20kv, and a secondary electron image was taken. The following abbreviations have been used: С (chaetiger) followed by a number referring to the segment order (e.g. С-1 means first chaetiger).
Results
The parapodia of Nephtyidae have two large, widely separated branches. The chaetae in each branch are arranged in a deep ‘U’ around the acicular lobe, with pre- and postacicular ‘U’-limbs pointing away from the interramal space constituting the pre- and postacicular rows (Rainer Citation1984, Citation1989, Citation1990, Citation1991). The literature usually refers to these as pre- and postacicular chaetae.
All nephtyid chaetae are simple. Under LM, four basic types of chaetae are easily discerned: barred (specific for Nephtyidae), capillary, chaetae with spines, and lyrate (present only in some Aglaophamus and Micronephthys, but absent in species investigated here). The short (growing) chaetae of all types are seldom seen, though they can be found on any chaetiger.
Epitokous individuals have increased numbers of chaetae, which are more elongated also; no specialized chaetae or parapodial modifications occur.
It should be noted that chaetae examined under LM (using oil immersion), often seem striated with parallel longitudinal stripes (A). SEM photos of fully formed and damaged chaetae reveal collagen fibrils within the chaetae giving this longitudinal striation (B–D).
Figure 1. Structure of neptyid chaetae. A–D, internal structure of postacicular chaetae: A, basal part of chaeta (A. malmgreni); B, broken serrate chaeta (N. hombergii); C,D, ruptured spinose chaeta with ‘combs’ (N. ciliata); E, basal part of barred chaeta (N. paradoxa); F, median part of barred chaeta (A. malmgreni); G, basal part of barred chaeta (N. ciliata); H, distal part of barred chaeta (N. ciliata); I, internal structure of broken barred chaeta (N. sp. nov); J, capillary chaeta (A. malmgreni); K, capillary chaeta (A. malmgreni); L,M, chaeta with tiny spines (apparently smooth using LM): L, N. cirrosa, M, N. assimilis; N, lyrate chaeta (A. dibranchis); O, lyrate chaeta (M. oculifera, LM) . Scale bars: all 10 μm. A,E,F and J drawn using LM; others SEM (except O).
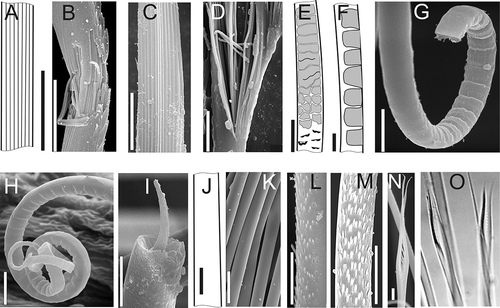
The collagen fibrils consist of coiled microfibrils, which are composed of triple helices containing the coiled protein chains (Hilbig Citation1986, Citation1989; Hausen Citation2005a, Citation2005b).
Barred chaetae (scalloped in profile, F, LM), unique to Nephtyidae, are present only in preacicular rows, first appearing in notopodia of C-1 and neuropodia of C-2.
These chaetae are very flexible and can coil because of their small diameter during the drying process for SEM (G,H). SEM photos show clearly that the barred striations are caused by constriction of the chaetae's body. Transverse sections examined using LM reveal no internal chambers and SEM photos show only a central band of fibrils surrounded by a friable substance (I).
Barred chaetae of C-1 to C-4 are almost completely transversely striated. On subsequent chaetigers the barred striations do not occur throughout the length of the chaetae; a small unbarred granular area occurs at the base of each chaeta (E). The barred chaetae of the posterior-most chaetigers are much thinner and longer and their unbarred part comparatively longer. At the most posterior chaetigers the barred striations on the long chaetae are weak.
Capillary chaetae first appear in the postacicular rows of C-1. At posterior chaetigers they usually form bunches above and below the acicular lobes. In addition, a small number are usually present in the postacicular rows. All capillary chaetae can be divided into thin and truly smooth (J,K), and thicker ones bearing very small scattered spines that are visible only by SEM (L), so we will regard them as chaetae with spines. Short smooth chaetae could be the emergent tips of developing chaetae of other types. Chaetigers with well-developed branchiae can bear up to 30 capillary chaetae, and these are more numerous toward the interramal spaces than the external margins.
Lyrate chaetae are located only in postacicular rows of some species of Aglaophamus and Micronephthys. They are absent in nephtyids from the Arctic Ocean. However, we did examine by LM the lyrate chaetae of M. oculifera in the collections of the National Museum of Wales (O) and A. dibranchis in the collection of the American Museum of Natural History by SEM (N).
Chaetae with spines: fully formed chaetae usually have a smooth basal region; however, spines exist on the ‘bases’ of short developing chaetae. Chaetae with spines are usually present in postacicular rows and are of several types.
In the first type, spines are small, sporadically scattered along the chaetal length. These spines are clearly visible using SEM but, using LM, the chaetae appear slightly striated or sometimes smooth (N. assimilis, N. cirrosa; L,M). Some authors (Hutchings & Glasby Citation1988; Nogueira et al. Citation2010) also noted (for Terebellomorpha) that most of the so-called smooth-tipped chaetae are finely denticulate at high magnification. In the studied species, this type of chaeta occurs only in some Aglaophamus and Nephtys. These chaetae tend to be grouped above and below the acicular lobes (N. assimilis; M). However, in N. cirrosa, such chaetae occur in the middle of postacicular rows (L), and the bunches above and below the acicular lobes are represented by capillary chaetae only.
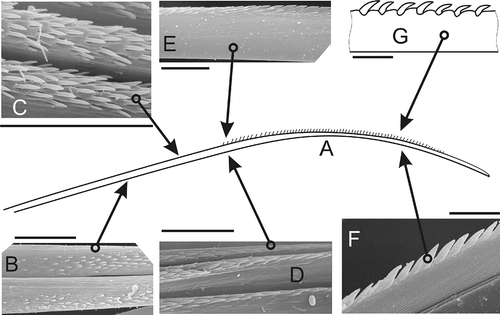
The second type has spines in one lateral row (serrated). Such chaetae have smooth basal regions (A), then small sporadically scattered spines appear prior to forming a single lateral row of large spines (B–D). One or two small spines may initially accompany each large spine, but these soon disappear (E–G). The chaetal tips lack spines. Older chaetae may lose spines due to breakage (H,I).
Figure 2. Serrate chaeta. A, sketch of general view (N. kersivalensis); B, start of small scattered spines (N. hombergii); C, irregular rows of spines (N. kersivalensis); D,E, small spines transitioning into single lateral row of large spines (N. hombergii); F, lateral row of large spines (N. hombergii); G, same (N. hysticis). Scale bars: all 10 μm. A and G drawn using LM, others SEM.
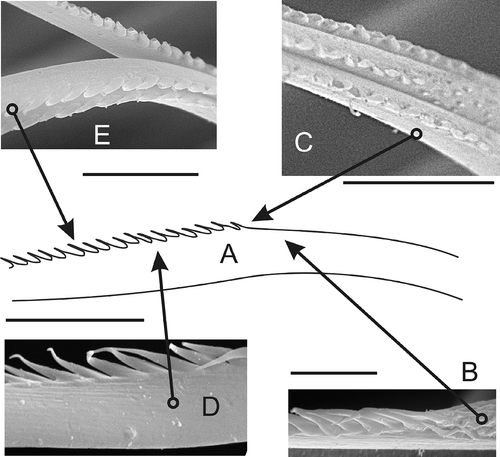
The third type – the geniculate chaetae – was described for N. cirrosa. They are similar to the second type, but smaller and curved in the middle (A–E, 6A–C).
Figure 3. Geniculate chaeta (N. cirrosa). A, sketch of median portion by LM; B,C, small scattered spines transitioning into single lateral row of large spines; D,E, middle part of geniculate chaeta. Scale bars: all 10 μm. A drawn using LM, others SEM.
San Martin (Citation1982) described a fourth type of chaetae with spines; dentate chaetae on notopodia of C-1 in Micronephthys maryae. Such chaetae were absent in the material examined.
The nephtyid species studied have a fifth type in which the spines form very characteristic transversal rows (‘combs’) (A,D,L). Some authors (Rainer & Hutchings Citation1977; Rainer Citation1984, Citation1989, Citation1990, Citation1991; Rainer & Kaly Citation1988; Hilbig Citation1994, Citation1997) separated spinose and spinulose chaetae. The only difference between these types is the size of spines: fine in spinulose and coarse in spinose. However, this difference is subjective; fine for one seems coarse for another, and vice versa. Among species studied by SEM we cannot easily separate spinose and spinulose forms, so we prefer to call all of them spinose. These have the smooth basal regions (B), although scattered spines soon appear (C,J), combining first in irregular (C,K) and then regular rows ( D,L) along the chaetae. The spines in such rows merge basally, forming combs with 8 or 9 teeth in Nephtys (D,L, 5A–L) and up to 22–25 teeth in Aglaophamus (E); combs can sometimes detach entirely (M). In the distal regions of the chaetae, the size of the comb spines decrease and their number reduce to 3–4 per row (E), before they become scattered in irregular rows, and then separate small spines (F). The tips are smooth (G).
Figure 4. Spinose chaeta with combs. A, sketch of general view; B, base of chaeta, showing longitudinal striation of collagen fibrils; C,J,K, scattered spines combine in irregular, then regular rows; D,L, middle part of chaeta with combs; E, reduced combs in subdistal region of chaeta; F, small scattered spines toward distal part of chaeta; G, smooth tip of chaeta showing longitudinal striation of collagen fibrils; H,I, examples of optical aberrations regarding relative length of spines; L,M, detached combs. Scale bars: all 10 μm. M, N. longosetosa, others A. malmgreni; A–I, drawn using LM, others SEM.
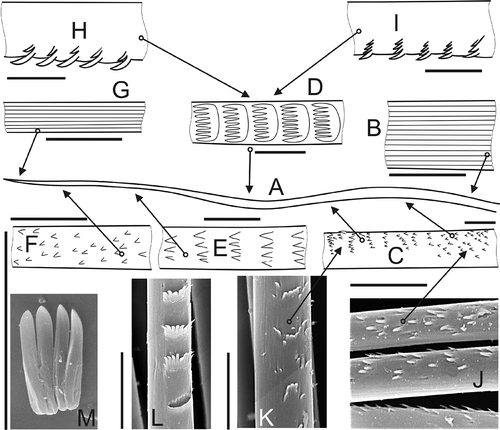
Figure 5. Middle parts spinose chaetae of arctic and north-boreal Nephtys. A,B, N. caeca; C,D, N. ciliata; E,F, N. longosetosa ; G,H, N. paradoxa ; I,J, N. pente; K,L, N. rickettsi. Scale bars: all 10 μm; SEM.
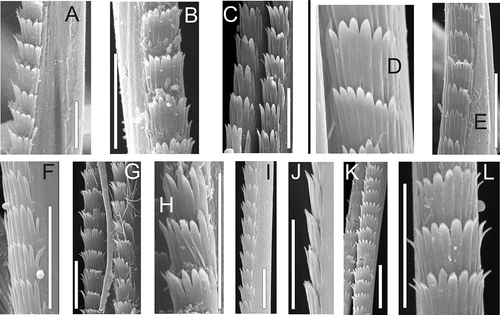
Figure 6. Serrate chaetae of south boreal-lusitanian Nephtys. A–C, N. cirrosa, geniculate chaetae; D–F, N. hombergii; G–I, N. hystricis; J–L, N. kersivalensis; M–O, N. assimilis A,D,E,G,J,K,M,N, lower parts, others middle parts; SEM. Scale bars: all 10 μm.
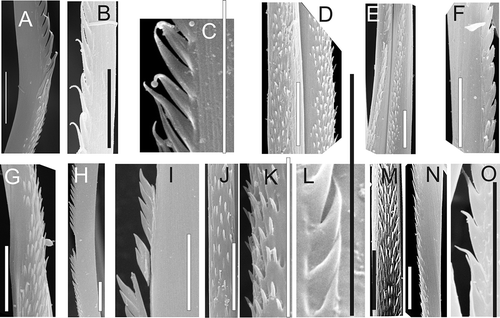
Figure 7. Spinose chaetae (middle parts) showing differences between genera and species. A,B, A. malmgreni; C–E, A. rubellus; F–H, N. ciliata. Scale bars: all 10 μm; SEM.
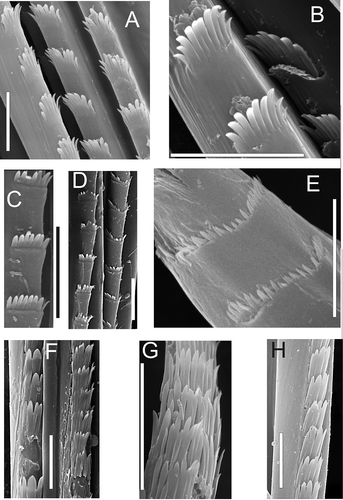
In the literature, drawings (using LM) of chaetae with transverse rows of spines may be found, where the apical spines seem much larger than the others (H,I, 9A). However, we believe that these are optical aberrations and the spines are actually of similar size, as in other spinose (type 5) chaetae (Paraketsova Citation2000).
Discussion
Both examined species of Aglaophamus (A. malmgreni and A. rubellus) have spinose chaetae (A–E). In Nephtys, such chaetae are found in all arctic and north-boreal species (with species ranges include at least the Norwegian Sea, and often more northern areas): N. caeca, N. ciliata, N. longosetosa, N. paradoxa, N. pente and N. rickettsi (A–L, 7F–H). However, in all south boreal-lusitanian species (with species ranges including regions to the south from the Norwegian Sea) – N. assimilis, N. cirrosa, N. hombergii, N. hystricis and N. kersivalensis – the chaetae are without any combs. They are serrate (type 2) with only single lateral rows of spines (A–G, 6D–O), geniculate (type 3) (A–E, 6A–C) or with small, scattered spines along the chaetal length (type 1) (L,M).
It is remarkable that these two groups of species perfectly agree with the results of Ravara et al. (Citation2010) based on the phylogenetic analysis of other morphological characters.
In Micronephthys, the pattern is somewhat the opposite: arctic and north-boreal species (M. minuta and M. neotena) have only serrate chaetae (A–G), while the south boreal-lusitanian M. hartmannschroederae has both serrate and spinose chaetae (A,B).
Figure 8. Serrate chaetae of arctic and north-boreal Micronephthys. A–D, M. minuta; E–G, M. neotena. A,G, lower parts, others middle parts. Scale bars: all 10 μm; SEM.
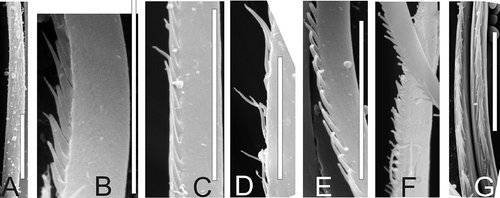
Figure 9. Chaetae of south boreal-lusitanian Micronephthys hartmannschroederae. A, spinose; B, serrate. Scale bars: all 10 μm. Both LM drawings, middle parts.
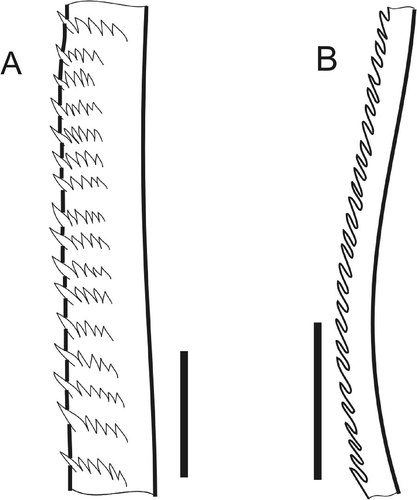
Obvious differences in the structure of spinose chaetae are present only in species of Aglaophamus. The arctic-atlanto-pacific eurybathic A. malmgreni (A,B) can be readily distinguished from the more southern Faroe–Icelandic shallow-water A. rubellus (C–E). The chaetae of the latter have 2–3 times more spines per comb than those of A. malmgreni. Furthermore, the comb spines of A. rubellus are 2–3 times smaller. In SEM photos we can also see a difference between genera. In Aglaophamus the combs are well-separated (A–E), while in Nephtys the combs are much closer and often overlap (F–H).
Acknowledgements
We would like to thank G. Davidovich and A. Bogdanov (Electron Microscopy Laboratory, Biology Faculty, Moscow State University, Russia); Dr M. Siddall and Dr C. LeBeau (American Museum of Natural History, New York, USA); Dr A. Mackie (National Museum of Wales, Cardiff, UK), Dr G. Hartmann-Schröder (Zoological Museum and Institut of Hamburg University, Germany), Dr G. Paterson, A. Muir, E. Sherlock (National History Museum, London, UK), Dr J. Kongsrud (National History Museum, Bergen, Norway), Dr J. Sorensen (Kaldbak Marine Biological Laboratory, Kaldbak, Faroe Islands, Denmark); Dr M. Aguado (Universidad Autonoma de Madrid, Madrid, Spain); Dr A. Sikorsky (Akvaplan-Niva, Tromsö, Norway); Dr A. Ravara (Universidade de Aveiro, Aveiro, Portugal) and Dr E. Kupriyanova (Marine Invertebrates Australian Museum, Sydney, Australia) for the useful hints, critical remarks and for the use of material and Dr D. Hall and T. Worsfold (Unicomarine, Thomson, Letchworth, UK) for the loan of material. A special thanks to Lisa Grubb (Marine Ecological Surveys Ltd, Bath, UK) for correcting our English.
References
- Banse , K. 1959 . Polychaeten aus Rovinj (Adria) . Zoologischer Anzeiger , 162 : 295 – 313 .
- NYu , Dnestrovskaya and Jirkov , IA. 2001 . “ Nephtyidae Grube, 1850 ” . In Polychaeta of the Arctic Ocean , Edited by: Jirkov , IA . 181 – 212 . Moscow : Yanus-K . [in Russian]
- Ehlers , E. 1868 . “ Die Borstenwürmer (Annelida Chaetopoda) nach systematischen und anatomischen Untersuchungen dargestellt ” . In Leipzig: Wilhelm Engelmann Volume 1 , 269 – 748 . pls 12–24
- Ehlers , E. 1887 . Reports on the results of dredging, under the direction of L.F. Pourtalès during the years 1868–1870, and of Alexander Agassiz, in the Gulf of Mexico (1877–78), and in the Caribbean Sea (1878–79), in the U.S. Coast Survey Steamer ‘Blake’, Lieut.-Com. C.D, Sigsbee, U.S.N., and Commander J.R. Bartlett, U.S.N., commanding. XXXI. Report on the annelids . Memoirs of the Museum of Comparative Zoology at Harvard College , 15 : 1 – 335 . pls 1–60
- Fauchald , K. 1965 . Some Nephtyidae (Polychaeta) from Australian waters . Records of the Australian Museum , 26 : 333 – 340 .
- Fauchald , K. 1968 . Nephtyidae (Polychaeta) from the Bay of Nha Trang, South Viet Nam . Naga Report Scientific results of marine investigations of the South China Sea and the Gulf of Thailand 1959–1961 , 4 : 5 – 33 .
- Hartman , O. 1938 . Review of the annelid worms of the Family Nephtyidae from the northeast Pacific, with descriptions of five new species . Proceedings of the United States National Museum , 85 : 143 – 158 .
- Hartman , O. 1950 . Goniadidae, Glyceridae and Nephtyidae . Allan Hancock Pacific Expeditions , 15 : 1 – 181 .
- Hartman , O. 1968 . Atlas of the errantiate polychaetous annelids from California , 828 Los Angeles, CA : Allan Hancock Foundation, University of Southern California .
- Hartmann-Schröder , G. 1959 . Zur Ökologie der Polychaeten des Mangrove-Estero-Gebietes von El Salvador . Beiträge zur neotropischen Fauna , 1 : 69 – 183 .
- Hausen , H. 2005a . Chaetae and chaetogenesis in polychaetes (Annelida) . Hydrobiologia , 535/536 : 37 – 52 .
- Hausen , H. 2005b . Comparative structure of the epidermis in polychaetes (Annelida) . Hydrobiologia , 535/536 : 25 – 35 .
- Hilbig , B. 1986 . Light and electron microscopical studies of the cuticle, epidermis, and setae of some Eunicida (Annelida, Polychaeta) I . Cuticle. Zoologische Jahrbucher Abteilung fur Anatomie und Ontogenie der Tiere , 114 : 1 – 41 .
- Hilbig , B. 1989 . Vergleichende licht- und elektronenmikroskopische Untersuchungen an Cuticula, Epidermis und Borsten einiger Eunicida (Polychaeta, Annelida). III. Borsten. Zoologische Jahrbűcher . Abteilung fűr Anatomie und Ontogenie der Tiere , 119 : 281 – 301 .
- Hilbig , B. 1994 . “ Family Nephtyidae Grube, 1850 ” . In Taxonomic atlas of the benthic fauna of the Santa Maria Basin and Western Santa Barbara Channel , Edited by: Blake , J and Hilbig , B . Vol. 4 , 329 – 362 . Santa Barbara, CA : Santa Barbara Museum of Natural History .
- Hilbig , B. 1997 . “ Family Nephtyidae Grube, 1850. In: Blake JA, Hilbig B ” . In Taxonomic atlas of the benthic fauna of the Santa Maria Basin and Western Santa Barbara Channel 4. The Annelida Part 1 – Oligochaeta and Polychaeta: Phyllodocida (Phyllodocidae to Paralacydoniidae). Revised , Edited by: Scott , PH . 317 – 347 . Santa Barbara, CA : Santa Barbara Museum of Natural History .
- Hutchings , P and Glasby , CJ. 1988 . The Amphitritinae (Polychaeta: Terebellidae) from Australia . Records of the Australian Museum , 40 : 1 – 60 .
- Imajima , M and Takeda , Y. 1985 . Nephtyidae (Polychaeta) from Japan. I. The genera Inermonephthys, Micronephthys, and Aglaophamus . Bulletin of National Science Museum, Tokyo, Series A (Zoology) , 11 : 57 – 90 .
- Imajima , M and Takeda , Y. 1987 . Nephtyidae (Polychaeta) from Japan. II. The Genera Dentinephtys and Nephtys. Bulletin of the National Science Museum, Tokyo . Series A (Zoology) , 13 : 41 – 77 .
- Mackie , ASY. Micronephthys oculifera (Polychaeta: Nephtyidae), a remarkable new species from Hong Kong, China . Proceedings of the Sixth International Polychaete Conference . Curitiba, Brazil 1998. Edited by: Reish , DJ . Vol. 67 , pp. 517 – 528 . Bulletin of Marine Science .
- Martin , D , Gil , J and Lana , PC . Inermonephtys brasiliensis sp. nov. (Polychaeta: Nephtyidae) from SE Brazil, with a redescription of I. palpata Paxton, 1974 . Proceedings of the Ninth International Polychaete Conference . 2007 , Portland, Maine, USA. Edited by: Maciolek , NJ and Blake , JA . Vol. 2 , pp. 165 – 177 . Zoosymposia .
- McIntosh , WC. 1885 . Report on the Annelida Polychaeta collected by H.M.S. Challenger during the years 1873–76 . Report on the Scientific Results of the Voyage of H.M.S. Challenger, Zoology , 12 : 1 – 554 . pls 1–55, 1A–39A
- McIntosh , WC. 1900 . Notes from the Gatty Marine Laboratory, St. Andrews, No. XX. 1. On the reproduction and developement of Pholoe minuta, O. Fabr. 2. On the British Nephthydidae. 3. On Nephthys (Aglaophanus) inermis, Ehlers, from the ‘Porcupine’. 4. On the Nephthydidae of the Gulf of St. Lawrence, Canada . The Annals and Magazine of Natural History, Series 7 , 5 : 254 – 268 . pls 7, 8
- Nogueira , JMM , Hutchings , PA and Fukuda , M. 2010 . Morphology of terebelliform polychaetes (Annelida: Polychaeta: Terebelliformia), with a focus on Terebellidae . Zootaxa , 2460 : 1 – 185 .
- Paraketsova , NYu. The structure of setae in Arctic Nephtyidae . Materials of international scientific conference ‘Aquatic ecosystems and organisms – 2’ . June 23–24 2000 , Moscow. pp. 63 Moscow : MAX Press. 3 . [in Russian]
- Rainer , SF. 1984 . Nephtys pente sp. nov. (Polychaeta: Nephtyidae) and a key to Nephtys from northern Europe . Journal of the Marine Biological Association of the United Kingdom , 64 : 899 – 907 .
- Rainer , SF. 1989 . Redescription of Nephtys assimilis and N. kersivalensis (Polychaeta: – Phyllodocida) and a key to Nephtys from northern Europe . Journal of the Marine Biological Association of the United Kingdom , 69 : 875 – 889 .
- Rainer , SF. 1990 . The genus Nephtys (Polychaeta: Phyllodocida) in northern Europe: Redescription of N. hystricis and N. incisa . Journal of Natural History , 24 : 361 – 372 .
- Rainer , SF. 1991 . The genus Nephtys (Polychaeta: Phyllodocida) of northern Europe: A review of species, including the description of N. pulchra sp. n. and a key to the Nephtyidae . Helgoländer Meeresuntersuchungen , 45 : 65 – 96 .
- Rainer , S and Hutchings , P. 1977 . Nephtyidae (Polychaeta: Errantia) from Australia . Records Australian Museum , 31 : 307 – 347 .
- Rainer , SF and Kaly , UL. 1988 . Nephtyidae (Polychaeta: Phyllodocida) of Australia: New species from the North West Shelf, and a key to Australian species . Journal of Natural History , 22 : 685 – 703 .
- Ravara , A , Wiklund , H , Cunha , MR and Pleijel , F. 2010 . Phylogenetic relationships within Nephtyidae (Polychaeta, Annelida) . Zoologica Scripta , 39 : 394 – 405 .
- San Martin , G. 1982 . Una neuva especie de Nephtyidae (poliquetos: errantes) del Mediterraneo: Micronephthys maryae n. sp . Cahiers de Biologie Marine , 23 : 427 – 434 .