Abstract
Populations of Alitta (=Neanthes) succinea (Frey and Leuckart, 1847) collected from two closely situated locations on the Romanian coast of the Black Sea were analysed from March 2007 to May 2008 in order to elucidate their population dynamics and genetics. The mean density was 655 ind. m−2, presenting two peaks, the first in June 2007 with 950 ind. m−2 and the second in November 2007 with 1633 ind. m−2. The mean biomass over entire period of survey was 4.33 g dry weight (DW) m−2, with a minimum of 1.15 g DW m−2 in September 2007 and a maximum of 8.08 g DW m−2 in June 2007. The decrease of density and biomass in winter is determined by the mortality of adults, whereas the increase in numerical abundance in late spring–early summer and especially in the autumn can be explained by the recruitment of juveniles. The analysis of the size frequency histograms indicated the existence of two recruitment periods: the first in May–June and the second in September–November. From June 2007 to May 2008, the annual secondary production, mean biomass and the production/biomass ratio (P/) for the entire population were estimated to be 5.66 g DW m−2 year−1, 4.22 g DW m−2 and 1.34 year−1, respectively.
Extracts for isozyme analysis were prepared from 23 individuals from the polluted Danube–Black Sea Canal (population C) and 25 individuals from relatively pristine area situated at only 2 km distance (population A). A genetic analysis was conducted with 29 DNA markers on 10 individuals from population A and 10 individuals from population C. The value of Nei's genetic index was h = 0.1873 for population A, h = 0.2099 for population C and h = 0.2172 for the total, while the gene flow was Nm = 1.12. The results showed that the two sampled populations are genetically close and could be considered as a single population and genetic pool.
Introduction
The polychaete Alitta (= Neanthes) succinea (Frey and Leuckart, 1847), also known as ‘pileworm’ or ‘clam worm’, is a cosmopolitan coastal species which is commonly encountered in brackish-water bays, marshes, lagoons and estuaries from tropical and equatorial regions (Rioja Citation1946). This worm is considered native for the Northern Atlantic, but has been presumably introduced in many parts of the world: Mediterranean Sea (Laubier Citation1962; Castelli et al. Citation1995), Sea of Azov (Stark Citation1959), Atlantic coasts of Africa (Day Citation1967), Central and South America (Orensanz & Estivariz Citation1971; Elias et al. Citation2003), Pacific coast of North America, Salton Sea (Detwiler et al. Citation2002; Swan et al. Citation2007), and southern Australia (Wilson Citation1984). In the Black Sea, Alitta succinea was recorded for the first time by Annenkova (Citation1929) in the relic Lake Paliastomi, situated near the city of Poti (Caucasian coast), which is connected to the sea by a narrow channel. At the Romanian coast the species was reported for the first time by Dumitrescu (Citation1957). In the 1980s–1990s, the species became extremely common in local benthic communities due to severe eutrophication and pollution of the northwestern Black Sea (Ţigănuş Citation1986, Citation1988, 1992; Surugiu Citation2000, Citation2003, 2005a, 2005b). Losovskaya (Citation1977, Citation1988) even speak about the establishment in some areas of the north-western part of the Black Sea of a new biocoenosis with Alitta succinea which replaced the mussel biocoenosis.
Alitta succinea inhabits different types of substrate and occurs commonly in dock-fouling communities of local marinas (Dumitrescu Citation1962; Pettibone Citation1963; Detwiler et al. Citation2002; Surugiu Citation2005a). This species is very eurythermal and euryhaline, being capable of supporting salinities ranging between 0.14 and 80 PSU (Kuhl & Oglesby Citation1979; Neuhoff Citation1979; Rasmussen Citation1994) and temperatures between 0.9 and 36°C (Losovskaya Citation1977). As well, this worm is tolerant to hypoxia and temporary anoxia and is resistant to the presence of the hydrogen sulphide in pore water (Miron & Kristensen Citation1993; Rasmussen Citation1994; Como & Magni Citation2009).
Alitta succinea is an active worm, but it usually hides during daylight in mucus-lined U-shaped burrows, emerging to feed at night. The worm is a typical surface deposit-feeder, feeding on detritus and plant material (Stark Citation1959; Losovskaya Citation1977; Kisseleva Citation1981). The reproduction of Alitta succinea was studied by Zeeck et al. (Citation1990, Citation1998a, Citation1998b), Ram et al. (Citation1999) and Hardege et al. (Citation2003). During reproduction, both sexes transform into epitokes, which are reproductive forms called heteronereids. Reproductive swarming is thought to be triggered by a complex set of exogenous cues including temperature, salinity, photoperiod and lunar period (Hardege et al. Citation1990).
Alitta succinea is a major link between detritus accumulated at the water–sediment interface and organisms from higher trophic levels, such as fish and waterfowl (Stark Citation1959; Detwiler et al. Citation2002). This species is often used as live bait by recreational fishermen (Surugiu Citation2008). It is also known as an opportunistic species, reaching very high densities in areas subjected to severe disturbance and organic enrichment (Grassle & Grassle Citation1974; Pearson & Rosenberg Citation1978; Elias et al. Citation2003; Surugiu Citation2005b, Citation2009). The species is a biological model used in experimental systems rich in organic matter (Tenore Citation1982; Bartoli et al. Citation2000; Swan et al. Citation2007).
Despite the fact that Alitta succinea can serve as an important bioindicator of perturbations in the Black Sea, its population biology and genetics is poorly known. Therefore, the purpose of this study is to provide first set of data on the population dynamics and genetic structure of Alitta succinea from Romanian coast of the Black Sea.
Material and methods
Study area
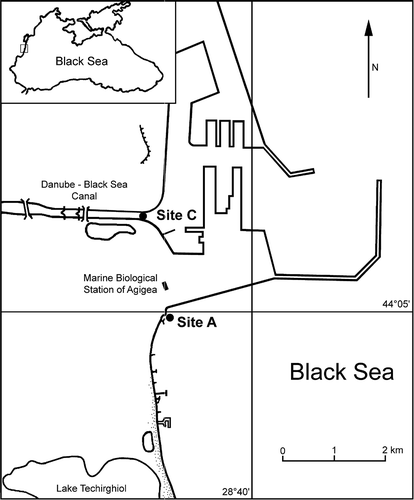
The survey was carried out from March 2007 to May 2008 in two sites called station A (Agigea) and station C (marine part of the Danube–Black Sea Canal). Station C is placed at the end of the Danube–Black Sea Canal where it opens towards the port of Constanta South (44°05′55.05′′N; 28°38′19.75′′E). In this station occurs a mixing of freshwater coming from the Danube and seawater coming from the Black Sea (salinity range = 3.2–15.1 PSU). The water temperature varied between 1.0°C in February 2008 and 28.5°C in August 2007. Because the Danube brings water rich in nutrients and because of reduced water circulation, this area experiences episodic severe eutrophication events, followed by hypoxia or even anoxia. During the survey period, the dissolved oxygen concentration varied between 4.20 mg l−1 in August 2007 and 10.73 mg l−1 in January 2008. The substrate of station C is composed of large boulders overgrown by aggregates of Mytilus galloprovincialis and green algae (mainly Enteromorpha sp. and Cladophora sp.). The high rates of sedimentation cause the clogging of this artificial hard substrate by fine, muddy particles. Station A is placed close to the fisheries of Agigea (44°04′53.05′′N; 28°38′28.92′′E), at only 2 km distance from the station C, but separated from this by a 5.5-km long dam (). This station is considered healthier than station C because it is situated outside of the nearby Constanţa South seaport. Thus, station A is characterized by more stable salinity (15.6 PSU in October 2007 and 17.9 PSU in June 2007), temperature (9.0°C in April 2007 and 24.6°C in August 2007) and dissolved oxygen conditions (6.2 mg l−1 in September 2007 and 11.7 mg l−1 in July 2007) as well as by much attenuated water pollution. The seabed of station A is composed by Sarmatic limestone plates with algal cover.
Population dynamics
To study the population structure of Alitta succinea it was necessary to have a sufficient number of individuals each sampling month to elucidate the size frequency distribution. Each month, three replicate samples were collected in station C from 0.5 to 1 m depth by scraping off the mussel epibionts with a knife from an area of 20 × 20 cm. Specimens were separated using sieves with a mesh size of 0.5 mm, preserved with 4% neutral formalin and later stored in 70% ethanol. Size frequency was studied by measuring the length of the first three chaetigers (L3) of all individuals with an ocular micrometer mounted on a stereomicroscope to the nearest 25 μm. The fresh weight of the individuals (FW) was determined by blotting preserved specimens on a filter paper and subsequent weighing with an analytical balance of ± 0.0005 g sensitivity. For the determination of dry weight (DW) the worms were dried in an oven at 65–70°C to a constant weight and weighted on an analytical balance to the nearest 0.001 g.
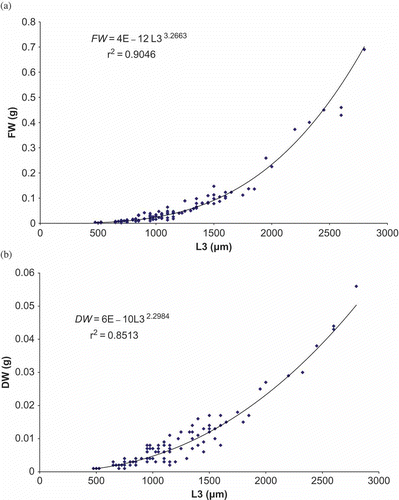
The size frequency histograms were obtained by the FISAT II Software package with ELEFAN I Program (Pauly & David Citation1981; Gayanilo et al. Citation1988, Citation2005; Gayanilo & Pauly Citation1989). The relationship between the length of the first three chaetigers (L3) and FW and between the length L3 and the DW of 100 intact individuals were compared (). For the fresh weight, the relationship was FW = 4E–12L33.2663 (r 2 = 0.9046; N = 100) and for the dry weight the relationship was DW = 6E–10L32.2984 (r 2 = 0.8513; N = 100).
Figure 2. Relationship between the length L3 and the fresh weight (a) and between the length L3 and the dry weight (b) of Alitta succinea from station C, Romanian coast of the Black Sea (N = 100 individuals).
The secondary production (P) was estimated by the method of Crisp (Citation1971) with the equation P = [(N
1 + N
2)/2]·(W
2 – W
1), where N
1 and N
2 represents the number of individuals in a cohort at time 1 and 2, and W
1 and W
2 are the mean fresh weight of a cohort at time 1 and time 2. The secondary production and biomass ratio (P/) was calculated for a period of one year.
Population genetics
Isozymes. Extracts for isozymes analysis were prepared from 23 individuals from station C and 25 individuals from station A. Preparations for gel electrophoresis were obtained by whole worms' homogenization in a 1 μl mg−1 fresh weight buffer solution (Tris 0.2 M, EDTA Na2 1mM, β-mercaptoethanol 25mM, pH 7). The homogenate was then centrifuged for 15 min at 10,000 g and the supernatant subjected to an electrophoresis on starch or polyacrylamide gel.
Electrophoresis was carried out as previously described by Acquaah (Citation1992). Two different methods were used to resolve six enzymes: (I) for isocitrate-dehydrogenases (IDH), malate-dehydrogenases (MDH), lactate dehydrogenases (LDH), leucine aminopeptidases (LAP) and phosphoglucomutases (PGM), horizontal 11% starch gel with a continuous buffer system histidine-citrate pH 6.5 (Cardy & Beversdorf Citation1984) were conducted during 5 h at 300 V; (II) for esterases (EST), vertical discontinuous polyacrylamide gel (9% acrylamide TBE pH 8.3 running gel; TBE pH 6.9 stacking gel; Acquaah Citation1992) was used. Enzyme activity was visualized according to standard staining procedures (Acquaah Citation1992).
Genotype frequencies were obtained by direct count from the enzyme electrophoresis patterns on PGM zymograms. Allele frequencies, observed (Ho) and expected (He) heterozygosity values, inbreeding coefficient (Fis) and genetic differentiation coefficient (Fst) between the two populations studied, were estimated using the GENETIX v4.05 software.
DNA extraction and PCR amplification
Total DNA was extracted from 100 mg of tissue from 10 individuals for each population using ‘DNeasy Blood & Tissue Kit’ (QUIAGEN). Amplification of genomic DNA was performed in an Eppendorf MasterCycler using arbitrary decamers (Williams et al. Citation1990). Amplifications were performed in a total volume of 25 μl reaction mixture containing 100 μM dNTP (Promega), 2 mM MgCl2, Green Flexi 1X buffer, 20 nM primer (MWG Biotech), 0.2 U GoTaq® Flexi DNA Polymerase (Promega) and 50 ng of genomic DNA template. The thermocycler was programmed for 45 cycles of 30 s 94°C, 30 s 36°C, 1 min 72°C with a pre-cycle of 2 min at 94°C and a final extension at 72°C for 10 min. Primer sequences and annealing temperatures are: RAPD08: GAAACACCCC (36°C); RAPD10: AGGGCCGTCT (36°C). These primers were chosen randomly as only four DNA sequence data were available from Genbank for Alitta succinea (28S and 18S ribosomal RNA and omega and theta glutathione S-transferase mRNA). The amplified products were separated according to size by electrophoresis on 1.4% agarose gels buffered with 0.5× TBE, stained with ethidium bromide, then visualized under ultraviolet light and recorded using a video camera. Negative control samples containing all reaction mixture except DNA were also included in each polymerase chain reaction (PCR) run in order to check that no DNA contamination occurred. Repeatability was assayed for each primer on random samples.
Genetic analysis of Random Amplification of Polymorphic DNA (RAPD) data
Only reproducible and strong amplified bands were scored as present (1) or absent allele (0). Bands of similar molecular size, scored for the same primer, were assumed to be homologous. All marker data were converted into a binary character matrix and a set of standard measures of genetic diversity were computed from this matrix using the POPGEN v1.32 software (Yeh et al. Citation1999). The percentage of polymorphic loci (PPB), Nei's genetic diversity h (Nei Citation1973) and Shannon's index I (Lewontin Citation1972) were calculated for both groups and at the population level. The coefficient of genetic differentiation (Gst) and the average of gene flow among population (Nm) were estimated at the population level. An UPGMA (unweighted pair group method with arithmetic averaging) cluster analysis of distance values was obtained based on the genetic distance matrix generated between pairs of individuals according to Nei (Citation1972).
Results
Population dynamics
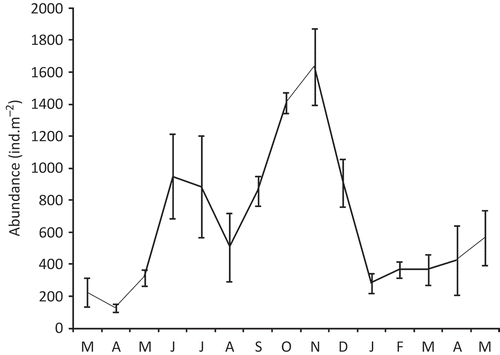
A total of 1171 individuals were collected during the 15 months of sampling. The mean density of Alitta succinea was 655 ind. m−2, with two peaks, the first in June 2007 with 950 ind. m−2 and the second in November 2007 with 1633 ind. m−2 (). The smallest densities were observed in April 2007 with 125 ind. m−2 and January 2008 with 283 ind. m−2.
Figure 3. Temporal variation of density of the population of Alitta succinea from station C, Romanian coast of the Black Sea (mean values ± SD).
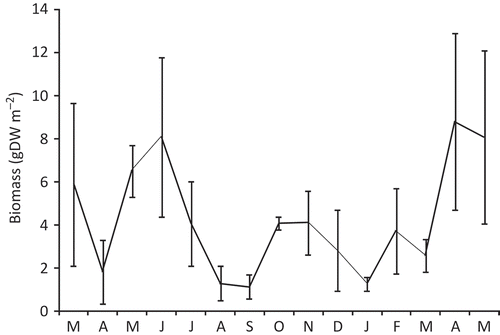
The biomass showed temporal variations, with the highest values in June 2007 and April 2008 with 8.08 g DW m−2 and 8.79 g DW m−2, respectively, and the lowest values in August 2007 and September 2007 with 1.31 g DW m−2 and 1.15 g DW m−2, respectively (). The mean biomass over the entire period of survey was 4.33 g DW m−2. The decrease of density and biomass reflects, most probably, the mortality of adults in winter and the increase of density in late spring–early summer and in the autumn reflects the recruitment of juveniles.
Figure 4. Temporal variations of the biomass (wet weight) of the population of Alitta succinea from station C, Romanian coast of the Black Sea (mean values ± SD).
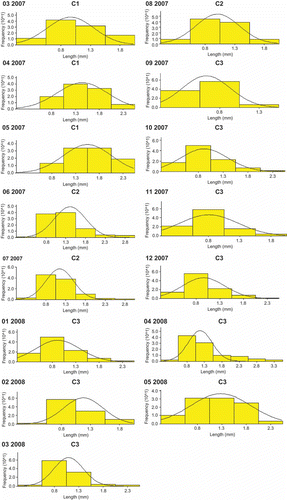
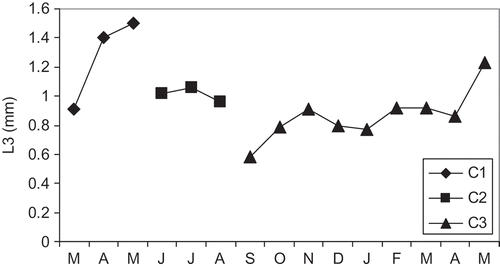
The individual length L3 of worms varied from 0.275 to 3.0 mm. The maximum mean value of L3 was 1.54 mm for cohort C1 in May 2007 and the minimum mean value was 0.60 mm for cohort C3 in September 2007. The fresh weight (FW) of undamaged worms varied from 0.004 to 0.690 g, while the dry weight (DW) varied from 0.001 to 0.056 g ().
The size frequency histograms were separated into 7 classes based on the length L3 with a 0.5-mm class interval (). All size frequency histograms showed unimodal groups with normal distribution. Three of such groups, corresponding to cohorts (C1, C2 and C3) were observed: a first cohort C1 which disappeared in May 2007, a second cohort C2 occurring from June to August 2007 and a third cohort C3 from September 2007 to May 2008 (). These results suggest that there are two generations per year, the first that recruits in autumn and disappears in the spring of the next year and the second which appears in late spring–early summer and disappears in autumn. The lifetime for the wintering cohort could be 9–10 months, while for the summer cohort the lifetime is only 2–3 months, depending on the ecological conditions and inter-annual variations (). Total production of cohorts of Alitta succinea over a period of 12 months is shown in . Thus, from June 2007 to May 2008, the mean annual secondary production of the whole population was estimated to 5.66 g DW m−2 year−1, mean biomass was 4.22 g DW m−2 and the ratio P/ was 1.34 year−1.
Table I. Secondary production of Alitta succinea from the Danube-Black Sea Canal, Romanian coast of the Black Sea (ΔW, weight variation of the cohort between t
1 and t
2; , mean number of individuals of the cohort between t
1 and t
2)
, mean number of individuals of the cohort between t
1 and t
2)
Population genetics
Isozymes. Enzymatic activity, bands resolution and inter-individual variability were compared for six different enzymatic systems tested (). The enzymatic patterns for IDH, MDH and PGM were used for genetic interpretation. No variation in IDH (single band) and MDH banding patterns was found.
Table II. Different tested enzymatic systems: enzymatic activity, resolution and patterns variability (+ low, ++ mean, +++ strong) for Alitta succinea
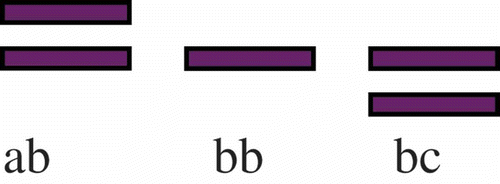
Considering the PGM system, high enzymatic activity and well-separated bands were observed. PGM isozyme patterns showed one or two bands located at three different levels () and are characteristic of a monomeric enzyme with a monolocus biparental codominant inheritance presenting 3 alleles (a, b, c). shows the observed (Ho) and expected (He) heterozygosity values, the Fis inbreeding coefficient and the Fst differentiation coefficient estimated from PGM pattern analysis. No significant differences were observed between the expected (He) and the observed (Ho) heterozygosities (the Fis value was non-significant for both populations) showing that both populations are in Hardy–Weinberg equilibrium. On the other hand, the non-significant Fst value (0.00126) is indicative for non-significant differences in genetic structure between the two compared populations.
Table III. Genotype, allele frequencies and observed (Ho) and expected (He) heterozygosity estimates for Alitta succinea (site A: Agigea and site C: Danube–Black Sea Canal)
Figure 7. PGM enzymatic patterns and corresponding genotypes of Alitta succinea, Romanian coast of the Black Sea.
Considering EST, the obtained patterns were extremely complex to interpret as two zones are distinguished in the gel: an anodic zone showing eight different bands with a great variability, and, in the middle of the gel, another zone presenting well-resolved, separated bands but with a weak activity (). The other tested enzymatic systems were not considered later in this study as they presented a weak activity (LAP) or a low resolution (LDH). Nevertheless, this system remains interesting for future work.
Figure 8. EST variable patterns in the anodic zone of the gel of Alitta succinea, Romanian coast of the Black Sea.
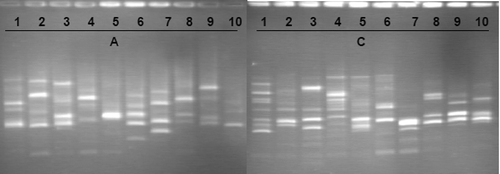
RAPD markers. Two RAPD primers were used to amplify DNA of 10 individuals from each group (station C and station A). The RAPD amplification yielded 29 reproducible and reliable bands: 13 with RAPD8 primer and 16 with RAPD10 primer (). Of the bands, 82.76% were polymorphic in the group A and 79.31% in the group C. Both groups have close Nei's genetic diversity h values: 0.1873 and 0.2099, respectively, for A and C groups (). The Shannon's Information index was 0.3066 for group A and 0.3314 for group C. Considering together both groups, 100% of the amplified markers were polymorphic; the Nei's genetic diversity (h) and the Shannon's Information index (I) were, respectively, 0.2172 and 0.3523. The genetic differentiation measured by Gst was 0.3076 and the gene flow Nm was 1.12.
Table IV. Genetic parameters for Alitta succinea (site A: Agigea and site C: Danube–Black Sea Canal) based on 29 RAPD loci analysis (h, Nei's gene diversity; I, Shannon's information index; Nm, estimate of gene flow from Gst (Nm = 0.5 (1 – Gst)/ Gst))
Figure 9. Electrophoresis agarose gel profiles of RAPD products amplified with RAPD8 primer from DNA of 20 Alitta succinea individuals sampled at Agigea (population A; individuals 1 to 10) and in the Danube–Black Sea Canal (population C; individuals 11 to 20), Romanian coast of the Black Sea.
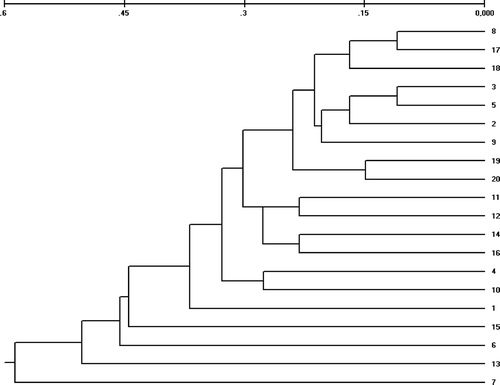
The matrix of Nei's genetic distance generated using RAPD markers was used to build an UPGMA-based dendrogram (). No clear clustering of the individuals from each population was observed.
Discussion
Population dynamics
The densities of Alitta succinea population from the Danube–Black Sea Canal can be compared to those observed in other studies (). They are higher than those noted by Neuhoff (Citation1979) in the Baltic Sea, are comparable to those observed by Losovskaya (Citation1977) in soft sediments of the NW Black Sea or by Rasmussen (Citation1973) in Denmark, but are much lower than those found in the Salton Sea (Detwiler et al. Citation2002). The numerical abundance of Alitta succinea in the Danube–Black Sea Canal seems to be influenced especially by the recruitment of juveniles from plankton and by the development of clusters of Mytilus galloprovincialis. Sudden increase in the number of individuals was observed shortly after the late spring–early summer and especially after the autumn reproduction period. There is also a positive correlation between populations of Alitta succinea and mussels because the worms thrive on ooze accumulated between byssuses of mussels and are almost absent on bare rocks. Although Detwiler et al. (Citation2002) shows that in the Salton Sea the main factor influencing the distribution and abundance of Alitta succinea is the oxygen concentration at the sediment surface, in the Danube–Black Sea Canal this factor affects worms indirectly, by killing mussels which are less tolerant to hypoxia. This is emphasized by the fact that during the study the oxygen levels in the water column did not dropped below 4.20 mg l−1.
Table V. Main characteristics of the populations of Alitta succinea in the world ordered by latitude
In the literature there are few data concerning the biomass of Alitta succinea. Our results on wet weight are similar to data obtained in other parts of the world (Table V). Thus in the Sea of Azov mean annual biomass varied from 0.22 to 2.32 g m−² (Stark Citation1959), while in the Baltic Sea standing stock ranged between 24.5 and 119.7. Regarding the annual fluctuations of biomass, Neuhoff (Citation1979) indicated that these are determined by timing and duration of the reproduction period. Thus, in the Kiel Fjord, Alitta succinea attained the highest values at the beginning of spawning, whereas the lowest values were found during or immediately after the spawning period. The considerable loss of biomass at the end of the breeding period is caused by the death of the ripe worms immediately after spawning. This is also true for our study, which showed an increase in biomass values of the standing stock immediately prior to the late spring–early summer and autumn breeding periods and a gradual decline during late summer and early winter.
There are no data available regarding the production/biomass ratio of Alitta succinea in the Black Sea or elsewhere. The only study carried on seasonal dynamics of polychaetes from Black Sea is that of Kisseleva (Citation1971) for Platynereis dumerilii population from Cystoseira meadows of Sevastopol Bay, which estimated the P/ ratio to be 2.87. In the most-studied species, Nereis diversicolor, the P/
ratio can vary from 1.1 to 7.9 for the adults and from 3.5 to 9.0 for the juveniles (Möller Citation1985). The lowest value of P/
= 1.1 ratio was observed in the UK by Humphreys (Citation1985). On the contrary, the highest value of ratio P/
= 7.9 was observed in the Canal of Mira, Ria de Aveiro, in Portugal by Abrantes et al. (Citation1999). According to Morin and Bourassa (Citation1992), the annual P/
ratio expresses the renovation rate of the biomass in a population, decreasing as the average individual biomass goes up and increasing with a rise in temperature. The relationship between the life span and the P/
ratios is strongly influenced by factors affecting the population structure and a wide range of P/
ratios may exist within a single species (Warwick Citation1980). Also, the annual P/
ratio of a population usually decreases with the age of the individuals. Thus, a population dominated by older individuals will have a lower P/
ratio than one dominated by younger individuals (Möller Citation1985). The higher P/
ratios in some populations could be a consequence of factors such as a shorter life span, a high productivity of the ecosystem and a high predation pressure which keeps the populations at a young stage (Sprung Citation1994). The ratio P/
= 1.34 for the Alitta succinea population from the Romanian coast of the Black Sea represents an average value for two cohorts with different life spans.
Data on the species' mode of reproduction are very scarce and based on old and incomplete observations. To our knowledge, the present study is the first providing data on the life cycle of Alitta succinea. Fauvel (Citation1923) stated that during reproduction sexually ripe individuals transform into epitokes, which are more active reproductive forms called heteronereids that are filled with gametes. At the Romanian Black Sea coast individuals of Alitta succinea in different stages of epitoky were observed in benthos from June to August (Surugiu Citation2008). Both sexes at this stage swarm together, following the release of pheromones stimulated by the lunar cycle (Hardege et al. Citation1990). During swarming, heteronereids swim to the surface and release gametes through ruptures in the body wall, allowing fertilization to occur (Ram et al. Citation1999). In US coastal waters swarming has been observed from March to October (Pettibone Citation1963). Carpelan and Linsley (Citation1961) indicated that in the Salton Sea, although there is an evidence that spawning of Alitta succinea takes place all year round, the peak of spawning activity occurs in spring, with a secondary maximum during autumn and winter. Neuhoff (Citation1979) reported that in the Kiel Fjord there is only one extended spawning period from June to September. Vorobiev (Citation1949) indicated that in the Sea of Azov the reproduction of the species takes place in May–June and August–October, which matches perfectly with our results. After spawning, individuals die. Within 24 h after fertilization eggs develop into pelagic trochophore larvae, which by adding segments transforms into nectochaete larvae. In Romanian coastal waters nectochaetes of Alitta succinea were found in plankton samples from June to August (Elian Citation1969). After about 2 weeks, these nectochaetes begin to settle on the bottom (Carpelan & Linsley Citation1961). Thus the recruitment of juveniles usually occurs 2 weeks after breeding, although some pelagic larvae can postpone their settlement until finding of suitable places. According to Grassle and Grassle (Citation1974), the life cycle takes a minimum of one year to complete. However, our study revealed that at the Romanian Black Sea coast there are two generations per year: the summer generation with a life span of 2–3 months and the winter generation with a life span of 9–10 months. The shorter life cycle of the summer generation could be explained by higher turnover due to raised water temperature, more abundant food supply and increased pressure from predators. Ecological plasticity in the mode of reproduction has been observed for several species of polychaetes and particularly for nereidid polychaetes (see review by Giangrande Citation1997). For example, individuals of Perinereis cultrifera reproduce with or without the morphological modifications which are characteristic of epitoky depending on geographical origin. This species is particularly interesting since it occupies the same habitat as Alitta succinea (rocky shores) and is present in our study site.
Population genetics
The present study provides the first set of population genetic data to assess the distribution of genetic diversity in two groups of A. succinea individuals sampled from two different sites of Romanian Black Sea.
The results obtained from the PGM isozymes showed that both populations seem to be in a similar situation to the Mediterranean Sea A. succinea populations studied by Abbiati and Maltagliati (Citation1992), who found for the PGM locus three alleles, a Hardy–Weinberg equilibrium for each population (non-significant Fis values) and no genetic differentiation between them, as the differentiation coefficient Fst obtained from PGM isozyme analysis showed no significant differences between both populations. However, the observed heterozygosity levels are higher than those observed for the Mediterranean populations.
Only a few genetic studies have been conducted using RAPD data on polychaetes, principally to test the cosmopolitan status of species (Schmidt & Westheide Citation2000; Westheide et al. Citation2003) and we have no reference values of population genetic parameters obtained for this species with DNA markers. Moreover, only four DNA sequences were found on the GenBank database for A. succinea, but these sequences are not suitable to assess genetic diversity. Genetic diversity and gene flow were studied in deep-sea hydrothermal vents of the Pacific coast in three Alvinellidae species: Alvinella caudata, Alvinella pompejana, Paralvinella grasslei with Nm comprised between 3.4 and 6.7 (Vrijenhoek Citation1997).
The population genetic parameters estimated here from PGM isozymes and RAPD markers analyses showed that both groups have close and relatively high genetic diversity levels. This result is in accordance with the dendrogram obtained from RAPD data showing no cluster between the studied individuals and therefore no peculiar genetic structure of both populations. This first attempt to use isozymes and RAPD markers in order to estimate the genetic diversity of A. succinea from the Black Sea provides very informative data about the degree of genetic diversity and of genetic exchange which seems to be relatively high between both populations in accordance with the geographical proximity of the sampled sites of this species. The individuals in the two areas sampled are genetically closed and could be considered as belonging to the same population.
Acknowledgements
This work has been carried with the financial support from the ‘Programme Hubert Curien’, 'Programme d'Action Intégrée ‘Brâncuşi’ (Project No. 14906K) during the years 2007 and 2008. The authors thank Dr. Maria Cristina Gambi and the two anonymous referees for their useful comments and suggestions on the manuscript.
References
- Abbiati , M and Maltagliati , F. 1992 . Genetic population structure of Neanthes succinea (Polychaeta: Nereididae) . Journal of the Marine Biological Association of the United Kingdom , 72 : 511 – 517 .
- Abrantes , A , Pinto , F and Moreira , MH. 1999 . Ecology of the polychaete Nereis diversicolor in the Canal de Mira (Ria de Aveiro, Portugal): Population dynamics, production and oogenic cycle . Acta Oecologica , 72 : 267 – 284 .
- Acquaah , G. 1992 . Practical protein electrophoresis for genetic research , 131 Portland: Dioscorides Press .
- Annenkova , NP. 1929 . Polychaeten aus dem Reliktsee Paläostom (West Kaukasus) und den mit ihm verbundenen Flüssen . Doklady Akademii Nauk SSSR , 21 : 138 – 140 .
- Bartoli , M , Nizzoli , D , Welsh , DT and Viaroli , P. 2000 . Short-term influence of recolonisation by the polychaete worm Nereis succinea on oxygen and nitrogen fluxes and denitrification: A microcosm simulation . Hydrobiologia , 431 : 165 – 174 .
- Cardy , BJ and Beversdorf , WD. 1984 . “ A procedure for the starch gel electrophoretic detection of isozymes of soybean (Glycine max L. Merr.) ” . In Department of Crop Science Technical Bulletin 119/8401 , Ontario, , Canada : University of Guelph .
- Carpelan , LH and Linsley , RH. 1961 . The spawning of Neanthes succinea in the Salton Sea . Ecology , 42 : 189 – 190 .
- Castelli , A , Abbiati , M , Badalamenti , F , Bianchi , CN , Cantone , G , Gambi , MC , Giangrande , A , Gravina , MF , Lanera , P Lardicci , C . 1995 . “ Annelida Polychaeta, Pogonophora, Echiura, Sipuncula ” . In Checklist delle specie della fauna italiana , Edited by: Minelli , A , Ruffo , S and La Posta , S . Vol. 19 , 1 – 45 . Bologna : Calderini .
- Çinar , ME , Katağan , T , Koçak , F , Öztürk , B , Ergen , Z , Kocatas , A , Önen , M , Kirkim , F , Bakir , K Kurt , G . 2008 . Faunal assemblages of the mussel Mytilus galloprovincialis in and around Alsancak Harbour (Izmir Bay, eastern Mediterranean) with special emphasis on alien species . Journal of Marine Systems , 71 : 1 – 17 .
- Como , S and Magni , M. 2009 . Temporal changes of a macrobenthic assemblage in harsh lagoon sediments . Estuarine, Coastal and Shelf Science , 33 : 638 – 646 .
- Crisp , DJ. 1971 . “ Energy flow measurements ” . In Methods for the study of marine benthos , Edited by: Holme , NA and Mc Intyre , AD . 197 – 279 . Oxford : Blackwell Scientific Publications .
- Day , JH. 1967 . A monograph on the Polychaeta of Southern Africa , 877 London : British Museum (Natural History) .
- Detwiler , PM , Coe , M and Dexter , D. 2002 . The benthic invertebrates of the Salton Sea: Distribution and seasonal dynamics . Hydrobiologia , 473 : 139 – 160 .
- Dumitrescu , E. 1957 . Contribuţii la studiul polichetelor din Marea Neagră, litoralul românesc . Buletinul Ştiinţific, Secţia Biologie, Seria Zoologie , 9 : 119 – 130 .
- Dumitrescu , E. 1962 . Nouvelle contribution à l'étude des Polychètes de la Mer Noire . Travaux du Muséum d'Histoire Naturelle ‘Grigore Antipa’ , 3 : 61 – 68 .
- Elian , L. 1969 . Contribuţii la studiul larvelor de polichete din dreptul litoralului românesc al Mării Negre . Studii şi Cercetări de Biologie, Seria Zoologie , 21 : 3 – 10 .
- Elias , R , Rivero , MS , Palacios , JR and Vallarino , EA. 2003 . Sewage-induced disturbance on polychates inhabiting intertidal mussel beds of Brachidontes rodriguezii off Mar del Plata (SW Atlantic, Argentina) . Scientia Marina , 70S3 : 187 – 196 .
- Fauvel , P. 1923 . Polychètes errantes. Faune de France , Paris : Lechevaliers .
- Gayanilo , FC and Pauly , D. 1989 . Announcing the release of version 1.1 of the Complete ELEFAN Software Package . Fishbyte , 7.2 : 20 – 31 .
- Gayanilo , FC , Soriano , M and Pauly , D. 1988 . A draft guide to the ELEFAN ICLARM Software Project Vol. 2:70 ,
- Gayanilo , FC , Sparre , P and Pauly , D. 2005 . FISAT II FAO ICLARM. Outils d’évaluation des stocks , 198 Version révisée .
- Giangrande , A. 1997 . Polychaete reproductive patterns, life cycles and life histories: an overview . Oceanolography and Marine Biology, An Annual Review , 35 : 323 – 386 .
- Grassle , JF and Grassle , JP. 1974 . Opportunistic life history and genetic systems in marine benthic polychaetes . Journal of Marine Research , 32 : 253 – 284 .
- Hardege , JD , Bartels-Hardege , H , Muller , CT and Beckmann , M. 2003 . Peptide pheromones in female Nereis succinea . Peptides , 25 : 1517 – 1522 .
- Hardege , JD , Bartels-Hardege , HD , Zeek , E and Grimm , FT. 1990 . Induction of swarming in Nereis succinea . Marine Biology , 104 : 291 – 295 .
- Humphreys , TJ. 1985 . Production of Nereis diversicolor in an upper estuarine creek . Journal Biological Education , 19 : 141 – 146 .
- Kisseleva , MI. 1971 . Dynamique et production de la population des polychètes Platynereis dumerilii dans la biocoenose des cystoséires en Mer Noire . Travaux du Muséum d'Histoire Naturelle ‘Grigore Antipa’ , 11 : 49 – 58 .
- Kisseleva , MI. 1981 . Benthos of the soft bottoms of the Black Sea. Kiev , 168 Naukova Dumka . [In Russian.]
- Kuhl , DL and Oglesby , LC. 1979 . Reproduction and survival of the pileworm Nereis succinea in higher Salton Sea salinities . Biological Bulletin , 157 : 153 – 165 .
- Laubier , L. 1962 . Quelques annélides polychètes de la lagune de Venice . Description de Prionospio caspersi n. sp. Vie et Milieu , 13 : 123 – 159 .
- Lewontin , RC. 1972 . “ The apportionment of human diversity ” . In Evolutionary biology 6 , Edited by: Dobzhansky , T , Hecht , MK and Steere , WC . 381 – 398 . New York, NY : Appleton-Century-Crofts .
- Losovskaya , GV. 1977 . The ecology of polychaetes of the Black Sea. Kiev , 92 Naukova Dumka . [In Russian.]
- Losovskaya , GV. 1988 . Long-term changes in the composition and distribution of polychaetes in the north-western part of the Black Sea . Gidrobiologichesky Zhurnal , 24 : 21 – 25 . [In Russian.]
- Miron , G and Kristensen , E. 1993 . Factors influencing the distribution of nereid polychaetes . The sulphide aspect. Marine Ecology Progress Series , 93 : 143 – 153 .
- Möller , P. 1985 . Production and abundance of juveniles Nereis diversicolor and oogenic cycle of adults in shallow waters of Western Sweden . Journal of the Marine Biological Association of the UK , 65 : 603 – 616 .
- Morin , A and Bourassa , N. 1992 . Modèles empiriques de la production annuelle et du rapport P/B d'invertébrés benthiques d'eau courante . Canadian Journal of Fisheries and Aquaculture Science , 49 : 532 – 539 .
- Nei , M. 1972 . Genetic distance between populations . American Naturalist , 106 : 283 – 292 .
- Nei , M. 1973 . Analysis of gene diversity in subdivided populations . Proceedings of the National Academy of Sciences of the United States of America , 70 : 3321 – 3323 .
- Neuhoff , HG. 1979 . Influence of temperature and salinity on food conversion and growth of different Nereis species (Polychaeta Annelida) . Marine Ecology Progress Series , 1 : 255 – 262 .
- Orensanz , JM and Estivariz , MC. 1971 . Los anélidos poliquetos de aguas salobres de la Provincia de Buenos Aires . Revista del Museo de La Plata, Zoologia , 11 : 95 – 114 .
- Pauly , D and David , N. 1981 . ELEFAN I a basic program for the objective extraction of growth parameters from length–frequency data . Kommission für Meeresforschung , 28 : 205 – 211 .
- Pearson , TH and Rosenberg , R. 1978 . Macrobenthic succession in relation to organic enrichment and pollution of the marine environment . Oceanography and Marine Biology an Annual Review , 16 : 229 – 311 .
- Pettibone , MH. 1963 . Marine polychaete Worms of the New England region. 1. Aphroditidae through Trochochaetidae . US National Museum Bulletin , 227 ( 1 ) : 356
- Ram , JL , Muller , CT , Beckmann , M and Hardege , JD. 1999 . The spawning pheromone cysteine–glutathione disulfide arouses a multicomponent nuptial behaviour and electrophysiological activity in Nereis succinea males . The FASEB Journal , 13 : 945 – 952 .
- Rasmussen , E. 1973 . Systematics and ecology of the Isefjord marine fauna (Denmark) . Ophelia , 11 : 1 – 507 .
- Rasmussen , E. 1994 . Namalycastis abiuma (Müller in Grube) 1871, an aberrant Nereidid Polychaete of a Georgia salt marsh area and its faunal associations . Gulf Research Reports , 9 : 17 – 28 .
- Rioja , E. 1946 . Estudios anelidológicos. XV. Neréidos de Agua Salobre de los Esteros del Litoral del Golfo de México . Anales del Instituto de Biología de México , 17 : 205 – 214 .
- Schmidt , H and Westheide , W. 2000 . Are the meiofaunal polychaetes Hesionides arenaria and Stygocapitella subterranea true cosmopolitan species? Results of RAPD–PCR investigations . Zoologica Scripta , 29 : 17 – 27 .
- Sprung , M. 1994 . Macrobenthic secondary production in the intertidal zone of the Ria Formosa, a lagoon in Southern of Portugal . Estuarine, Coastal and Shelf Science , 38 : 539 – 558 .
- Stark , IN. 1959 . Nereis succinea in the Sea of Azov . Zoologichesky Zhurnal , 38 : 1634 – 1348 . [In Russian.]
- Surugiu , V. 2000 . Des modifications survenues dans la structure des peuplements d'annélides polychètes d'Agigea dans les 30 dernières années . Analele Ştiinţifice ale Universităţii ‘Alexandru Ioan Cuza’ Iaşi , 46 : 73 – 80 .
- Surugiu , V. 2003 . Near-shore polychaete assemblages of the southern part of the Romanian Black Sea coast . Revue Roumaine de Biologie , 48 : 55 – 67 .
- Surugiu , V. 2005a . Inventory of inshore polychaetes from the Romanian coast (Black Sea) . Mediterranean Marine Science , 6 : 51 – 73 .
- Surugiu , V. 2005b . The use of polychaetes as indicators of eutrophication and organic enrichment of coastal waters: A study case – Romanian Black Sea coast . Analele Ştiinţifice ale Universităţii ‘Alexandru Ioan Cuza’ Iaşi , 51 : 55 – 62 .
- Surugiu , V. 2008 . Populaţiile de polichete de la litoralul românesc al Mării Negre , 281 Iaşi : Universităţii ‘Alexandru Ioan Cuza’ .
- Surugiu , V. 2009 . The influence of sewage pollution on polychaetes associated with mussel beds of the southern Romanian Black Sea coast . Geo-Eco-Marina , 15 : 77 – 87 .
- Swan , BK , Watts , JM , Reifel , KM and Hurlbert , SH. 2007 . Role of the polychaete Neanthes succinea in phosphorus regeneration from sediments in the Salton Sea, California . Hydrobiologia , 576 : 111 – 125 .
- Tenore , KR. 1982 . Comparison of the ecological energetics of the polychaetes Capitella capitata and Nereis succinea in experimental systems receiving similar levels of detritus . Netherlands Journal of Sea Research , 16 : 46 – 54 .
- Ţigănuş , V. 1986 . Structure des peuplements de polychètes de substrat sableux sous condition de forte eutrophisation en Mer Noire . Rapports et Procès-Verbaux des Réunions de la Commission Internationale pour l'Exploitation Scientifique de la Mer Méditerranée , 30 : 20
- Ţigănuş , V. 1988 . Distribution des peuplements des polychètes les plus fréquentes du secteur marin devant les embouchures du Danube . Rapports et Procès-Verbaux des Réunions de la Commission Internationale pour l'Exploitation Scientifique de la Mer Méditerranée , 31 : 23
- Ţigănuş , V. 1992 . Evolution des populations de certaines especes de mass de Polychetes de la Zone Marine rounaine . Rapports et Procès-Verbaux des Réunions Commission Internationale pour l'Exploitation Scientifique de la Mer Méditerranée , 33 : 54
- Vorobiev , VP. 1949 . Benthos of the sea of Azov. Tr. AzCherNIRO, 13 , 193 Simferopol : Krymizdat Press . [In Russian, not seen.]
- Vrijenhoek , RC. 1997 . Gene flow and genetic diversity in naturally fragmented metapopulations of deep-sea hydrothermal vent animals . Journal of Heredity , 88 : 285 – 293 .
- Warwick , RM. 1980 . Population dynamics and secondary production of benthos. In Tenore KR, Coull BC . Marine benthic dynamics. Belle W. Baruch Library in Marine Science , 11 : 1 – 24 .
- Westheide , W , Haβ-Cordes , E , Krabush , M and Müller , M. 2003 . Ctenodrilus serratus (Polychaeta: Ctenodrilidae) is a truly amphi-atlantic meiofauna species – Evidence for molecular data . Marine Biology , 142 : 637 – 642 .
- Williams , JGK , Kubelik , AR , Livak , KJ , Rafalski , JA and Tingey , SV. 1990 . DNA polymorphisms amplified by arbitrary primers are useful as genetic markers . Nucleic Acids Research , 18 : 6531 – 6535 .
- Wilson , RS. 1984 . Neanthes (Polychaeta: Nereididae) from Victoria with descriptions of two new species . Proceedings of the Royal Society of Victoria , 96 : 209 – 226 .
- Yeh FC, Yang R, Boyle T. 1999. POPGENE, Version 1.32. Microsoft Window-Based Freeware for Population Genetic Analysis. University of Alberta: Edmonton. http://www.ualberta.ca/Bfyeh/index.htm (http://www.ualberta.ca/Bfyeh/index.htm)
- Zeeck , E , Hardege , JD and Bartels-Hardege , HD. 1990 . Sex pheromones and reproductive isolation in two nereid species Nereis succinea and Platlynereis dumerilii . Marine Ecology Progress Series , 67 : 183 – 188 .
- Zeeck , E , Harder , T and Beckmann , M. 1998a . Inosine, L-glutamic acid and L-glutamine as components of a sex pheromones complex of the marine polychaete Nereis succinea (Annelida: Polychaeta) . Chemoecology , 8 : 77 – 84 .
- Zeeck , E , Müller , CT , Beckmann , M , Hardege , JD , Papke , U , Sinnwell , V , Schröder , FC and Francke , W. 1998b . Cysteine glutathione disulfide, the sperm release pheromone of the marine polychaete Nereis succinea (Annelida: Polychaeta) . Chemoecology , 8 : 33 – 38 .