Abstract
Developmental genetic studies of cephalochordates, which give evolutionary insights into the origin of the vertebrates from the invertebrates, require a supply of embryos and larvae. To date, such studies have been limited to the genus Branchiostoma. My purpose here is to establish a practical method for obtaining developmental stages of Asymmetron, a second and relatively inaccessible cephalochordate genus. Reproductive periodicity of Asymmetron lucayanum was studied for a total of 1626 specimens in 22 collections made between November 2008 and December 2010 in the lagoon at Bimini, Bahamas. Water temperatures at the collection site were also measured. The lancelets were collected by sieve at early afternoon low tides and transported to the laboratory for determination of sex, body length, gonad index, and capacity to spawn (by dark stimulation on the evening of collection). The sex ratio did not differ significantly from 1:1, and the minimum length at sexual maturity was about 10 mm. During spring and autumn, when water temperatures were moderate (mid to high 20soC), lancelets placed in the dark would spawn predominantly 1 day before the date of the new moon. In contrast, during the winter and summer, dark stimulation did not induce spawning, regardless of the moon phase. It is likely that spawning is depressed in months when water temperatures are near their annual maxima and minima. The linkage of temperature and moon phase to spawning raises the possibility that cultures of A. lucayanum maintained in the laboratory under appropriate environmental conditions could provide a year-round, on-demand source of cephalochordate embryos and larvae.
Keywords:
Introduction
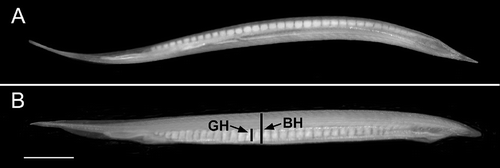
Cephalochordates, commonly called lancelets or amphioxus, are the most basal clade of the phylum Chordata, the other two being tunicates and vertebrates (Delsuc et al. Citation2008). In lancelets, the adult anatomy and genome are vertebrate-like, but simpler (Garcia-Fernàndez Citation2006; Schubert et al. Citation2006; Putnam et al. Citation2008). These features make lancelets particularly interesting to evolutionary biologists seeking insights into the invertebrate-to-vertebrate transition (Kubokawa et al. Citation2010; Litman et al. Citation2010; Yu Citation2010). Moreover, because lancelets, unlike vertebrates, have not undergone any whole-genome duplications, cephalochordates are favourable for studying mechanisms of development that produce a vertebrate-like creature (Holland et al. Citation2008; Holland Citation2010; Koop et al. Citation2010). Kon et al. (Citation2007) estimate that there are about 30 described living species of cephalochordate. The genus Branchiostoma (approximately two dozen species) has been studied intensively, but little is known about the biology of the other two genera – Asymmetron (six species) and Epigonichthys (one species) – which have especially pronounced left–right asymmetry as adults. For example, their gonads are found only along the right side of the body (,B).
Figure 1. Living adult male of A. lucayanum. A, ventral view showing testes running along right side of body; B, right side view with gonad height (GH) and body height (BH) indicated; the percentage of the former divided by the latter is the gonad index. Scale bar: 2 mm.
The asymmetries of lancelets raise questions about the development and evolution of such features. One promising approach to such questions is molecular genetics. For example, asymmetries of Asymmetron and Branchiostoma differ somewhat even at the early larval stages, with the first row of gill slits forming on the right in the former and on the left in the latter (Holland & Holland Citation2010); it would be interesting to determine whether such anatomical differences are preceded in development by differences in the expression domains of such genes as Nodal and Lefty, which help establish the left–right axis of chordates (Yu et al. Citation2002). Moreover, the germ plasm of Branchiostoma segregates shortly after fertilization and localizes to a single cell during cleavage; subsequently, at the gastrula stage, this cell divides and its progeny migrate posteriorly to give rise to the germ cells of the gonads (Wu et al. Citation2011). A similar study of germ plasm would be interesting in Asymmetron in which gonads develop only on the right side of the body. In addition, comparisons between Asymmetron and Branchiostoma genomes should be valuable for evolutionary studies of gene regulation and non-coding RNAs, including micro-RNAs (miRNAs). Recently, Heimberg et al. (Citation2010) have demonstrated the importance of miRNAs for elucidating deep phylogenetic relationships among major chordate groups. Approximately 92 Branchiostoma-specific miRNAs have been found by Campo-Paysaa et al. (Citation2011), and it would be interesting to determine how many of these are shared with Asymmetron and whether they regulate the same genes as in Branchiostoma.
Molecular genetic studies require an adequate supply of embryos and larvae for constructing cDNA libraries and in situ hybridizations. Unfortunately, lancelets in the genera Asymmetron and Epigonichthys are difficult subjects for embryological work because the adults typically live sub-tidally and far from civilization. The most favourable of these markedly asymmetric species is Asymmetron lucayanum Andrews, Citation1893 at Bimini, Bahamas, where specimens can be collected by wading at low tide and a marine laboratory is close at hand. In addition, some aspects of the reproductive biology of this species were published by Andrews (Citation1893), who obtained one spawning in late June and reported that the animals tended to leave their burrows and swim on evenings near the spawning time; he also suggested that the lancelets might cease spawning during the hot summer months. Because Andrews spent only about 6 weeks on Bimini (Andrews et al. Citation1945), he gained no additional insights into the environmental cues for spawning of A. lucayanum. There was no further work on the lancelets in Bimini until I found them where they had originally been discovered over a century earlier. From this beginning, Holland and Holland (Citation2010) spawned them in the laboratory and described their embryos and early larvae.
The purpose of the present study was to discover how environmental factors influence the timing of spawning of the population of A. lucayanum in Bimini. Lancelets were collected periodically over 2 years to determine fluctuations in their gonad indices and their capacity for spawning when stimulated by darkness – already known to be the proximate trigger of spawning in three species of Branchiostoma (Fang et al. Citation1992; Fuentes et al. Citation2004; Holland & Yu Citation2004). The salient result is that the A. lucayanum will spawn if brought into the laboratory around new moon dates in spring and fall. The linkage of spawning to moon phase and water temperature also raises the possibility that cultures of A. lucayanum, even in laboratories far from the sea coast, could provide a reliable supply of embryos and larvae if temperature and light cycles were appropriately manipulated.
Materials and methods
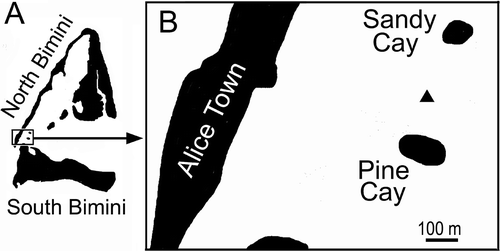
Specimens of A. lucayanum were collected during early afternoon low tides in the lagoon between North and South Bimini, Bahamas (). Dates of collection (with number of animals in parentheses) were: 17 November 2008 (22); 23 January 2009 (29); 9 March 2009 (57); 21 April 2009 (47), 29 May 2009 (36); 5 July 2009 (87); 1 September 2009 (89); 15 November 2009 (106); 31 January 2010 (55); 26 March 2010 (54); 11 May 2010 (117), 12 May 2010 (79); 13 May 2010 (58); 25 May 2010 (66); 26 May 2010 (65); 7 August 2010 (70); 8 August 2010 (98); 9 August 2010 (75); 6 October 2010 (118); 7 October 2010 (110); 4 December 2010 (85); 5 December 2010 (103). Seawater temperature was measured at each collection. In addition, water temperature was automatically recorded every 2 h from March 2009 to March 2010 with a HOBO pendant temperature logger (Onset Computer Corp., Bourne, MA) attached to a sunken boat just below the level of the lowest low tide about 50 m from the collection site. The logger data were smoothed by calculating moving averages over 15-day intervals. The exact site of the lancelet collections is indicated by the triangle in (global positioning system coordinates 25.72297N, 79.29388W) between Sandy Cay and Pine Cay. The site is located in an extensive seagrass bed, where the depth of the water falls to approximately 0.3 m at low tide. The lancelets were collected by scooping the substrate to a depth of a few cm with a 20 cm diameter geology sieve (0.71 mm mesh; American grade 25). The sieve allowed much of the sediment (carbonate mud and sand with a mean grain size of approximately 0.5 mm) to pass while retaining all lancelets longer than about 12 mm; some shorter specimens were also retained, but not quantitatively.
Figure 2. A, map of Bimini, Bahamas; B, enlargement of rectangle in A, with collection site for A. lucayanum indicated by triangle.
Captured lancelets were transferred, via a plastic spoon, to a seawater-filled thermos and transported the same afternoon to the nearby Bimini Biological Field Station (‘Sharklab’), where they were pipetted away from residual sediment and transferred to clean seawater at room temperature (about 26oC). Each lancelet was placed alive in a small Petri dish in a few millilitres of seawater, and the body length was measured to the nearest millimetre with a ruler. The animal was then examined in right side view under a dissecting microscope and classified as male (testes filled with homogeneous mass of sperm), female (ovaries filled with oocytes), or without detectable gonads. An ocular micrometer was used to measure the heights of the body and one gonad (BH and GH, respectively, in ) at a level halfway between the anterior and posterior ends of the body. The percentage of the gonad height to the animal height was the gonad index (a measure of relative ripeness, but not representing the actual percentage of gonad volume to total body volume). The lancelets were kept under continuous illumination in the laboratory on the evening of collection until dark induction of spawning was attempted. The lancelets were placed in a darkened 20 cm diameter dish containing 500 ml of seawater at 21:00 h (a time chosen to give me a chance to finish gathering the gonad index data for the afternoon's sample). Usually, all the animals in a collection were put in the dark, but, when spawning seemed likely, only a subset of the individuals with the largest gonads was so treated – this minimized the number of shed eggs ingested by the adults. After 90 min of darkness, the dish was observed for evidence of spawning, as judged by the opacity from shed sperm and the presence of early embryos. When spawning occurred, the number of males and females that had emptied their gonads was recorded.
Results
The 22 collections from 17 November 2008 to 5 December 2010 comprised 1626 specimens of A. lucayanum with lengths ranging from 8 to 25 mm. There were 806 females, 717 males and 103 animals with gonads too small to be seen under the dissecting microscope. The proportion of animals with undetectable gonads was greatest in the smaller size classes (). For all 1523 sexable animals, the sex ratio did not vary significantly from 1:1 (Chi-square test; df = 1; p = 0.023). Moreover, no hermaphroditic specimens were detected. A plot of animals with visible gonads versus body length showed that the minimum length at sexual maturity was about 10 mm (). For all 22 collections, the gonad index and spawning data are summarized in and . If one considers all the animals with visible gonads – i.e. over 90% of the lancelets – the gonad indices ranged from 1 to 55%. Moreover, in any given collection, there was always a broad spectrum of gonad indices between the smallest and the largest.
Figure 3. Minimum size at sexual maturity of A. lucayanum shown by plotting percentage of animals with visible gonads in each 1 mm length class. The total number of specimens in each length class is indicated.
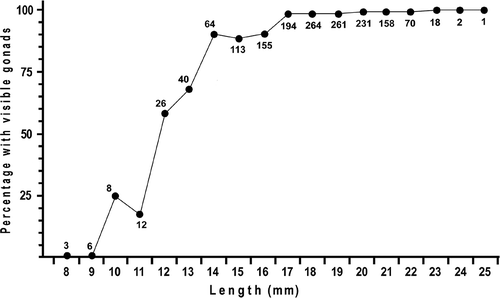
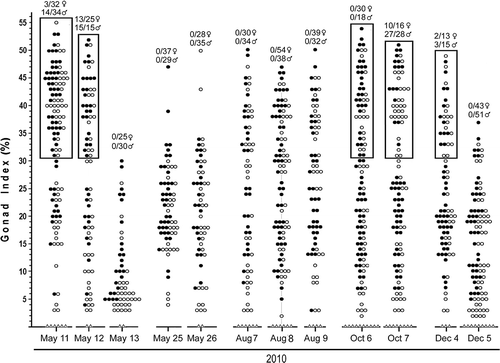
Figure 4. Summary of gonad indices for collections of A. lucayanum from 17 November 2008 to 26 March 2010. Filled and empty circles are gonad indices, respectively, for females and males; triangles indicate no gonads visible. For each collection, numbers above the gonad index data show how many females and males spawned when placed in the dark the evening of collection (on 15 November 2010, only specimens represented by underlined circles were placed in the dark).
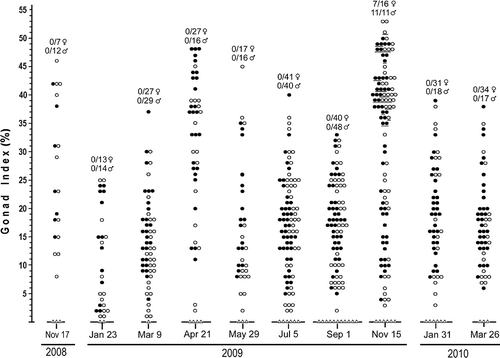
Figure 5. Summary of gonad indices for collections of A. lucayanum from 11 May 2010 to 5 December 2010; symbols are as in . For each collection, numbers above the gonad index data show how many females and males spawned when placed in the dark the evening of collection (on some dates only specimens represented by circles in boxed regions were placed in the dark).
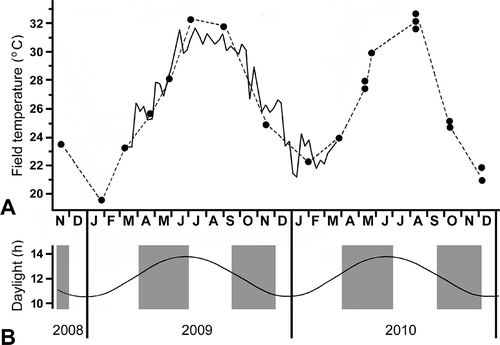
At the beginning of this work, it was unclear which, if any, environmental factors might be correlated with the reproduction of A. lucayanum at Bimini. The only available information was the suggestion by Andrews (Citation1893) that the animals might not spawn during the heat of the summer. Therefore, special attention was given to measuring the water temperature in the field. Over the 2 years of the present study, water temperatures in the Bimini lagoon fluctuated between winter lows (around 20oC) and summer highs (in the low 30soC). The 15-day moving averages of temperatures from the HOBO logger (, solid line) do not reflect the extreme minimum (13.1oC in January 2010) and maximum (35.2oC in July 2010).
Figure 6. Environmental conditions at collection site. A, seawater temperature measured individually on days of collection (filled circles connected by dashed line) or measured every 2 h for a year by a HOBO pendant logger (solid line, presented as moving averages over 15-day intervals); B, annual fluctuation in day length; shaded areas indicate times of year when spawnings of A. lucayanum take place near new moon dates.
In the absence of any definite information about the reproductive biology of A. lucayanum, the monitoring of the gonad indices followed no elegant protocol at the beginning: I simply began collecting lancelets in Bimini at roughly two-monthly intervals. No clear insights were gained for the first year of the study. Then, on the evening of 15 November 2009, I fortuitously obtained a spawning. During the late afternoon, I noticed that the lancelets collected earlier in the day were tending to swim instead of lying as usual on the bottom of their dish. As already mentioned, Andrews (Citation1893) had noticed that the lancelets were especially likely to swim around the time of spawning. I used a pipette to capture 16 swimming females and 11 swimming males, all with relatively large gonad indices (represented by the underlined circles in ). After the selected lancelets were placed together in a darkened dish at 21:00 h, 7 of the 16 females and all of the 11 males had spawned by 22:30 h; with the exception of one animal, all of the gonads were completely emptied at spawning (Holland & Holland Citation2010). Spawning took place 1 day before the date of the new moon and was the first indication that this might be a major environmental factor timing the reproduction of A. lucayanum in Bimini.
The spawning of 15 November 2009 permitted me to make sense out of some of data from 2008–2009 and to undertake a more focused study of the suspected influence of environmental factors on reproduction during 2010. summarizes the relations between the average gonad index, moon phase, seawater temperature, and spawning. The data for some of the collections are particularly informative. For example, in , the seawater was cool and the average gonad index was low, even though the new moon was only 2.5 days later. Presumably, at this cold time of year, low temperatures slowed gametogenesis, and the population would thus not spawn around the new moon date. Conversely, in , the seawater temperature was moderate, the gonad index was high and a major spawning probably did take place around the time of the next new moon, 3.3 days later. illustrates a time of year when seawater temperature was moderate: the average gonad index was elevated on the afternoon of collection, and a large spawning took place the same evening, 1 day before the new moon.
Figure 7. Collection-by-collection summary of seawater temperature, number of days until next new moon, average gonad index of A. lucayanum (white bar), and percentage of spawning females and males (black bars).
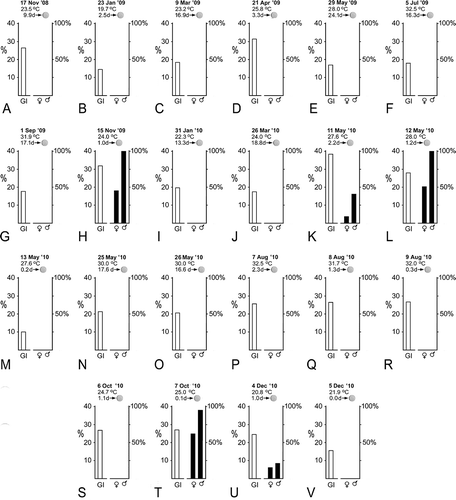
Figure 7K–M shows a 3-day series when the water temperature was moderate. Over this period, the gonad index declined slightly 2 days before the new moon (minor spawning) and then markedly 1 day before the new moon (major spawning); by the new moon date, the population had spawned out. The changes in the individual gonad indices between 12 and 13 May () reflect this spawning event in detail. Gonad indices over 30% were measured for about half the animals collected on 12 May, but for none of the animals collected on 13 May, whereas, in the latter collection, there was a marked increase in specimens with small gonad indices (between 3 and 7%). In addition, –M (along with ,T,U) indicates that, all things being equal, males are more likely than females to spawn after collection; although only about half the ripe females spawned in the laboratory in , there were no ripe females left in the field the next day (). Evidently, the stress of collection and handling are more likely to disrupt the physiological processes leading up to spawning in females than in males.
Figure 7P–R, a 3-day series when the seawater was exceptionally warm, shows that the gonad index is not particularly elevated and that no spawning occurred 1 or 2 days before the new moon or on the new moon date itself. ,T shows an instance where the water temperature was moderate and spawning took place not 1 day before the new moon, but on the new moon date itself. ,V shows the situation when the water temperature was approaching its winter lows. There was a minor spawning of both sexes in the laboratory 1 day before the new moon. However, the decline in the average gonad index between 4 and 5 December indicates that a higher proportion of the lancelets probably spawned in the field than in the laboratory, possibly because the reproductive physiology of animals at the end of the autumn breeding season is more prone to disruption by the stress of collection.
In summary, the data summarized in , and lead to three major conclusions. First, when seawater temperatures hover around their warmest in summer and their coolest in winter, no lancelets in the Bimini population of A. lucayanum spawn at any time. Second, when seawater temperatures are moderate during the spring and fall, dark stimulation will induce the riper animals (up to half of the adult population) to spawn around the time of the new moon. More exactly, the major spawning can be 0, 1, or 2 days before the new moon, but most often 1 day before the moon date. Third, the spawning of males in the laboratory tends to reflect the intensity of male spawning in the field, while some females that would normally spawn in the field may fail to do so if brought into the laboratory.
Relatively unripe lancelets are present in all samples, even when ripe animals predominate and spawning is imminent. There is no known reason for this lack of gametogenic synchrony, which has been documented previously for other cephalochordates (Stokes & Holland Citation1996). Such heterogeneity makes it difficult to determine the length of time that a spawned-out individual requires to refill its gonads and spawn again. There are two possibilities, not necessary mutually exclusive. At least some of the spawned lancelets might refill their gonads and spawn again a month later. On the other hand, on a given spawning date, the lancelets shedding their gametes might enter an extended period of reproductive rest, so that only the unshed animals – i.e. those with small to medium gonad indices – participate in the next spawning.
Discussion
The timing of reproductive events has ultimate causes and proximate causes (Baker Citation1938). Examples of ultimate causes include selection to maximize fertilization and/or larval survival. Proximate causes include reception of environmental signals, endogenous rhythms (sometimes multiple and interacting), endocrinology, physiology, and molecular genetics. Because of such complexity, the general principles of chronobiology have been slow to emerge, although good progress is currently being made in relating cryptochrome photoreception to the rhythmic expression of clock genes (Levy et al. Citation2007; Reitzel et al. Citation2010). The multiplicity of causes of reproductive periodicity is reflected in a great diversity in the resulting patterns of reproduction in marine animals – from mass spawning on only a single date per year (Holland Citation1981) to continual spawning of some individuals throughout the year (Pearse & Phillips Citation1968), with almost every intervening possibility (Mercier & Hamel Citation2009; Takemura et al. Citation2010). Moreover, closely related species or even neighbouring populations within a species can use strikingly different environmental cues to time their reproductive behavior (Neumann Citation1966). For example, spawnings of A. lucayanum at new moon within a particular range of water temperature are more similar to the pattern in Australian coral trout (Samoilys & Squire Citation1994) than to the pattern in other cephalochordates that have been investigated.
The results of the present study, although too species-specific to constitute a major advance in chronobiology, are significant for another reason: they raise the possibility that cultures of adult cephalochordates maintained in the laboratory could provide a year-round source of embryos for developmental genetic studies. As mentioned in the introduction, cephalochordates, with their stripped-down, vertebrate-like morphology and genome, are providing important new insights into chordate mechanisms of development and into the evolutionary origin of the vertebrates from the invertebrates. Up to now, these studies have employed three amphioxus species (the Floridian Branchiostoma floridae, the European B. lanceolatum, and the Asian B. belcheri). In the field, these species have a single breeding season of only a few months each year. Moreover, within the limits of the ripe period, the animals spawn on dates that do not reliably correspond to well-defined environmental events (Cerfontaine Citation1906; Fang et al. Citation1992; Stokes & Holland Citation1996; Zhang et al. Citation1999; Yasui et al. Citation2007). Although Fuentes et al. (Citation2004, Citation2007) developed valuable culture conditions for greatly increasing the number of spawning dates of B. lanceolatum during the ripe period of the year, no laboratory manipulations have yet succeeded in extending the natural breeding season of any Branchiostoma species for more than about a month (Wu et al. Citation1994). It is possible that these species have a genetically fixed annual reproductive cycle that resists resetting by the manipulation of environmental factors in the laboratory.
For amphioxus of the genus Branchiostoma, it is widely agreed that water temperature is the most influential environmental factor influencing the limits of the ripe period each year (Willey Citation1891; Hatschek Citation1893; Wilson Citation1893; Cerfontaine Citation1906; Conklin Citation1932; Bone Citation1958; Azariah Citation1965; Frankenberg Citation1968; Courtney Citation1975; Futch & Dwinell Citation1977; Flood et al. Citation1982; Wu et al. Citation1994). Similarly for A. lucayanum, water temperature plays a major part in setting the boundaries of the reproductive period in Bimini. Hot summer and cold winter water temperatures could well be major factors delimiting the spring and autumn breeding periods when temperatures are moderate (, shaded areas). It cannot be ruled out that changing day length might also play some part in controlling the reproduction of A. lucayanum. However, this control, if it exists, would be rather complex, because day length is on the increase during the spring spawning period and on the decrease during the autumn spawning period ().
The results of the present study may well be the first step toward establishing breeding laboratory cultures of A. lucayanum. For example, a culture of the adult lancelets, if maintained at a moderate temperature (e.g. 27oC) on a 12 h daylight cycle might be induced to spawn in the laboratory by mimicking the moonlight cycle during the dark periods. Spawning would take place once a month at the time of the simulated new moon. By staggering moonlight regimes from one culture to the next, spawnings could be obtained as frequently as needed. The developmental stages of A. lucayanum have recently been described (Holland & Holland Citation2010), and the laboratory culture of the adults for year-around, on-demand spawning would make this lancelet a valuable model for studying chordate developmental mechanisms and evolution.
Acknowledgements
I am indebted to Linda Holland for her helpful criticism, to Greg Rouse for photographing specimens of A. lucayanum alive and to Chuck Messing for his unstinting hospitality in Fort Lauderdale, Florida, the jumping-off place for Bimini. At the Bimini Biological Field Station (‘Sharklab’), the following staff members and volunteers helped in the laboratory and the field: Jimiane Ashe, Jim Barley, Amanda Brown, Erika Cironte, Tyler Clavelle, Christina Comfort, Sheri Connolly, Christopher Crooks, Kat Geldhill, Lindsay Graff, Katie Grudecki, Merika Huhn, Grant Johnson, Emily Marcus, Michael Neuhaus, Steve Pollett, Kristyn Rubertus, Samantha Sherman, Kristine Stump, and Sean Williams. Above all, I am indebted to Sharklab's director, Samuel H. ‘Doc’ Gruber, for his support and inspiring enthusiasm for all things marine biological.
References
- Andrews , EA. 1893 . An undescribed acraniate, Asymmetron lucayanum . Studies from the Biological Laboratory, Johns Hopkins University , 5 : 213 – 247 . + pl XIII–XIV
- Andrews , EA , Bigelow , RP and Morgan , TH. 1945 . Three at Bimini . Scientific Monthly , 61 : 333 – 344 .
- Azariah , J. 1965 . On the seasonal appearance of fin rays and their bearing on the reproductive cycle of Branchiostoma lanceolatum . Journal of the Marine Biological Association of India , 7 : 459 – 461 .
- Baker , JR. 1938 . “ The evolution of breeding seasons ” . In Evolution, essays on aspects of evolutionary biology , Edited by: De Beer , G . 161 – 177 . Oxford : Clarendon .
- Bone , Q. 1958 . Observations upon the living larva of amphioxus . Pubblicazioni della Stazione Zoologica di Napoli , 30 : 458 – 471 .
- Campo-Paysaa , F , Semon , M , Cameron , RA , Peterson , KJ and Schubert , M. 2011 . MicroRNA complements in deuterostomes: Origin and evolution of micro RNAs . Evolution & Development , 13 : 15 – 27 .
- Cerfontaine , P. 1906 . Recherches sur le développement de l'amphioxus . Archives de Biologie, Liège , 22 : 229 – 418 .
- Conklin , EG. 1932 . The embryology of amphioxus . Journal of Morphology , 54 : 69 – 141 .
- Courtney , WAM. 1975 . The temperature relationships and age-structure of North Sea and Mediterranean populations of Branchiostoma lanceolatum . Symposia of the Zoological Society of London , 36 : 213 – 233 .
- Delsuc , F , Tsagkogeorga , G , Lartillot , N and Philippe , H. 2008 . Additional molecular support for the new chordate phylogeny . Genesis , 46 : 592 – 604 .
- Fang , YQ , Qi , X and Hong , GY. 1992 . Effects of photoperiod on gonadal maturation and spawning . Acta Oceanologia Sinica , 143 : 128 – 132 .
- Flood , PR , Gosselck , F and Braun , JG. 1982 . Larvas de ‘Branchiostoma’ en el area de ‘upwelling’ del NW de Africa . Boletin del Instituto Espanol de Oceanografia , 7 : 73 – 86 .
- Frankenberg , D. 1968 . Seasonal aggregation in amphioxus . BioScience , 18 : 877 – 878 .
- Fuentes , M , Benito , E , Bertrand , S , Paris , M , Mignardot , A , Godoy , L , Jimenez-Delgado , S , Oliveri , D , Candiani , S Hirsinger , E . 2007 . Insights into spawning behavior and development of the European amphioxus (Branchiostoma lanceolatum) . Journal of Experimental Zoology , 308B : 484 – 493 .
- Fuentes , M , Schubert , M , Dalfo , D , Candiani , S , Benito , E , Gardenyes , J , Godoy , L , Moret , F , Illas , M Patten , I . 2004 . Preliminary observations on the spawning conditions of the European amphioxus (Branchiostoma lanceolatum) in captivity . Journal of Experimental Zoology , 302B : 384 – 391 .
- Futch , CR and Dwinell , SE. 1977 . Nearshore marine ecology at Hutchinson Island, Florida: 1971–1974. IV. Lancelets and fishes. Florida . Marine Research Publications , 24 : 1 – 23 .
- Garcia-Fernàndez , J. 2006 . Amphioxus: A peaceful anchovy fillet to illuminate chordate evolution . International Journal of Biological Science , 2 : 30 – 31 .
- Hatschek , B. 1893 . The amphioxus and its development , London : Swann Sonnenschein .
- Heimberg , AM , Cowper-Sallari , R , Sémon , M , Donoghue , PCJ and Peterson , KJ. 2010 . MicroRNAs reveal the interrelationships of hagfish, lampreys, and gnathostomes and the nature of the ancestral vertebrate . Proceedings of the National Academy of Sciences USA , 107 : 19379 – 19383 .
- Holland , LZ , Holland , ND and Gilland , E. 2008 . Amphioxus and the evolution of head segmentation . Integrative and Comparative Biology , 45 : 630 – 646 .
- Holland , LZ and Yu , JK. 2004 . Cephalochordate (amphioxus) embryos . Procurement, culture, basic methods. Methods in Cell Biology , 74 : 195 – 215 .
- Holland , ND. 1981 . “ A model linking environmental signals to the spawning time of Comanthus japonica (Echinodermata, Crinoidea) ” . In Advances in invertebrate reproduction , Edited by: Clark , WH and Adams , TS . 75 – 78 . New York, NY : Elsevier .
- Holland , ND and Holland , LZ. 2010 . Laboratory spawning and development of the Bahama lancelet, Asymmetron lucayanum (Cephalochordata): Fertilization through feeding larvae . Biological Bulletin , 219 : 132 – 141 .
- Holland , PWH. 2010 . From genomes to morphology: A view from amphioxus . Acta Zoolologica Stockholm , 91 : 81 – 86 .
- Kon , T , Nohara , M , Yamanoue , Y , Fujiwara , Y , Nishida , M and Nishikawa , T. 2007 . Phylogenetic position of a whale-fall lancelet (Cephalochordata) inferred from whole mitochondrial genome sequences . BMC Evolutionary Biology , 7 : 127
- Koop , D , Holland , ND , Semon , M , Alvarez , S , Rodriguez de Lara , A , Laudet , V , Holland , LZ and Schubert , M. 2010 . Retinoic acid signaling targets Hox genes during amphioxus gastrula stage: Insights into early anterior–posterior patterning of the chordate body plan . Developmental Biology , 338 : 98 – 106 .
- Kubokawa , K , Tando , Y and Roy , S . 2010 . Evolution of the reproductive endocrine system in chordates . Integrative and Comparative Biology , 50 : 53 – 62 .
- Levy , O , Appelbaum , L , Leggat , W , Gothlif , Y , Hayward , DC , Miller , DJ and Hoegh-Guldberg , O. 2007 . Light-responsive cryptochromes from a simple multicellular animal, the coral Acropora millepora . Science , 318 : 467 – 470 .
- Litman , GW , Rast , JP and Fugmann , SD . 2010 . The origins of vertebrate adaptive immunity . Nature Reviews Immunology , 10 : 543 – 553 .
- Mercier , A and Hamel , JF . 2009 . Endogenous and exogenous control of gametogenesis and spawning in echinoderms . Advances in Marine Biology , 55 : 1 – 302 .
- Neumann , D. 1966 . Die lunare und tägliche Schlüpfperiodik der Mücke Clunio. Steuerung und Abstimmung auf die Schlüpfperiodik . Zeitschrift fur Vergleichende Physiologie , 53 : 1 – 61 .
- Pearse , JS and Phillips , BF. 1968 . Continuous reproduction in the Indo-Pacific sea urchin Echinometra mathaei at Rottnest Island, Western Australia . Australian Journal of Marine and Freshwater Research , 19 : 161 – 172 .
- Putnam , NH , Butts , T , Ferrier , DE , Furlong , RF , Hellstein , U , Kawashima , T , Robinson-Rechavi , M , Shoguchi , E , Terry , A Yu , JK . 2008 . The amphioxus genome and the evolution of the chordate karyotype . Nature , 453 : 1064 – 1072 .
- Reitzel , AM , Behrendt , L and Tarrant , AM. 2010 . Light entrained rhythmic gene expression in the sea anemone Nematostella vectensis: The evolution of the animal circadian clock . PLoS One , 5 : e12805
- Samoilys , MA and Squire , LC. 1994 . Preliminary observations on the spawning behavior of coral trout, Plectropomus leopardus (Pisces: Serranidae), on the Great Barrier Reef . Bulletin of Marine Science , 54 : 332 – 342 .
- Schubert , M , Escriva , H , Xavier-Neto , J and Laudet , V. 2006 . Amphioxus and tunicates as evolutionary model systems . Trends in Ecology and Evolution , 21 : 269 – 277 .
- Stokes , MD and Holland , ND. 1996 . Reproduction of the Florida lancelet (Branchiostoma floridae): Spawning patterns and fluctuations in gonad indexes and nutritional reserves . Invertebrate Biology , 115 : 349 – 359 .
- Takemura , A , Rahman , MS and Park , YJ. 2010 . External and internal controls of lunar-related reproductive rhythms in fishes . Journal of Fish Biology , 76 : 7 – 26 .
- Willey , A. 1891 . The later larval development of amphioxus . Quarterly Journal of Microscopical Science , 32 : 183 – 234 .
- Wilson , EB. 1893 . Amphioxus and the mosaic theory of development . Journal of Morphology , 8 : 579 – 639 .
- Wu , HR , Chen , YT , Su , YH , Luo , YJ , Holland , LZ and Yu , JK. 2011 . Asymmetric localization of germline markers Vasa and Nanos during early development in the amphioxus Branchiostoma floridae . Developmental Biology , 353 : 147 – 159 .
- Wu , XH , Zhang , SC , Wang , YY , Zhang , BL , Qu , YM and Jiang , XJ. 1994 . Laboratory observations on spawning, fecundity and larval development of amphioxus (Branchiostoma belcheri tsingtauense) . Chinese Journal of Oceanology and Limnology , 12 : 289 – 294 .
- Yasui , K , Urata , M , Yamaguchi , M , Ueda , H and Henmi , Y. 2007 . Laboratory culture of the oriental lancelet Branchiostoma belcheri . Zoological Science , 34 : 514 – 520 .
- Yu , JK. 2010 . The evolutionary origin of the vertebrate neural crest and its developmental gene network – Insights from amphioxus . Zoology , 113 : 1 – 9 .
- Yu , JK , Holland , LZ and Holland , ND. 2002 . An amphioxus nodal gene (AmphiNodal) with early symmetric expression in the organizer and mesoderm and later asymmetrical expression associated with left–right axis formation . Evolution and Development , 4 : 418 – 425 .
- Zhang , SC , Zhu , JT , Jia , CH and Li , GR. 1999 . Production of fertile eggs and sperms in laboratory-reared amphioxus Branchiostoma belcheri tsingtauense . Chinese Journal of Oceanology and Limnology , 17 : 53 – 56 .