Abstract
The deep-sea genus Axiokebuita (Annelida, Scalibregmatidae) has hitherto been considered to contain two species, Axiokebuita minuta (Hartman, Citation1967) and Axiokebuita millsi Pocklington and Fournier, Citation1987, each rarely recorded in the literature and both supposedly having bipolar distributions. From the study of some types, other museum collection material, plus newly collected specimens from the slope depths of the Bellingshausen Sea and off the Antarctic Peninsula (Antarctica), Iceland and Iberian Peninsula, as well as from hydrothermal vents of the southeastern Pacific Ocean, the taxonomic history of the genus is revisited. Based on the results of this study, the features traditionally used to distinguish between the two species are actually the same in both. Therefore, A. millsi is proposed to be synonymised with A. minuta, which is redescribed and considered the only valid species of the genus, leaving Axiokebuita monotypic. Two previously unnoticed body sensory structures, i.e. a ciliated neck organ and a prostomial depression, were observed under scanning electron microscopy.
Introduction
The genus Axiokebuita (Scalibregmatidae) was erected by Pocklington and Fournier (Citation1987) for a new species collected from the slope depths off eastern Canada, Axiokebuita millsi Pocklington and Fournier, Citation1987. These authors also proposed the inclusion of Kebuita minuta Hartman, Citation1967, described from South Orkney Islands, in this new genus. They also indicated that some Antarctic specimens, previously identified as Kebuita minuta by Hartman (Citation1978) and Blake (Citation1981), were actually Axiokebuita millsi. As a result of this, A. millsi was considered to have a bipolar distribution and this fact was reflected in the etymology of the genus (‘Axio’ in Latin meaning ‘the pole’). According to Pocklington and Fournier (Citation1987), the two species are essentially distinguished by the presence of a globular notopodial cirrus in A. millsi, which was supposed to be absent in A. minuta. Recently, Persson and Pleijel (Citation2005) collected additional specimens of Axiokebuita from Norwegian waters. They studied the taxonomic affinities between both species and also assessed the phylogenetic relationships of the genus with other scalibregmatids using the nuclear genes 18S rDNA and 28S rDNA. They concluded that the Norwegian specimens could not be referred to either of the two known species due to delineation problems between the taxa and the poor condition of the holotype of A. minuta. They believe that ‘without access to further specimens in good conditions from type locality of A. minuta, it is difficult to state if the separation between the two can be corroborated by other features’.
In the present study, new material of Axiokebuita mainly collected in the Bellingshausen Sea (BS) and Weddell Sea (WS) and west coast of the Antarctic Peninsula (BENTART–06 and ANDEEP II cruises) was studied. These specimens were compared with type specimens of both species, other material from southwest Iceland (BIOICE project) and northwestern Iberian Peninsula (DIVA-Artabria II project) continental slope, hydrothermal vents of the southeast Pacific Ocean, as well as relevant museum specimens. As a result of this, Axiokebuita millsi is proposed as a junior synonym of A. minuta and a redescription of the species is presented.
Materials and methods
The present work is largely based on Antarctic specimens collected during the BENTART project (2006 cruise) aboard the R/V Hesperides (2 January to 17 February 2006). A total of 13 stations located in the Bellingshausen Sea and Antarctic Peninsula were sampled using a modified version (Cartes et al. Citation1994) of the Macer-GIROQ suprabenthic sled (Brunel et al. Citation1978; Dauvin & Lorgere Citation1989). Description of sampling methodology can be found in San Vicente et al. (Citation2007) and Corbera et al. (Citation2009). Data concerning sampling positions, dates and water depth are reported in . Sorting of samples was carried out onboard and specimens were fixed in 4% formalin and then transferred in 70% ethanol. Specimens used for scanning electron microscopy (SEM) were dehydrated via a graded ethanol series, critical-point dried using CO2, covered with gold in a sputter coater and examined and photographed under a JEOL JSM-6400 scanning electron microscope at the SAIN (Servicio de Apoio á Investigación), Universidade da Coruña, Spain (UDC). This material was augmented by one specimen from hydrothermal vents of the southeastern Pacific Ocean and the loan of two specimens from the BIOICE and DIVA-Artabria II projects in Iceland and the Iberian Peninsula, respectively. Comparative material included several ANDEEP II specimens from the Weddell Sea and South Sandwich Islands (Antarctica) and material from Museum collections, including Hartman's and Pocklington and Fournier's type material.
Table I. Coordinates and depth (referred to the start of the tow) of locations where new material of Axiokebuita was sampled. (*) Alvin dive number
Genomic DNA was extracted from a single specimen collected in the southeast Pacific and ethanol-preserved, using a Qiagen DNeasy tissue kit (Valencia, CA). Just over 1000 base pairs of the small subunit ribosomal DNA (18S) and approximately 650 base pairs of the mitochondrial COI gene were amplified using primers and protocols as outlined in (Osborn & Rouse Citation2011). All sequencing was carried out by Advanced Studies in Genomics, Proteomics and Bioinformatics (ASGPB) at the University of Hawaii at Manoa using Applied Biosystems BigDye terminator chemistry and an ABI 3730XL sequencer.
Specimens were deposited in the Museo Nacional de Ciencias Naturales, Madrid (MNCN), Scripps Institution of Oceanography (SIO-BIC) and Stazione Zoologica Anton Dohrn, Napoli (SZN). For comparative purposes, additional material from the German ANDEEP II expeditions to the Antarctica (Brandt & Hilbig Citation2004) and the coast of Norway (Persson & Pleijel Citation2005) was loaned by the Deutsches Zentrum für Marine Biodiversitätsforschung-Senckenberg Forschungsinstitut und Naturmuseum (DZMB) and the Naturhistoriska Riksmuseet-Stockholm (SMNH), respectively.
Results
-
Family Scalibregmatidae Malmgren, 1867
-
Genus Axiokebuita Pocklington and Fournier, Citation1987
-
Axiokebuita minuta (Hartman, Citation1967)
-
Kebuita minuta. Hartman Citation1967: 131–132; Hartman Citation1978: 179, fig. 27a,b; Blake Citation1981: 1153–1154, fig. 11a,b.
-
Axiokebuita millsi. Pocklington and Fournier Citation1987: 108, , Blake & Hilbig 1995: 242, fig. 13a–c.
Figure 1. Positions of all the worldwide records of Axiokebuita extracted from the literature, and newly collected material from Iceland (BIOICE cruise), Spain (DIVA–Artabria II cruise), SE Pacific (ALVIN dive) and Antarctica (BENTART–06 cruise). The boxed area is represented at a larger scale showing in detail the positions in the northwest Antarctic littoral. Encircled dot and triangle show positions of the type localities of A. millsi and A. minuta, respectively.
-
Axiokebuita. sp. Persson and Pleijel Citation2005: 8, ,
Material examined
This study is based on 74 specimens mostly collected in the Antarctic Seas during Spanish and German cruises but also in the Atlantic Ocean (southwest Iceland, northwest Spain) and the eastern Pacific Ocean (near Easter Island) (, ). In addition, for comparison purposes, type specimens of both species and nine Norwegian non-type specimens from museum collection were also examined.
Axiokebuita minuta
ICELAND: Southwest coast (BIOICE project, 1 specimen; MNCN 16.01/13603), IBERIAN PENINSULA: northwest coast (DIVA-Artabria II, corals with sand, 1 specimen used for DNA extraction). SOUTH-EAST PACIFIC (DSV Alvin; around 700 km northwest of Easter Island, rock fissures and mussel clumps near active venting areas, 1 specimen; middle part used for DNA sequencing, the anterior and posterior MNCN 16.01/13602). ANTARCTICA: Scotia Sea (USNM 56625, 62°25′S, 56°30′–56°32′W, 300 m deep, coll. USNS Eltanin, 13 March 1964; 1 paratype including 2 slides with parapodia). Weddell Sea (ANDEEP II cruise): DZMB 79-0077 and 79-0081(stat. 131-3, 2 specimens); MNCN 16.01/13601 (DZMB 79-0079; 1 specimen in SEM stub). Bellingshausen Sea and West Antarctic peninsula (BENTART–06 cruise): MNCN 16.01/13596 (stat. MB30, 30 specimens). MNCN 16.01/13600 (stat. MB30, 5 specimens in 3 SEM stubs). MNCN 16.01/13597 (stat. MB31, 17 specimens). MNCN 16.01/13598 (stat. MB35, 5 specimens). MNCN 16.01/13599 (stat. PA42, 1 specimen). SZN 013 (stat. MB31, 1 specimen for TEM analysis; stat. MB 34, 3 specimens; MB38, 3 specimens).
Axiokebuita millsi
ANTARCTICA: Ross Sea (USNM 60574, 73°59′S, 170°41′E–73°58′E, 170°58′S, 608 m deep, coll. USNS Eltanin, 13 January 1968; 1 paratype of A. millsi). South Sandwich Islands (ANDEEP II cruise): DZMB 79–0126, 79–0127 and 79–0128 (stat. 143–1, 3 specimens).
Axiokebuita
spec. NORWAY: Trondheim's fjord: SMNH 75817 (Rødberg, 63°28.36′N, 10°00.04′E, 180–250 m, Lophelia reef, triangular dredge, coll. F. Pleijel, 21 Feb 2003, 2 specimens). SMNH 75819 and 75820 (same locality data, 26 Feb 2003, 6 – one in SEM stub – and 1 specimens, respectively).
Description of Bellingshausen specimens (BENTART–06)
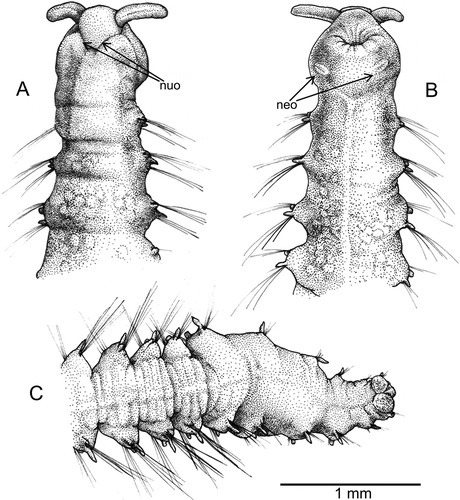
Body elongated, slightly tapered at both ends, swollen in middle (, ). Specimens 8–13 mm long and 1.0–1.8 mm wide at widest part for 22–24 chaetigers. Prostomium pentagonal, with straight frontal margin bearing two distinct laterally directed and well delineated projections (palps) (A, A); eyes lacking. Mid-dorsal prostomial depression observed with the SEM (A,B). Nuchal organs bordering posterior part of prostomium (A, A,C). Basal part of everted proboscis with longitudinal folds (B, A). Peristomium complete ring, dorsally incised giving a biannulate appearance slightly larger than following segments (D). Segment 1 achaetigerous; similar to following segments (D). A pair of ciliated slits located ventrally on peristomium behind buccal opening (B, D,E, B,C). Each segment with four annuli (A). Parapodia similar throughout the body. Notopodia and neuropodia jointed on a vertically orientated parapodial extension; all chaetigers bearing notopodia provided with a small knob located ventrally to chaetal bundle (Hartman's ‘interramal cirri’ and Blake's ‘postchaetal lamella’; see Discussion) and neuropodia provided with digitate, distally pointed cirrus located dorsally to chaetal bundle (F, B–D). A ciliated band visible with the SEM in the dorsal part of the notochaetal knob (C). Notochaetae less numerous than neurochaetae; notochetae disappear in the last three body chaetigers (B). Two types of chaetae in both parapodial rami: long, slender capillaries in ventral part of notopodium and dorsal part of neuropodium and shorter and thinner capillaries in dorsal part of notopodium and ventral part of neuropodium (E, C–F). Pygidium with two pad-shaped lobes (C), densely covered with conical papillae (F); each papilla terminating in four pores (E,F).
Figure 3. Axiokebuita minuta, Antarctic (Bellingshausen Sea) specimens from BENTART–06 cruise. MNCN 16.01/13600, (A) prostomium in dorsal view, (B) detail of prostomial depression, (C) detail of nuchal organ, (D) anterior end, ventro-lateral view, (E) detail of neck organ ciliation, (F) chaetiger 7. neo, neck organ; dk, dorsal knob; nuo, nuchal organ; prd, prostomial depression; pag, palp groove; vc, ventral cirrus.
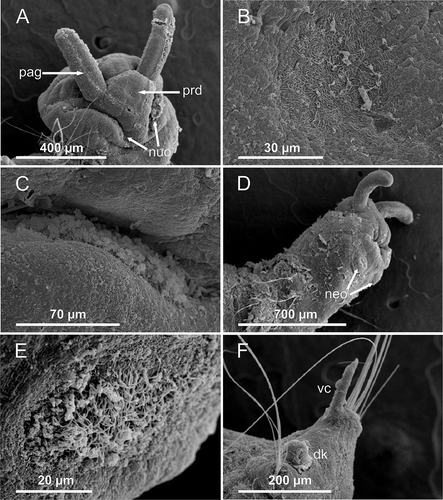
Figure 4. Axiokebuita minuta, Antarctic (Bellingshausen Sea) specimens from BENTART–06 cruise. MNCN 16.01/13600, (A) anterior end, antero-ventral view, (B) ventral neck organ, (C) detail of neck organ ciliation, (D) anterior end, dorsal view, (E) neurochaetae and ventral cirrus from chaetiger 14, (F) right pygidial lobe. as, achaetigerous segment; fs, first chaetiger; neo, neck organ; nuo, nuchal organ; pag, palp groove; per, peristomium; pf, proboscideal folds; pr, prostomium.
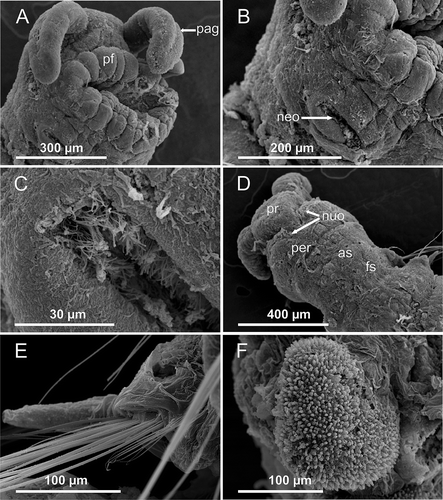
Figure 5. Axiokebuita minuta, Antarctic (Bellingshausen Sea) specimens from BENTART–06 cruise. MNCN 16.01/13600, (A) general view of the animal, (B) chaetiger 3, (C) chaetiger 20 notopodial knob with ciliation, (D) chaetigers 21–23 and pygidial lobe, (E,F) detail of pygidial adhesive papillae. dk, dorsal knob; pl, pygidial lobe; vc, ventral cirrus.
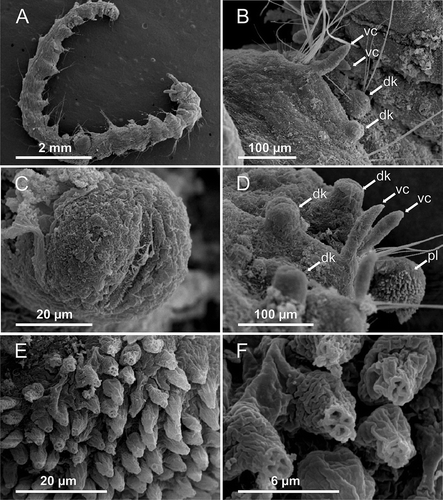
Figure 6. Axiokebuita minuta, Antarctic (Bellingshausen Sea) specimens from BENTART–06 cruise. MNCN 16.01/13600, (A) mid-body segments, dorsal view, (B) five posterior end segments, dorsal view, arrows marking three posterior achaetous notopodial rami, (C) detail of notochaetae from posterior chaetiger, (D) detail of scale covering of long notochaetae, (E) detail of short notochaetae, (F) neurochaetae from same chaetiger.
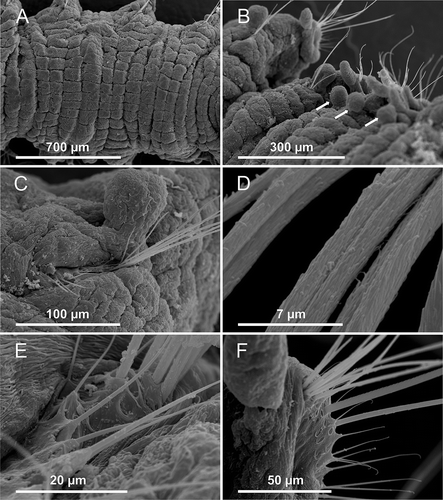
The studied specimens from the Norwegian waters (), Weddell Sea and South Sandwich Island waters () have the same body characteristics as those in the Bellingshausen Sea with the only exception of body size and chaetae. The European specimens are smaller (3–5 mm) than the BS specimens, but not that of the Weddell Sea (4–5 mm) and Ross Sea (6–7 mm) (see Pocklington & Fournier Citation1987), although in all cases the number of chaetigers is the same (22–25). In the North Atlantic specimens chaetae are longer than in Antarctic specimens (A vs. D) and also the difference between long and short chaetae on each parapodial rami is much less pronounced (F vs. C,E).
Figure 7. Axiokebuita spec. sensu Persson and Pleijel (Citation2005), Norwegian specimen. SMNH 75819, (A) anterior end, ventro-lateral view, (B) detail of neck organ, (C) neuropod of chaetiger 4, (D) neuropodia of chaetigers 5–10, (E) chaetiger 14 neurochaetae, (F) detail of neurochaetal serration, (G) detail of cuticle porous, (H) posterior end, latero-dorsal view. neo, neck organ.
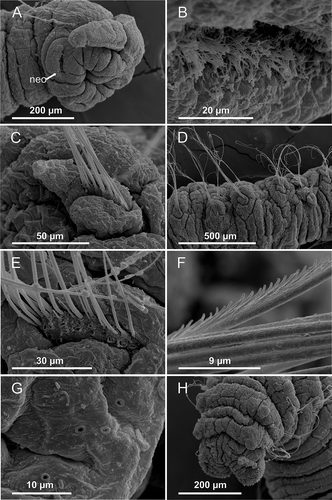
Figure 8. Axiokebuita minuta sensu Schüller and Ebbe (Citation2007), Antarctic specimen from ANDEEP-II cruise. MNCN 16.01/13601, (A) anterior end, ventro-lateral view, (B,C) chaetigers 7 and 9, (D) chaetigers 18–19. neo, neck organ; dk, dorsal knob; vc, ventral cirrus.
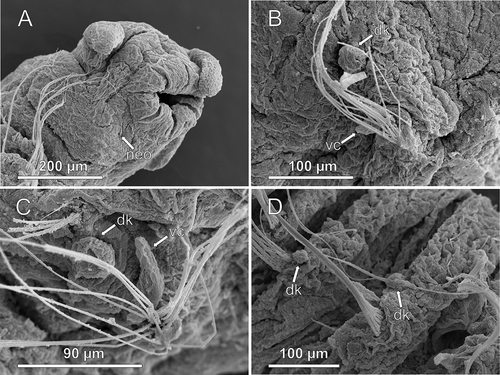
Re-examination of one paratype of A. minuta (USNM 56625) and one paratype of A. millsi (USNM 56625) did not reveal any difference among both species. The holotype of A. minuta (USNM 55553) was not examined but, as reported Persson and Pleijel (Citation2005), the specimen is too poorly preserved to permit unequivocal assessment of any difference with A. millsi (e.g. presence or absence of notopodial lobes). Axiokebuita minuta paratype USNM 56625 is composed of two specimens: one is an Axiokebuita but the other is another scalibregmatid with furcate chaetae, from which the slide was made from (see Discussion). The Axiokebuita specimen is posteriorly incomplete, but the presence of globular notopodial cirri was confirmed. In A. millsi paratype USNM 60574 (slide and specimen) none of the supposed differences with A. minuta were apparent.
DNA sequence data
Unfortunately it was not possible to get adequate DNA extraction from any material except the specimen from the hydrothermal vents from the southeastern Pacific Ocean hydrothermal vent specimen. The specimen was divided into three fragments and the median fragment was used for molecular analysis. The anterior part, 4.0 mm long and 1.5 mm in its wider part, consists of prostomium, peristomium and 10 chaetigers. The posterior part was 2.0 mm long and 0.8 mm wide and 5 chaetigers. All prostomial, parapodial and pygidial features characteristic of A. minuta are present in this specimen. For this specimen, the partial 18S rDNA sequence (Genbank n. JN256053) showed 99% similarity with the 18s rDNA sequence for Axiokebuita sp. sequenced by Persson and Pleijel (Citation2005), with only 6 bases different and 2 gaps. We also sequenced COI for the southeastern Pacific Ocean specimen and make this available on Genbank (n. JN256052) for future comparisons, but there are currently no other COI sequences available for Axiokebuita. DNA extractions for the specimen from the Iberian peninsula were attempted, but unfortunately failed to recover any usable DNA.
Reproduction
Pocklington and Fournier (Citation1987) reported ovigerous females in specimens of A. millsi in July (North Atlantic) and January (Antarctica). Large eggs were observed through the tegument inside the body cavity in some of collected specimens in January 2006 in the BS. A single putative male specimen was used for TEM analysis (data no shown), but it did not reveal the occurrence of spermatids, or early or mature spermatozoa (M.C. Gambi, personal observation).
Ecology
The BS specimens were collected at various stations during the BENTART–06 cruise, exclusively with a suprabenthic sledge and occurred only on the lower net (located about 50 cm from the bottom). For the same stations where benthic material was collected with a box corer and Agassiz trawl many other polychaetes were sampled, but not a single specimen of Axiokebuita occurred (Parapar et al. Citation2011a). Therefore the species probably thrives in the upper few centimetres of the sediment, and is so delicate that other tougher sampling gear destroyed the material. The species was collected in various stations located on the continental slope and deeper (from 620 to over 3000 m depth). The occurrence of Axiokebuita in deep hydrothermal vents habitat has previously been documented in the northeast Pacific for specimens identified as A. millsi by Blake and Hilbig (Citation1990). These authors felt that Axiokebuita was unlikely to be a true vent taxon in terms of only being found at these habitats. The second record here of Axiokebuita from hydrothermal vents does not disprove this assertion until further material is acquired from various habitat and localities.
Distribution
On the basis of material collected and examined from museum collections, A. minuta appears to be present in all world oceans except the Indian Ocean, which may be due simply to the different sampling efforts. In terms of latitudinal distribution, the species seems to prefer high-latitude waters both boreal and austral, although again this may also be biased by lack of sampling. As presently known it is vertically distributed from 305 down to 3049 m depth.
Taxonomic remarks
The genus Kebuita Chamberlin, 1919 (Scalibregmatidae) was erected for Kebuita glabra (Ehlers, 1887), from off Havana (Cuba). Hartman (Citation1967) did the first description of Kebuita minuta based on specimens from the South Orkney Islands (type locality) and off the Antarctic Peninsula (). Shortly after, and based on two specimens collected in the Weddell Sea, she presented a more detailed description along with the first drawings of the taxon (Hartman Citation1978) (A,B); however, with no mention of any notopodial features. Blake (Citation1981), in the context of a study on a large collection of scalibregmatids from western South America, Antarctica and sub-Antarctic regions, revised Hartman's material, presenting an improved description and illustration of the anterior body region. He described in detail the peristomium, as well as a developed ring overlapping the prostomium dorsally and with a mid-dorsal notch (C). Hartman's ‘interramal cirrus’ (B arrow) is named by Blake ‘postchaetal lamella’ (D arrow), although still there was no reference to any structure in the notopodial lobe. The furcate chaetae reported by Hartman (Citation1967, Citation1978) were not confirmed by Blake (Citation1981).
Figure 9. Line drawings of Axiokebuita from the earliest to the most recent illustrations in the literature, all redrawn from original, (A) anterior end in dorsal view, arrow showing Hartman's ‘antennae’ (now ‘palps’), (B) postmedian parapodium, arrow showing Hartman's ‘interramal cirrus’ (from Hartman Citation1978 as Kebuita minuta), (C) anterior end in dorsal view, arrow showing Blake's ‘peristomial mid-dorsal notch’, (D) two anterior chaetigers in lateral view, arrow marking Blake's ‘postsetal lamella’ behind neurochaetae (from Blake Citation1981 as Kebuita minuta), (E) semi-diagrammatic latero-dorsal view of complete specimen (holotype), (F) parapodium, arrow showing ‘globular notopodial postchaetal lamella’, (G) detail of serrated chaetae (from Pocklington & Fournier Citation1987 as Axiokebuita millsi), (H) anterior end in dorsal view, (I) middle parapodium, (J) neurochaeta (from Blake & Hilbig Citation1990 as Axiokebuita millsi). [(A & B) From figure 27, Hartman, O., Polychaeta from the Weddel Sea quadrant, Antarctica. The biology of Antarctic seas. Antarctic Research Series, 26:125–223, 1978. © 1978 American Geophysical Union. Reproduced by permission of American Geophysical Union; (C & D) from figure 11, Blake, J.A. Citation1981. The Scalibregmatidae (Annelida: Polychaeta) from South America and Antarctica collected chiefly during the cruises of the R/V Hero and USNS Eltanin. Proceedings of the Biological Society of Washington, 94:1131–1162, 1981. © 1981 Biological Society of Washington. Reproduced by permission of Biological Society of Washington; (E, F & G) from and , Pocklington, P. & Fournier, J.A. Citation1987. Axiokebuita millsi, new genus, new species, (Polychaeta : Scalibregmatidae) from eastern Canada. Bulletin of the Biological Society of Washington, 7:108–113, 1987. Reproduced by permission of Biological Society of Washington; (H, I & J) from figure 13, Blake, J.A. & Hilbig, B., Polychaeta from the vicinity of deep-sea hydrothermal vents in the Eastern Pacific. II. New species and records from the Juan de Fuca and Explorer Ridge Systems. Pacific Science 44:219–253, 1990. © 1990 University of Hawaii Press. Reproduced by permission of University of Hawaii Press.] Scale bars: (A) and (B) no scale; (C) = 300 μm; (D) = 200 μm; (E) = 1 mm; (F) = 30 μm; (G) = 4 μm; (H) = 1 mm; (I) = 200 μm; (J) = 10 μm.
![Figure 9. Line drawings of Axiokebuita from the earliest to the most recent illustrations in the literature, all redrawn from original, (A) anterior end in dorsal view, arrow showing Hartman's ‘antennae’ (now ‘palps’), (B) postmedian parapodium, arrow showing Hartman's ‘interramal cirrus’ (from Hartman Citation1978 as Kebuita minuta), (C) anterior end in dorsal view, arrow showing Blake's ‘peristomial mid-dorsal notch’, (D) two anterior chaetigers in lateral view, arrow marking Blake's ‘postsetal lamella’ behind neurochaetae (from Blake Citation1981 as Kebuita minuta), (E) semi-diagrammatic latero-dorsal view of complete specimen (holotype), (F) parapodium, arrow showing ‘globular notopodial postchaetal lamella’, (G) detail of serrated chaetae (from Pocklington & Fournier Citation1987 as Axiokebuita millsi), (H) anterior end in dorsal view, (I) middle parapodium, (J) neurochaeta (from Blake & Hilbig Citation1990 as Axiokebuita millsi). [(A & B) From figure 27, Hartman, O., Polychaeta from the Weddel Sea quadrant, Antarctica. The biology of Antarctic seas. Antarctic Research Series, 26:125–223, 1978. © 1978 American Geophysical Union. Reproduced by permission of American Geophysical Union; (C & D) from figure 11, Blake, J.A. Citation1981. The Scalibregmatidae (Annelida: Polychaeta) from South America and Antarctica collected chiefly during the cruises of the R/V Hero and USNS Eltanin. Proceedings of the Biological Society of Washington, 94:1131–1162, 1981. © 1981 Biological Society of Washington. Reproduced by permission of Biological Society of Washington; (E, F & G) from figures 1 and 2, Pocklington, P. & Fournier, J.A. Citation1987. Axiokebuita millsi, new genus, new species, (Polychaeta : Scalibregmatidae) from eastern Canada. Bulletin of the Biological Society of Washington, 7:108–113, 1987. Reproduced by permission of Biological Society of Washington; (H, I & J) from figure 13, Blake, J.A. & Hilbig, B., Polychaeta from the vicinity of deep-sea hydrothermal vents in the Eastern Pacific. II. New species and records from the Juan de Fuca and Explorer Ridge Systems. Pacific Science 44:219–253, 1990. © 1990 University of Hawaii Press. Reproduced by permission of University of Hawaii Press.] Scale bars: (A) and (B) no scale; (C) = 300 μm; (D) = 200 μm; (E) = 1 mm; (F) = 30 μm; (G) = 4 μm; (H) = 1 mm; (I) = 200 μm; (J) = 10 μm.](/cms/asset/5757a7e4-2ee1-4a9b-8005-efd352f62fc8/tizo_a_598350_o_f0009g.gif)
Pocklington and Fournier (Citation1987) described the new genus Axiokebuita and the species A. millsi from slope depths off eastern Canada (). They reported for the first time the ‘globular notopodial postsetal lamellae’ (E,F arrow) in the parapodia, two types of neurochaetae (long and short) and confirm the lack of furcate chaetae in both A. millsi and K. minuta. The authors revised part of Hartman's (Citation1978) and Blake's (Citation1981) Antarctic specimens, previously identified as K. minuta, and found that they all should belong to A. millsi because of the presence of the globular notopodial lamellae. Nevertheless, because they did not revise all Antarctic material, they maintained both species as valid but now included K. minuta in the genus Axiokebuita. Following Hartman's (Citation1967) original description of Axiokebuita minuta, the species was proposed by Pocklington and Fournier (Citation1987) to differ from A. millsi in lacking the globular notopodial lamellae, in having the neuropodial postschaetal lamellae restricted to posterior chaetigers, and in having a simple pygidial ring, rather than a bilobed pygidium.
According to the aforementioned criteria, Blake and Hilbig (Citation1990) reported A. millsi from hydrothermal vents of the northeast Pacific () because of the presence of ‘globular postchaetal notopodial lamella’. The authors also present the most accurate drawing to date available for the species (H,I), particularly regarding the prostomium/peristomium region, and reported neurochaetae with finely serrated shafts and slightly expanded bifid tips (J).
Persson and Pleijel (Citation2005) collected new specimens from Norway (), studying their affinities with both A. minuta (from Hartman's Citation1967 original material) and A. millsi (from Blake's Citation1981 material). The good condition of the Nordic specimens allowed a detailed description of the taxon Axiokebuita and the first SEM images. The main diagnostic characters of the genus were defined as follows: prostomium pentagonal with strait frontal margin bearing two palps; peristomium dorsally incised; each segment with four annuli; notopodia with a small knob ventral to chaetal bundle; neuropodia with digitate pointed cirrus dorsal to chaetal bundle; both noto- and neuro-chaetae capillary; two pad-shaped lobes on pygidium covered with papillae. Persson and Pleijel (Citation2005) presented for the first time a detailed description of the pygidial lobes and reported the probable presence of nuchal organs as ciliated bands bordering the posterior part of the prostomium. From the examination of Hartman's type, Persson and Pleijel (Citation2005) could not unambiguously refer the specimens from Norway to either A. minuta or A. millsi (but SEM pictures from Trondheim specimen in are named A. minuta) because the three differences proposed by Pocklington and Fournier (Citation1987) to distinguish between the two taxa were not confirmed, largely due to poorly preservation of the type specimens. Persson and Pleijel (Citation2005) also proposed that the previously unnoticed bilobed pygidium with adhesive papillae may constitute an apomorphy of Axiokebuita.
In this work, the study of paratypes of A. minuta and A. millsi along with newly collected specimens from the BS and Antarctic Peninsula, from near the type locality of A. minuta (), Norwegian specimens named as Axiokebuita sp. by Persson and Pleijel (Citation2005), Antarctic specimens identified by Schüller and Ebbe (Citation2007) and Schüller (Citation2008a, Citation2008b) as A. minuta and A. millsi, one specimen from the Pacific Ocean (Easter Island) and two from NW Spain and SW Iceland, respectively, led us to confirm the constant presence of all relevant taxonomic characters (notopodial knob, neuropodial cirrus and pygidial lobes with papillae) in all specimens. Therefore, it is difficult to state any definite morphological difference between the two nominal species, except that Norwegian specimens are much smaller than those from the Antarctica (3–5 vs. 9–16 mm), but with a similar number of chaetigers (22–25 vs. 22–24). Specimens from South Orkney Islands identified as A. millsi by Schüller and Ebbe (Citation2007) also have a notopodial knob, but this is less apparent than in specimens named as A. minuta by the same authors collected in the Central Weddell Sea.
Our examination of a relatively large number of specimens confirms the lack of furcate chaetae in Axiokebuita. Since Hartman (Citation1967, Citation1978), no one else has reported the presence of this type of chaetae; subsequent authors who have examined Hartman's material also did not mention the presence of those chaetae in the types. We examined a microscope slide from USNM 56625 paratype that has some parapodia (available on the web: http://collections.nmnh.si.edu/search/iz/), supposedly corresponding to the type series of Axiokebuita minuta deposited in the Smithsonian National Museum of Natural History, Washington, DC. In that slide, furcate chaetae are clearly present, but this body fragment corresponds to a different scalibregmatid species which was incorrectly assigned to A. minuta and lies in the same vial.
Two new, previously unreported structures in the genus Axiokebuita were found in this study (both occurring in all studied specimens): a prostomial depression and a ciliated ‘neck organ’. The depression observed with the SEM in the dorsal part of the prostomium in BS specimens (A,B) was initially supposed to be an artefact, but this structure could also be observed in boreal specimens (see A in Persson & Pleijel Citation2005); its location suggests a sensory role, but only a histological study would lead us to speculate about its function. Whereas nuchal organs are widely present in other scalibregmatids and many other polychaetes, the finding of the neck organ in this species reveals a probable new sensory structure in the family. Because of its location in the ventral part of the peristomium, its ciliated nature and the apparent possibility to modulate its degree of aperture (compare D,E vs. B,C), a chemosensory or mechanoreceptor role in the burrowing or food uptake could be suggested owing to that scalibregmatids are considered subsurface deposit-feeders (Schüller & Ebbe Citation2007).
Discussion
Axiokebuita was placed by Pocklington and Fournier (Citation1987) in group II of scalibregmatids as defined by Kudenov and Blake (Citation1978), and characterised by the maggot-like body and incised prostomium. This group included also Polyphysia Quatrefages, 1865 (with branchiae) and Lipobranchus Cunningham & Ramage, 1888 (without branchiae). The grouping of these genera by Kudenov and Blake (Citation1978) was not based on a phylogenetic study, so that hypothetical relation requires further study based on additional knowledge of the morphology of the genus.
Axiokebuita, as noted above, can be diagnosed by five possibly apomorphic features: a pentagonal prostomium with strait frontal margin bearing two projections; notopodia each with a small knob ventral to chaetal bundle; neuropodia each with a digitate pointed cirrus dorsal to the chaetal bundle; both noto- and neuro-chaetae capillary; two pad-shaped lobes on pygidium covered with papillae. A sixth possible apomorphy, the ciliated organ located in the ventral peristomium here named the ‘neck organs’, and perhaps the prostomial depression, seem to be highly characteristic of Axiokebuita, as their presence has not been reported in any other scalibregmatid genus.
The position and shape of the notopodial knob resembles the interramal papillae or ciliated organs usually present in other scalibregmatids such as in Sclerobregma Hartman, 1965 and Oligobregma Kudenov and Blake, Citation1978 (see Blake Citation1981). Rouse and Pleijel (Citation2001) proposed a possible homology of this structure with the lateral organs occurring in the same position in other taxa such as Opheliidae, Orbiniidae and Paraonidae within Scolecida and also in Canalipalpata (Spionidae and Pectinariidae) and Aciculata (Syllidae, Eunicidae and Amphinomidae) (Purschke Citation2005).
From the study of museum collections and newly collected material of both A. minuta and A. millsi, a clear separation between both taxa could not be established using classic morphological characters. Therefore, we propose to consider the two previous nominal species as a single ‘species’, and due to this to consider Axiokebuita millsi a junior synonym of A. minuta. Only the finding of new discriminating characters, either morphological or molecular, could in the future lead to the reconsideration of this conclusion.
The few records of this monotypic genus in the literature makes difficult to establish a definite pattern of geographic distribution. Although Schüller and Ebbe (Citation2007) defined Axiokebuita as potentially cosmopolitan, in the light of currently available data, it may rather have a bipolar (or antitropical) distribution associated with the deep slope of boreal Atlantic and Pacific and west and east Antarctic. The findings of new specimens seem to confirm this pattern of distribution, although there are significant exceptions given the specimens from the hydrothermal vents of the southeast and northeast Pacific. The southeast Pacific specimen was located near the tropics 23°S, and therefore extends this range to the deep bottoms of the Southern Pacific Ocean (), while the northeast Pacific records are from south of 50°N (Blake & Hilbig Citation1990). This discontinuous pattern, with a possible connection, through the deep waters, in temperate latitudes, has also been observed in other deep-sea polychaetes such as the opheliids Ammotrypanella and Ophelina (e.g. Schüller Citation2008a; Parapar & Moreira Citation2008; Parapar et al. Citation2011b). Kirkegaard (Citation1996) advocates the existence of a deep ocean current as described by Broecker and Denton (Citation1989) as a possible explanation for the broad distribution of some deepwater species. Glasby (Citation2005) suggests three reasons for this apparently disjunct distribution pattern: the result of long-distance dispersal, extinction events, or incorrect taxonomic assignments. As at the moment no discriminating morphological characters have been identified, while the molecular analysis is still limited to a few geographic locations, we cannot exclude the presence of sibling species which may have originated from this long distance dispersal and disjunct distribution. Therefore, the proposed synonymy and monotypic feature of the genus Axiokebuita should be further investigated and validated by additional analyses, especially at the molecular level.
In our case, however, a further reason could be proposed: the sampling effort and methodology. The sampling effort in deep ocean sediments is still much lower than in areas of continental shelf and slope (e.g. Parapar & Moreira Citation2009). In both BENTART and ANDEEP II cruises, specimens were collected with a suprabenthic sledge (Brenke Citation2005); other gears used (box corer, multibox corer and Agassiz trawl) in same areas and in different cruises did not collect Axiokebuita (Hilbig et al. Citation2006; Saiz et al. 2008, Parapar et al. Citation2011a). The high selectivity in the fauna collected by the suprabenthic sledge, primarily used to study of the suprabenthic community (mostly peracarid crustaceans; San Vicente et al. Citation2009), but also capable of collecting the first few centimeters of sediment, makes it very suitable for the capture of Axiokebuita. Suprabenthic sledges have so far not been very widely used, mainly in the European region (Mees & Hammerlynck Citation1992; San Vicente et al. Citation1993; Dauvin et al. Citation1994) and Antarctica (San Vicente et al. Citation2007, Citation2009). It is likely that a more widespread use of this gear may result in a different picture of the distribution of the genus Axiokebuita.
Acknowledgements
The authors wish to thank all colleagues involved in the BENTART-06 cruise, especially the chief scientist Ana Ramos Martos (Oceanographic Institute, Vigo), and Carles San Vicente (Generalitat de Catalunya) and Patrick Arnaud (Station Marine d'Endoume, France) and Jean-Claude Sorbe who allowed collection of the Axiokebuita specimens from the suprabenthic sledge samples. Thanks to R. Vrijenhoek, Chief Scientist of the PAR5 Cruise, for inviting GWR on the cruise and to the crews of RV Atlantis, and DSV Alvin, for collecting the specimens. The PAR5 cruise was funded by grants from the US National Science Foundation to R.C. Vrijenhoek (OCE- 0241613) and to C.L. Van Dover (OCE-0350554). We are also grateful to Juan Moreira (UAM-Madrid), Gudmundur V. Helgason (University of Iceland) and Gudmundur Gudmundsson (Icelandic Institute of Natural History) for providing DIVA-Artabria and BIOICE specimens, respectively. David Romero (UDC) prepared the line drawings, and Ada Castro and Belen López (SAIN, UDC) assisted with the use of the SEM. Thanks also to José Carvajal who did the DNA extractions and sequencing of the Axiokebuita samples. Our most sincere thanks to Karin Sindemark (Naturhistoriska Riksmuseet, Stockholm), Karin Meißner (Senckenberg Forschungsinstitute und Naturmuseen-SFN, Hamburg), Saskia Brix (DZMB), and Myriam Schüller (Rühr-Universität Bochum, Germany) for loaning Norwegian and ANDEEP specimens. Thanks are also given to Linda Ward (USNM, Washington) for making available to us the type material of both species and to Fredrik Pleijel (MET-Göteborgs Universitet, Sweden) for providing information on Norwegian specimens. The BENTART–06 cruise was funded by the Spanish Antarctic Programme GLC2004–01856/ANT.
References
- Blake , JA. 1981 . The Scalibregmatidae (Annelida: Polychaeta) from South America and Antarctica collected chiefly during the cruises of the R/V ‘Anton Brunn,’ R/V ‘Hero,’ and USNS ‘Eltanin.’ . Proceedings of the Biological Society of Washington , 94 : 1131 – 1162 .
- Blake , JA and Hilbig , B. 1990 . Polychaeta from the Vicinity of Deep-sea Hydrothermal Vents in the Eastern Pacific. II. New species and Records from the Juan de Fuca and Explorer Ridge Systems . Pacific Science , 44 : 219 – 253 .
- Brandt , A and Hilbig , B. , eds. 2004 . “ ANDEEP (Antarctic benthic DEEP-sea) biodiversity: Colonization history and recent community patterns: a tribute to Howard Sanders ” . In Deep-Sea Research II Vol. 51 , 1457 – 1919 .
- Brenke , N. 2005 . An epibenthic sledge for operations on marine soft bottoms and bedrock . Marine Technology Society Journal , 39 ( 2 ) : 10 – 19 .
- Broecker , WS and Denton , GH. 1989 . The role of ocean–atmosphere reorganizations in glacial cycles . Geochimica et Cosmochimica Acta , 53 : 2465 – 2501 .
- Brunel , P , Besner , M , Messier , D , Poirier , L , Granger , D and Weinstein , M. 1978 . Le traîneau Macer-GIROQ: Appareil amélioré pour l’échantillonnage quantitatif étagé de la petite faune nageuse au voisinage du fond. Internationale Revue der gesamte . Hydrobiologie , 63 : 815 – 829 .
- Cartes , JE , Sorbe , JC and Sardá , F. 1994 . Spatial distribution of deep-sea decapods and euphausiids near the bottom in the northwestern Mediterranean . Journal of Experimental Marine Biology and Ecology , 179 : 131 – 144 .
- Corbera , J , San Vicente , C and Sorbe , JC. 2009 . Cumaceans (Crustacea) from the Bellingshausen Sea and off the western Antarctic Peninsula: A deep-water link with fauna of the surrounding oceans . Polar Biology , 32 : 611 – 622 .
- Dauvin , JC , Iglesias , A and Lorgere , JC. 1994 . Circalittoral suprabenthic coarse sand community from the Western English channel . Journal of the Marine Biological Association of the United Kingdom , 74 : 543 – 562 .
- Dauvin , JC and Lorgere , JC. 1989 . Modification du traîneau Macer-GIROQ pour l'amélioration de l’échantillonnage quantitatif étagé de la faune suprabentique . Journal de Recherche Oceanographique , 14 : 65 – 67 .
- Glasby , C. 2005 . Polychaete distribution patterns revisited: an historical explanation . Marine Ecology , 26 : 235 – 245 .
- Hammerlynck , O and Mees , J. 1991 . Temporal and spatial structure in the hyperbenthic community of a shallow coastal area and its relation to environmental variables . Oceanologica Acta , 11 : 205 – 211 .
- Hartman , O. 1967 . Polychaetous annelids collected by the USNS ‘Eltanin’ and ‘Staten Island’ cruises, chiefly from Antarctic seas . Allan Hancock Monographs on Marine Biology , 2 : 1 – 387 .
- Hartman , O. 1978 . Polychaeta from the Weddell Sea Quadrant, Antarctica . Antarctic Research Series, American Geophysical Union , 26 : 125 – 233 .
- Hilbig , B , Gerdes , D and Montiel , A. 2006 . Distribution patterns and biodiversity in polychaete communities of the Weddell Sea and Antarctic Peninsula area (Southern Ocean) . Journal of the Marine Biological Association of the United Kingdom , 86 : 711 – 725 .
- Kirkegaard , JB. 1996 . Bathyal and abyssal polychaetes (Sedentary species I) . Galathea Reports , 17 : 57 – 78 .
- Kudenov , J and Blake , JA. 1978 . A review of the genera and species of the Scalibregmatidae (Polychaeta) with descriptions of one new genus and three new species from Australia . Journal of Natural History , 12 : 427 – 444 .
- Mees , J and Hammerlynck , O. 1992 . Spatial community structure of the winter hyperbenthos of the Shelde estuary, the Netherlands, and the adjacent coastal waters . Netherlands Journal of Sea Research , 25 : 757 – 770 .
- Osborn , KJ and Rouse , GW. 2011 . Phylogenetics of Acrocirridae and Flabelligeridae (Cirratuliformia, Annelida) . Zoologica Scripta , 40 : 204 – 219 .
- Parapar , J , López , E , Gambi , MC , Núñez , J and Ramos , A. 2011a . Quantitative analysis of soft-bottom polychaetes of the Belligshausen Sea and Gerlache Strait (Antartica) . Polar Biology , 34 : 715 – 730 .
- Parapar , J and Moreira , J. 2008 . Sobre la presencia del género Ophelina Ørsted, 1843 (Polychaeta, Opheliidae) en el litoral de la península Ibérica . Nova Acta Científica Compostelana , 17 : 117 – 134 .
- Parapar , J and Moreira , J. 2009 . Polychaeta of the ‘DIVA-Artabria I’ project (cruise 2002) in the continental shelf and upper slope off Galicia (NW Spain) . Cahiers de Biologie Marine , 50 : 57 – 78 .
- Parapar , J , Moreira , J and Helgason , GV. 2011b . Distribution and diversity of the Opheliidae (Annelida, Polychaeta) on the continental shelf and slope of Iceland, with a review of the genus Ophelina in northeast Atlantic waters and description of two new species . Organisms, Diversity and Evolution , 11 : 83 – 105 .
- Persson , J and Pleijel , F. 2005 . On the phylogenetic relationships of Axiokebuita, Travisia and Scalibregmatidae (Polychaeta) . Zootaxa , 998 : 1 – 14 .
- Pocklington , P and Fournier , JA. 1987 . Axiokebuita millsi, new genus, new species, (Polychaeta: Scalibregmatidae) from eastern Canada . Proceedings of the Biological Society of Washington , 7 : 108 – 113 .
- Purschke , G. 2005 . Sense organs in polychaetes (Annelida) . Hydrobiologia , 535/536 : 53 – 78 .
- Rouse , G and Pleijel , F. 2001 . Polychaetes , 354 Oxford : Oxford University Press .
- Saiz , JI , García , FJ , Manjón-Cabeza , ME , Parapar , J , Peña-Cantero , A , Saucède , T , Troncoso , JS and Ramos , A. 2009 . Community structure and spatial distribution of benthic fauna in the Bellingshausen Sea (West Antarctica) . Polar Biology , 31 : 735 – 743 .
- San Vicente , C , Castelló , J , Corbera , J , Jimeno , A , Munilla , T , Sanz , MC , Sorbe , JC and Ramos , A. 2007 . Biodiversity and structure of the suprabenthic assemblages from South Shetland Islands and Bransfield Strait, Southern Ocean . Polar Biology , 30 : 477 – 486 .
- San Vicente , C , Munilla , T , Corbera , J , Sorbe , JC and Ramos , A. 2009 . Suprabenthic fauna from the Bellingshausen Sea and western Antarctic Peninsula: Spatial distribution and community structure . Scientia Marina , 73 : 357 – 368 .
- San Vicente , C and Sorbe , JC. 1993 . Estudio comparado de las playas catalanas y vascas: metodología y resultados preliminares . Publicaciones Especiales del Instituto Español de Oceanografía , 1 : 299 – 304 .
- Schüller , M. 2008a . New polychaete species collected during the expeditions ANDEEP I, II, and III to the deep Atlantic sector of the Southern Ocean in the Austral summers 2002 and 2005 – Ampharetidae, Opheliidae, and Scalibregmatidae . Zootaxa , 1705 : 51 – 68 .
- Schüller , M. 2008b . Polychaeta (Annelida) of the Deep Southern Ocean. Biodiversity and Zoogeography , 209 Germany : VDM Verlag .
- Schüller , M and Ebbe , B. 2007 . Global distributional patterns of selected deep-sea Polychaeta (Annelida) from the Southern Ocean . Deep-Sea Research II , 54 : 1737 – 1751 .