Abstract
Biological invasions have become a major cause of biodiversity loss. Tracing the origin of the populations of alien species is essential to infer the dispersal pathway and finally to set conservation policies aimed at preventing new introductions. The Italian wall lizard, Podarcis sicula, is one of the reptile species most widely introduced, with allochthonous populations occurring from the United States to Turkey. For some of them, instances of geographic expansion and competition/hybridization with autochthonous Podarcis sp. have been indicated. In the Iberian Peninsula, five introduced populations are known: Lisbon (W Portugal), Noja, Cantabria (N Spain), La Rioja (N Spain) and Almería (S Spain) and Sant Celoni, Barcelona (NE Spain) while the species now widely ranges Menorca Island in the Balearics. Here we assess the origin of four populations (Barcelona population will not be included) by comparing in a phylogenetic framework the cytochrome b gene sequences of specimens from the introduced and native populations. The results from this study provide evidence for distinct sources, pathways, and timing of introduction in Iberian Peninsula and Balearics by P. sicula from Tuscany, Calabria, Sicily, and Sardinia. This finding underpins the fact that P. sicula holds considerable potential invasiveness and advises for conservation strategies based on a global and preventive plan for avoiding new introductions as well as on eradication and control measures when prevention fails.
Introduction
Long dispersal movements of fauna constitute natural phenomena in the evolution of biological communities (de Queiroz Citation2005). However, the increasing degree of anthropisation and human-mediated transport are leading to an enormous increase in the rates of animal translocations. This has resulted in a real epidemic of biological invasions - the process by which an alien species establishes, expands its geographic range and numbers, and exerts ecological or economic impacts in a new area (Brown et al. Citation2007). Although the effects of an introduction are difficult to predict, several general impacts are now well-known.
In the particular case of reptiles, there is evidence for ecological, evolutionary and social effects, including biotic homogenization, disruptions of food-webs, hybridization, disease, behavioural changes, effects on human health, between other numerous negative impacts (Ficetola et al. Citation2009; Kraus Citation2009). Among the ecological effects, the negative impact on native biota is to be remarked. This is the case of the snake Natrix maura which is assumed to be responsible for the decline of the Mallorcan midwife toad (Alytes muletensis) through the predation of larvae and adults (Guicking et al. Citation2005; Kraus Citation2009; Álvarez et al. Citation2010). An example of negative social effect is the introduction of the brown tree snakes (Boiga irregularis) in Guam, which increase the susceptibility of humans to disease and even death (Fritts et al. Citation1994; Rodda et al. Citation1997).
The Italian wall lizard, Podarcis sicula constitutes an outstanding case of reptile species largely introduced worldwide. Its natural range is restricted to the Italian Peninsula and Sicily. However, an introduction of the species either in historical times or even more recently has been postulated for the Tyrrhenian Islands, Corsica and Sardinia, and in the islands and coastal areas of the eastern Adriatic Sea (Corti Citation2006; Isailovic et al. Citation2008 in IUCN 2011). Moreover, P. sicula is certainly introduced in the Iberian Peninsula, in Cantabria (Meijide Citation1981), Almería (Mertens & Wermuth Citation1960), Lisbon (González de la Vega et al. 2001), La Rioja (Valdeón et al. Citation2010) and nearby Barcelona (Rivera et al. Citation2011); in Southern France, in Toulon and Château d'If Island (Morgue Citation1924; Orsini Citation1984); in Turkey, in Istanbul and Marmara Islands (Basoglu & Baran Citation1977); in North Africa, in Tunisia and Tripoli (Arnold & Ovenden Citation2002); and in the United States, in Philadelphia, Kansas, and New York (Conant Citation1959; Behler & King Citation1979; Conant & Collins Citation1991; Deichsel & Miller Citation2000).
Several ecological and behavioural traits make P. sicula a highly effective invader in the majority of the regions where it is introduced. Experimental results have demonstrated behavioural interference with P. melisellensis (Downes & Bauwens Citation2002), which has gone extinct after introduction of P. sicula in Adriatic islets (Nevo et al. Citation1972). Furthermore, hybridization between P. sicula and other Podarcis species has been demonstrated by means of genetic data at least with the endemic Podarcis of Sardinia (P. tiliguerta, Capula Citation2002), of the Aeolian islands (P. raffonei, Capula et al. Citation2002) and of Sicily (P. wagleriana, Capula Citation1993).
Indeed, this species is very eclectic in habitat use being found in natural grassy areas, roadside verges, scrublands meadows and costal dunes, but also in agro environments, inside pine plantations, and associated to parkland urban areas, stone walls and buildings (Capula Citation1994; Oliverio et al. Citation2001; Biaggini et al. Citation2006; Corti Citation2006). The Italian wall lizard usually uses trees and human manufactures as a refuge, so its accidental transportation by humans and the availability of an anthropised environment are important factors for its spread. The use of planted trees and human manufactures as refuges enhances its accidental transportation while the availability of an anthropic environment seems important for its spread (Corti & Lo Cascio Citation2002).
This proneness of P. sicula both to be dispersed by humans and to produce negative effects on native species makes necessary to look closely for their pattern of invasiveness. By looking separately for each case of invasion we can obtain valuable information for the prevention and management of this biological invasion. Molecular evidence supporting the allochthonous status of P. sicula have been provided for populations occurring in United States (Deichsel & Miller Citation2000; Oliverio et al. Citation2001) and Menorca (Podnar et al. Citation2005) and for the Iberian populations of La Rioja and Sant Celoni, Barcelona (Valdeón et al. Citation2010; Rivera et al. Citation2011). However, the origin of most of the introduced populations is still unclear. Though, tracing the origin of the introduced populations and identifying the pathways of their translocations constitute the baseline to forecast the colonization patterns, as well as to prevent new introductions (Pyron et al. Citation2008; Rodda et al. Citation2008; Dorcas et al. Citation2010).
In this work, we use a molecular marker to identify the geographic origin of all the introduced populations of P. sicula known from Iberian Peninsula and Balearic islands. We specifically aim to determine whether these geographically separated populations derive from a single event of introduction or if they have multiple origins. By tracing the origin of introduced populations we expect to infer the source(s) and pathway(s) of dispersal, crucial for developing conservation and management policies aimed at preventing negative effect on native species.
Material and methods
Populations sampled
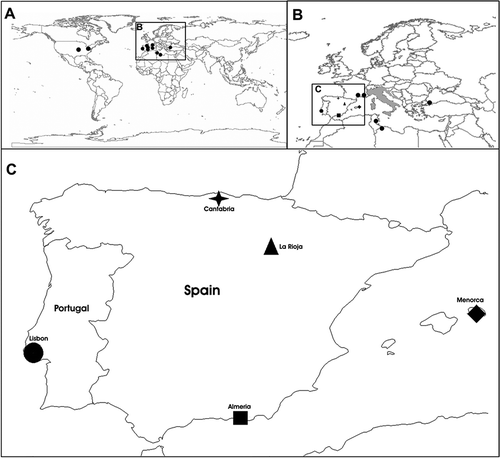
Four introduced populations are known from mainland Iberian Peninsula (): Lisbon (western Portugal, González de la Vega et al. 2001), Noja (Cantabria, northern Spain, Meijide Citation1981), Almería (southern Spain, Mertens & Wermuth Citation1960) and La Rioja (northern Spain, Valdeón et al. Citation2010) while the species is widespread in Menorca, Balearic Islands (Pérez-Mellado Citation2002). Very recently another population was found near Barcelona at Sant Celoni (Rivera et al. Citation2011). All Iberian localities, but La Rioja and Sant Celoni, are coastal, Lisbon and Almería being strictly urban whereas Noja is a sand dune area surrounded by urban habitat. La Rioja and Sant Celoni populations, located 100 km and 15 km inland respectively, are associated to old olive trees on sale in a plant nursery (Valdeón et al. Citation2010; Rivera et al. Citation2011).
Sampling and DNA extraction and amplification
The sampling includes all the Iberian and Balearic localities where the species has been reported to be introduced (). Tail tips were collected from 23 samples (2-7 samples from each locality, ) and then stored in pure ethanol.
Table I. Iberian localities where the introduced P. sicula populations were sampled. Geographic coordinates and sample size are also reported
Total genomic DNA was extracted from tail tissue following the standard saline method (Sambrook et al. Citation1989). A fragment of 684 bp of the mitochondrial gene cytochrome b (cyt b) was amplified by PCR using the primers GluDG-A and cb3H (Palumbi Citation1991). The amplification conditions consisted of an initial step of denaturing of 3 min at 94°C, followed by 35 cycles of denaturation of 30 s at 94°C, annealing of 30 s at 51°C and extension of 50 s at 72°C and a final extension step of 5 min at 72°C. The PCR products were purified and sequenced by an external service (by the company Macrogen® Korea).
Sequence data and phylogenetic analysis
Mitochondrial cytochrome b sequences from 39 individuals of P. sicula from the native range generated by Podnar et al. (Citation2005) were downloaded from GenBank (accession numbers AY185095, AY185094, AY770869–AY77090). Three additional sequences from (Podarcis muralis and P. melisellensis) were also downloaded from GenBank (accession numbers AY185096, AY185029 and AY185057) and included in the phylogenetic analyses as outgroups (following Podnar et al. Citation2005). We maintained geographic and haplotype designations as in Podnar et al. (Citation2005), in order to facilitate the comparison between our samples and those from previous studies. These sequences were aligned with those generated in the present study (accession numbers JX072938-JX072960) using the ClustalW software (Thompson et al. Citation1994).
We carried out a Maximum Likelihood (ML) phylogenetic analysis to infer the relationships between the Iberian and Balearic samples and those from the native range of P. sicula analyzed by Podnar et al. (Citation2005). The best model of sequence evolution was estimated by Mega 5 (Tamura et al. Citation2011) under the Bayesian Information Criterion (BIC) as the model HKY + Gamma (Hasegawa et al. Citation1985; Yang Citation1993). The ML tree was performed in Mega 5 with the heuristic search mode and the node support was calculated over 1000 bootstrap replicates. In addition to the tree-building method, we analyzed the genealogical relationships among native and non-native haplotypes by means of a statistical parsimony network using the program TCS 1.21 (Clement et al. Citation2000).
Results
The final alignment includes 65 sequences and 687 base pairs. A total of 42 haplotypes were identified among which 35 from the sequences from Podnar et al. (Citation2005) and eight from the five Iberian and Balearic introduced populations here studied. In particular, in Almería, La Rioja and Lisbon populations, two different haplotypes each were found, while in Cantabria and Menorca one haplotype occurred.
ML tree shows that lizards from Noja (Cantabria) are closely related to those from the “Tuscany” clade in Podnar et al. (Citation2005) (). Also, the lizards from Lisbon cluster together with Tuscanian samples, yet being fairly differentiated from them. Haplotypes from Almería and Menorca are related with those from Sicily and Sardinia and included within the “Sicula” haploclade. Moreover, the five Menorcan samples analyzed in this study showed the same haplotype as the single Menorcan sample analyzed by Podnar et al. (Citation2005). The two haplotypes found in the specimens from La Rioja cluster in a distinct clade, which is related to the “Monasterace” (Southeast Calabria) and “Sicula” haploclades. All the relationships above reported were highly supported by bootstrap analysis with BP values ranging from 86 to 99. Based on these relationships, the putative origin of each introduced population is discussed in the next section and depicted in
Figure 2. ML phylogenetic tree depicting the relationships between cytochrome b, haplotypes from native Podarcis sicula populations (Podnar et al. Citation2005) and those from the Iberian and Balearic introduced populations: Almeria, Cantabria, La Rioja, Lisbon, and Menorca. Specimens' localities are reported along with P. Sicula haploclades (grey boxes) as in Podnar et al. (Citation2005). Bootstrap support is indicated above the nodes of interest.
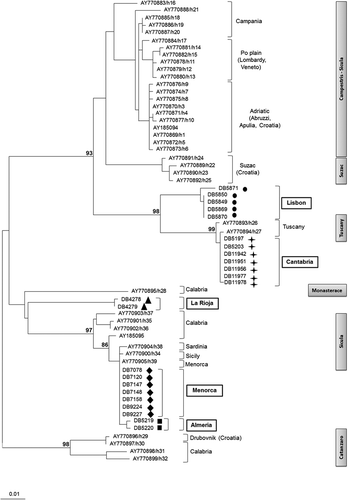
Figure 3. Origin of the Iberian and Balearic populations of Podarcis sicula as inferred from genetic analyses.
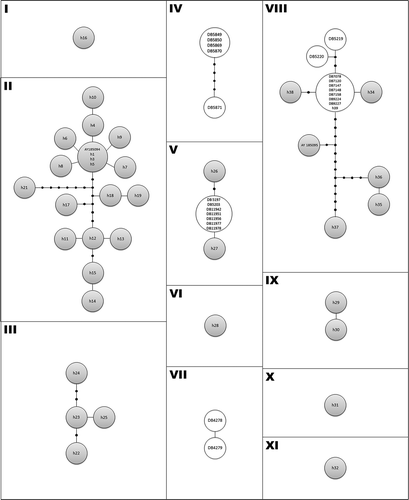
The network analysis shows 11 distinct networks and the maximum number of mutational steps allowing for a 95% parsimonious connection between haplotypes was estimated to be 11 (). The relationships between haplotypes as depicted by the networks corroborate the results from ML analysis. Cantabria lizards cluster together with those from Tuscany, Almería and Menorca samples group with samples from the Sicula clade, and La Rioja and Lisbon samples constitute two independent groups.
Figure 4. Statistical parsimony haplotype network depicting the genealogical relationships between native and non-native P. sicula haplotypes. See and Figure 2 for details on sampling localities and haplotypes codes. Circle size is proportional to the number of samples with the same haplotype. Black circles represent missing haplotypes. The eleven networks include samples from: (I) Campania - Campestris Sicula clade; (II) Po Plain + Campania + Adriatic – Campestris-Sicula clade; (III) Suzac (Croatia) - Suzac clade (IV); Lisbon (non-native); (V) Cantabria (non-native) + Tuscany - Tuscany clade; (VI) Calabria - Monasterace clade; (VII) La Rioja (non-native) (VIII) Almería (non-native) + Menorca (non-native) + Sardinia + Sicily + Calabria - Sicula clade; (IX) Drubovnik (Croatia) - Catanzaro clade; (X) Calabria - Catanzaro clade; and (XI) Calabria - Catanzaro clade.
Discussion
Origin, pathways and timing of the introduction
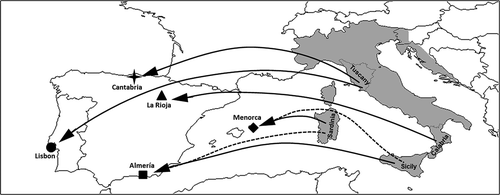
Phylogenetic analysis clearly indicates that the introduced Iberian and Balearic populations of P. sicula are related to native populations from distinct Italian regions, and not from other non-native (Iberian) populations. In turn, this result suggests multiple and independent introductions of the Italian wall lizard in the Iberian Peninsula and Menorca ().
As a whole, P. sicula from Ibero-Balearic localities clustered in two principal haploclades recovered by Podnar et al. (Citation2005), the Tuscany haploclade and the Sicula haploclade, which cluster haplotypes belonging to the P. s. campestris and the P. s. sicula+P. s. cetii subspecies respectively. On the other hand, haplotypes found in the population from La Rioja constitute an independent haploclade and are not included in any of those reported by Podnar et al. (Citation2005).
The Italian wall lizards occurring in Lisbon and Cantabria are likely originated from a Tuscan stock. In particular, Cantabrian lizards seem to be introduced from the Tuscany itself, and perhaps from Florence area since their haplotypes differ in only one-two nucleotide position from the haplotypes found by Podnar et al. (Citation2005) in the native populations from Florence. On the other hand, the lizards sampled in Lisbon were probably introduced from a proximate area to Tuscany as they are closely related to the lizards form Tuscany but fairly differentiated from them ().
Both Almería and Menorca lizards are related to the Sicilian and Sardinian populations of P. sicula. In both cases, it is difficult to disentangle whether the origin of these allochthonous populations is from Sicily or Sardinia, or even if Almería population derives from the Menorcan stock. Indeed, according to Podnar et al. (Citation2005), the Sardinian samples (assigned to the subspecies P. s. cetti) only differ from the Sicilian samples (assigned to the subspecies P. s. sicula) by three substitutions in a gene fragment of 687 nucleotides, which suggest and support the hypothesis of a historical colonization of Sardinia from Sicily (Lanza Citation1982). Thus, Menorcan samples could have been originated either from Sicily as suggested by Greca and Sacchi (Citation1957) or from Sardinia as suggested by Müller (Citation1905) and Eisentraut (Citation1950). Although Podnar et al. (Citation2005) would support the hypothesis of an introduction from Sicily as Menorcan haplotypes are somewhat closer to the Sicilian than to the Sardinian haplotypes (as the nucleotide differences between Menorcan samples and Sicilian/Sardinian samples are 2/3 respectively), the gene fragment they used does not have enough resolution for supporting this statement and the haplotype comparison is based only on single specimens from Menorca, Sicily and Sardinia. Additionally, the morphological inspections of Menorcan lizards (unpublished data from the authors) show a perfect match with the cetii morphotype occurring in Sardinia rather than with the sicula morphotypes observed in Sicily. Thus, taking into account this evidence, we would tentatively suggest a Sardinian origin for the Menorcan population of P. sicula, although the large spectrum of morphological variation of P. sicula advices for caution in the use of morphological diagnosis when inferring the origin of populations. Following a similar reasoning and based on morphological data, a tentative hypothesis of a Sicilian origin can be drawn for the lizards introduced in Almería.
Lizards from La Rioja constitute an independent clade, which is related to Monasterace and Sicula haploclades of Podnar et al. (Citation2005), although well differentiated from them. This result only partially agrees with those reported by Valdeón et al. (Citation2010), who also found La Rioja samples to be related with Catanzaro, Monasterace, and Sicula haploclades, but with a closer relationship with the former rather constituting an independent clade. However, it is important to notice that given the high evolutionary rate of the mitochondrial gene fragment analysed in this study, it provided a higher resolution than those used by those authors (12S and 16S genes combined). This evidence suggests not only that the source population of La Rioja would be located in an area close to Calabria (and perhaps in between Monasterace and Reggio Calabria), but also that the genetic diversity of P. sicula in the southern portion of its range has not been completely sampled, yet.
The pathways of introduction are known for some of the studied populations. Evidence for an anthropogenic introduction through the trade of old olive trees from southern Italy to Spain has been reported by Valdeón et al. (Citation2010) and Rivera et al. (Citation2011) for La Rioja and Sant Celoni populations respectively. Lisbon population appears to be introduced through the transport of several materials and ornamental plants during the Expo'98 event (González de la Vega et al. 2001). As already mentioned, the Italian wall lizard often uses trees and human manufactures as refuge and thermoregulatory site. Similarly the pathways of introduction for the other Iberian populations could have followed the human translocations of cargo in the maritime trade. Both Almería and Cantabrian populations maintained a route of maritime traffic with Italy during the Spanish Civil War between 1936 and 1939 (Rivera & Arribas Citation1993). Noja population has not a seaport, but may derive from the already extinct Santander population, which was closer to a seaport (Pleguezelos 2004). So it is possible that the primary pathway of introduction in Cantabria region was also derived from the development of maritime trade.
Even if based on circumstantial historical evidence, the timing of these introductions seems different from case to case. Noja (Cantabria) and Almería populations are considered to be introduced in the late XIX century or early XX century when an intensification of maritime traffic occurs (Mertens & Wermuth Citation1960; Meijide Citation1981; Henle & Klaver Citation1986; Olmedo Citation1997). Lisbon, La Rioja and Sant Celoni populations would be even more recent due to ornamental plants transportation (end of 90s and during the 2008/2009 period respectively; González de La Vega et al. 2001; Valdéon et al. 2010; Rivera et al. Citation2011). Finally, the origin of Menorcan population is probably dated to a more ancient time, likely back to the Middle Age (Mayol Citation1985) when the traffic between Italian Republics and Balearic Islands was intense, as the species is widespread across the entire islands (Pleguezuelos Citation2004).
Conservation implications
The results from this study provide evidence for distinct sources, pathways and timing of introduction in Iberian Peninsula and Menorca by P. Sicula from the central and the southern portion of its native distribution range. This evidence is crucial to design conservation strategies.
If introduction would come from a single source, conservation strategies would involve controlling in the original region/locality, which could be a feasible target. Since, in contrast, multiple independent events of introduction have been found here, a realistic conservation planning should be more global and preventive, addressed to the detection and control of alien lizards when arrived. The prevention of the introduction is, in fact, the most effective and cheaper way to avoid it and can be done by implementing inspection and quarantine measures of the commercial cargo and trade, since these two means are the main pathways of introduction.
When the prevention fails, an eradication and control program should be implemented, paying attention to both biological data, population size and distribution, and specific educational effort for the general public are needed for such programs to be successful. Introduced populations of P. sicula have been successfully eradicated from La Rioja (Valdeón et al. Citation2010) and Mallorca (Pinya & Carretero Citation2011; Mateo JA, pers. comm.) and it could be possible also for the Noja (Cantabria) population as its presence is localized. Conversely, the populations in Almería and Lisbon have already reached a size, which makes the full eradication not feasible (Pleguezelos 2004; Loureiro et al. Citation2008; personal observations), thus control measures should be taken to keep the population size stable and avoid its expansion. In the case of Menorca, P. sicula is naturalized since a long time, being nowadays the dominant species in the main island, so it is almost impossible to take an effective action. However, a substantial effect must be kept to prevent translocations toward the small surrounding islets of Menorca (as well as to other Balearic Islands) where it could be harmful for the autochthonous and endangered Podarcis lilfordi (Pinya & Carretero Citation2011).
Finally, on a general ground besides investigating the origin and the pathways of the introductions, resources have to be invested also for collecting more data about: (i) the current demographic status and spatial distribution of the introduced populations; (ii) the factors limiting or promoting the successful establishment after of the introduction and expansion (adopting Species Distribution Models approach to predict the invasion risk (Schulte et al. Citation2011)), as well as on (iii) the impacts of this species on native lizards. The integration of all this information would allow a deep comprehension and a successful management of this biological invasion.
Acknowledgements
We thank Ana Perera, Antigoni Kaliontzopoulou, Catarina Rato, Fátima Jorge, Enrique García-Muñoz, Michela Maura and Verónica Gomes for helping us in collecting samples. DS is supported by the FCT post-doctoral grant SFRH/BPD/66592/2009 and by the SYNTHESYS Project http://www.synthesys.info/ which financed by European Community Research Infrastructure Action under the FP7 Capacities Programme at the Museo Nacional de Ciencias Naturales of Madrid (CSIC). The study was supported by projects PTDC/BIA-BEC/101256/2008 and PTDC/BIA-BEC/102280/2008 (FCT, Portugal).
References
- Álvarez , C , Mateo , JA , Oliver , J and Mayol , J . 2010 . Los ofidios ibéricos de introducción reciente en las Islas Baleares . Boletín de la Asociación Herpetológica Española , 21 : 126 – 131 .
- Arnold , EN and Ovenden , DW. 2002 . A field guide to the reptiles and amphibians of Britain and Europe , London : Harper Collin .
- Basoglu , M and Baran , I. 1977 . Türkiye Sürüngenzleri I: The Reptiles of Turkey . Ege Üniversitesi Fen Fakültesi Kitaplar Serisi. Bornova , : 76
- Behler , JL and King , FW. 1979 . The Audubon society field guide to North American reptiles and amphibians , New York : Alfred A. Knopf .
- Biaggini , M , Dapporto , L , Paggetti , E and Corti , C. 2006 . “ Distribution of lacertid lizards in a Tuscan agro-ecosystem (Central Italy) ” . In Mainland and insular lacertid lizards: a Mediterranean perspective , Edited by: Corti , C , Lo Cascio , P and Biaggini , M . 13 – 21 . Italy : Firenze University Press .
- Brown , GP , Shilton , C , Philips Benjamim , L and Shine , R. 2007 . Invasion, stress and spinal arthritis in cane toads . Proceedings of the National Academy of Sciences of the United States of America , 104 : 17698 – 17700 .
- Capula , M. 1993 . Natural hybridization in Podarcis sicula and P. wagleriana (Reptilia: Lacertidae) . Biochemical Systematics and Ecology , 21 : 373 – 380 .
- Capula , M. 1994 . Population genetics of a colonizing lizard: Loss of variability in introduced populations of Podarcis sicula . Experentia , 50 : 691 – 696 .
- Capula , M. 2002 . Genetic evidence of natural hybridization between Podarcis sicula and Podarcis tiliguerta (Reptilia) . Amphibia-Reptilia , 23 : 313 – 321 .
- Capula , M , Luiselli , L , Bologna , MA and Ceccarelli , A. 2002 . The decline of the Aeolian wall lizard, Podarcis raffonei: causes and conservation proposals . Oryx , 36 : 66 – 72 .
- Clement , M , Posada , D and Crandall , KA. 2000 . TCS: a computer program to estimate gene genealogies . Molecular Ecology , 9 : 1657 – 1660 .
- Conant , R. 1959 . Lacerta colony still extant at Philadelphia . Copeia , 1959 : 335 – 336 .
- Conant , R and Collins , JT. 1991 . A field guide to reptiles and amphibians: Eastern and Central North America , 3rd , Boston : Houghton Mifflin Company .
- Corti , C. 2006 . “ Podarcis sicula. Lucertola campestre, Italian wall lizard ” . In Atlante degli Anfibi e dei Rettili d'Italia. Atlas of Italian Amphibians and Reptiles , Edited by: Sindaco , R , Doria , G , Razzetti , E and Bernini , F . 486 – 489 . Firenze : Polistampa .
- Corti , C and Lo Cascio , P . 2002 . The lizards of Italy and Adjacent Areas , Frankfurt am Main : Chimaira .
- Deichsel , G and Miller , LL. 2000 . Changes of Specific Status for the Green Lizard Lacerta, an alien lizard introduced in Topeka . Kansas Herpetological Society Newsletter , 119 : 10 – 11 .
- de Queiroz , A. 2005 . The resurrection of oceanic dispersal in historical biogeography . Trends in Ecology and Evolution , 20 : 68 – 73 .
- Dorcas , ME , Wilson , JD and Gibbon , JW. 2010 . Can invasive Burmese pythons inhabit temperate regions of the southeastern United States? . Biological Invasions , 13 : 793 – 802 .
- Downes , S and Bauwens , D. 2002 . An experimental demonstration of direct behavioural interference in two Mediterranean lacertid lizard species . Animal Behaviour , 63 : 1037 – 1046 .
- Eisentraut , M. 1950 . Das Fehlen endemischer und das Auftreten landfremder Eidechsen auf den beiden Hauptinseln der Balearen, Mallorca und Menorca . Zoologische Beiträge Berlin , 1 : 3 – 11 .
- Ficetola , GF , Thuiller , W and Padoa-Schioppa , E. 2009 . From introduction to the establishment of alien species: bioclimatic differences between presence and reproduction localities in the slider turtles . Diversity and Distributions , 15 : 108 – 116 .
- Fritts , TH , McCoid , MJ and Haddock , RL. 1994 . Syptoms and circumstances associated with bites by the brown tree snake (Colubridae: Boiga irregularis) on Guam . Journal of Herpetology , 28 : 27 – 33 .
- Gonzalez de la Vega , JP , Gonzalez-Garcia , JP , Garcia-Pulido , T and Gonzalez-Garcia , G. 2001 . Podarcis sicula (Lagartija italiana), primera cita para Portugal . Boletín de la Asociación Herpetológica Española , 12 : 9
- Greca , M and Sacchi , CF. 1957 . Problemi del popolamento animale nelle piccole isole mediterrenee . Annuario Museo Zoologico della Università di Napoli , 9 : 1 – 188 .
- Guicking , D , Griffiths , RA , Moore , RD , Jogger , U and Wink , M. 2005 . Introduced alien or persecuted native? Resolving the origin of the Viperine Snake (Natrix maura) on Mallorca . Biodiversity and Conservation , 15 : 3045 – 3054 .
- Hasegawa , M , Kishino , H and Yano , T. 1985 . Dating of the human–ape splitting by a molecular clock of mitochondrial DNA . Journal of Molecular Evolution , 22 : 160 – 174 .
- Henle , K and Klaver , CJJ. 1986 . “ Podarcis sicula (Rafinesque-Schmaltz, 1810) – Ruineneidechse ” . In Handbuch der Reptilien and Amphibien Europas, vol. 2/II: Echsen III , Edited by: Böhme , W . 254 – 342 . Wiesbaden : AULA-Verlag .
- Isailovic JC, Vogrin M, Corti C, Mellado VP, Sá-Sousa P, Cheylan M, Pleguezuelos J, Sindaco R, Romano A, Avci A. 2008. Podarcis siculus. In: IUCN 2011. IUCN Red List of Threatened Species. Version 2011. www.iucnredlist.org (http://www.iucnredlist.org) (Accessed: September 2011 ).
- Kraus , F. 2009 . Alien Reptiles and Amphibians: A Scientific compendium and analysis , New York : Springer .
- Lanza , B. 1982 . Ipotesi sulle origine del popolamento erpetologico della Sardegna . Lavori della Società Italiana di Biogeografia , 8 : 723 – 744 .
- Loureiro , A , Ferrand , N , Carretero , MA and Paulo , O , eds. 2008 . Atlas dos Anfíbios e Répteis de Portugal , Lisboa : Instituto da Conservação da Natureza e da Biodiversidade .
- Mayol , J. 1985 . Rèptils i amfibis de les Illes Balears. Manuales d'Introducció a la Naturalesa 6 , Palma de Maiorca : Editorial Moll .
- Meijide , M. 1981 . Una nueva población de Lacerta sicula rafinesque para el norte de España . Acta Vertebrata , 8 : 304 – 305 .
- Mertens , R and Wermuth , H. 1960 . Die Amphibien und Reptilien Europas , Frankfurt am Main : Verlag Waldemar Kramer .
- Morgue , M. 1924 . Note succinte sur les espèces de Lacerta muralis des îles du Golfe de Marseille . Bulletin de la Société Linnéenne de Lyon , 3 : 55
- Müller , L. 1905 . Ein neuer fundort der Lacerta serpa Raf . Zoologischer Anzeiger Leipzig , 28 : 502 – 504 .
- Nevo , E , Gorman , GC , Soulé , M , Yang , EJ , Clover , R and Jovanovic , V. 1972 . Competitive Exclusion between Insular Lacerta Species (Sauria, Lacertidae) . Oecologia , 10 : 183 – 190 .
- Oliverio , M , Burke , R , Bologna , MA , Wirz , A and Mariottini , P. 2001 . Molecular characterization of native (Italy) and introduced (USA) Podarcis sicula populations (Reptilia, Lacertidae) . Italian Journal of Zoology , 68 : 121 – 124 .
- Olmedo , G. 1997 . “ Podarcis sícula (Rafinisque, 1810) ” . In Distribución y biogeografía de los anfíbios y reptiles en España y Portugal, vol. 3 , Edited by: Pleguezelos , JM . 246 – 248 . Granada : Monografías de Herpetología .
- Orsini , JP. 1984 . A propos du Lézar sicilien Podarcis sicula en Provence . Bulletin du Centre de Recherche Ornithologique de Provence , 6 : 8
- Palumbi , S , Martin , A , Romano , S , Millan , WO , Stice , L and Grabowski , G. 1991 . The simple fool's guide to PCR (ver. 2) , Honolulu : University of Hawaii .
- Pérez-Mellado , V. 2002 . “ Podarcis sicula (Rafinesque, 1810). Lagartija italiana ” . In Atlas y libro rojo de los anfibios y reptiles de España , Edited by: Pleguezuelos , JM , Márquez , R and Lizana , M . 257 – 259 . Madrid : Dirección General de Conservación de la Naturaleza, Asociación Herpetológica Española (2a impresión) .
- Pinya , S and Carretero , MA. 2011 . The Balearic herpetofauna: A species update and a review on the evidence . Acta Herpetologica , 6 : 59 – 80 .
- Pleguezuelos , JM. 2004 . “ Las especies introducidas de anfibios y reptiles ” . In Atlas y libro rojo de los anfibios y reptiles de España , Edited by: Pleguezuelos , JM , Márquez , R and Lizana , M . 502 – 532 . Madrid : Dirección General de Conservación de la Naturaleza, Asociación Herpetológica Española (3a impresión) .
- Podnar , M , Mayer , W and Tvrtković , N. 2005 . Phylogeography of the Italian wall lizard, Podarcis sicula, as revealed by mitochondrial DNA sequences . Molecular Ecology , 14 : 575 – 588 .
- Pyron , RA , Burbrink , FT and Guiher , TJ. 2008 . Claims of potential expansion throughout the U . S. by invasive python species are contradicted by ecological niche models. PloS one , 3 : e2931
- Rivera , J and Arribas , O. 1993 . Anfibios y reptiles introducidos de la fauna española . Quercus , 84 : 12 – 16 .
- Rivera , X , Arribas , O , Carranza , S and Maluquer-Margalef , J. 2011 . An introduction of Podaricis sícula in Catalonia (NE Iberian Peninsula) on imported olive trees . Bulletín de la Societat Catalana d'Herpetologia , 19 : 79 – 85 .
- Rodda , GH , Fritts , TH and Chiszar , D. 1997 . The disappearance of Guam's wildlife: new insights for herpetology, evolutionary ecology and conservation . BioScience , 47 : 565 – 574 .
- Rodda , GH , Jarnevich , CS and Reed , RN. 2008 . What parts of the US mainland are climatically suitable for invasive alien pythons spreading from Everglades National Park? Biological Invasions , 11 : 241 – 252 .
- Sambrook , J , Fritsch , F and Maniatis , T. 1989 . Molecular Cloning: a laboratory manual , 2nd , New York : Cold Spring Harbor Laboratory, Cold Spring Harbor Laboratory Press .
- Schulte , U , Hochkirch , A , Lötters , S , Rödder , D , Schweiger , S , Weimann , T and Veith , M. 2011 . Cryptic niche conservatism among evolutionary lineages of an invasive lizard . Global Ecology and Biogeography , 21 : 298 – 211 .
- Tamura , K , Peterson , D , Peterson , N , Stecher , G , Nei , M and Kumar , S. 2011 . MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods . Molecular Biology and Evolution , 28 : 2731 – 2739 .
- Thompson , JD , Higgins , DG and Gibson , TJ. 1994 . CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice . Nucleic Acids Research , 22 : 4673 – 4680 .
- Valdeón , A , Perera , A , Costa , S , Sampaio , F and Carretero , MA. 2010 . Evidencia de una introducción de Podarcis sicula desde Italia a España asociada a una importación de olivos (Olea europaea) . Boletín de la Asociación Herpetológica Española , 21 : 122 – 126 .
- Yang , Z. 1993 . Maximum likelihood estimation of phylogeny from DNA sequences when substitution rates differ over sites . Molecular Biology and Evolution , 10 : 1396 – 1402 .