Abstract
Movements of Mediterranean Shags (Phalacrocorax aristotelis desmarestii, Payraudeau 1826) between Croatian breeding colonies and non-breeding areas such as the Gulf of Trieste increased consistently from the 1980s to become a migratory movement at the present time. In order to characterise the patterns of this migration and the behaviour of first-year Shags at their first migration, we analysed the recoveries of 812 birds that were colour-ringed at the most important Croatian breeding colonies in the northern Adriatic. Within a period of seven years, 568 ring-readings of 234 individual Shags were processed. Most sightings came from the Gulf of Trieste (43.0%) and the newly-discovered post-breeding area in Venice Lagoon (38.9%). Shags from the most distant colonies exploited mainly the Gulf of Trieste. The 48.3% of Shags sighted several times have been recorded in the same post-breeding area during subsequent years. This percentage increased to 93.4%, if we considered just the birds observed during successive years. Variations in the timing of migration within the post-breeding areas occurred and sustained the importance of the Slovenian coast during the return to breeding colonies. The ratio between first-year Shags and experienced Shags (immature and adults) was higher in Venice Lagoon and in general increased with the “novelty” of the site. A relevant portion of the Croatian breeding population moves to the northern Adriatic Sea after breeding. All the studied colonies concurred with this migration. We highlight a high fidelity to post-breeding sites, which reflects the quite sedentary “nature” of the species, and the role of first-year Shags in the discovery of new sites. We suggest that this migration is probably the later stage in a graded response to deterioration of the Shags’ feeding grounds within the breeding area.
Introduction
The European Shag (Phalacrocorax aristotelis Linnaeus, 1761) is a colonially breeding seabird endemic to the rocky coasts of the northeast Europe and Mediterranean (Wanless & Harris Citation2004). In the former area, the Atlantic subspecies Phalacrocorax aristotelis aristotelis Linnaeus, 1761 is distributed from Iceland and northern Scandinavia to the Iberian Peninsula (Nelson Citation2005). Iberian Shags were shown to be isolated from northern populations (Velando Citation1997; Velando & Freire Citation1999). The species has indeed always been considered as largely sedentary, although the immature may undergo post-breeding dispersive movements over short distances (Del Hoyo et al. Citation1992). For example, extensive studies on ringing and recovering have shown that no Icelandic Shags have been recovered elsewhere (Gardarsson Citation1982; Petersen Citation1998), and no adults ringed in the UK have been recovered in mainland Europe (Harris & Swann Citation2002). Furthermore, 95% of the Shags were recorded within a radius of 8 km from the natal colony in eastern Britain (Aebischer Citation1995).
Historical recoveries of Shags ringed at colonies in north Europe recorded median winter movements that were generally less than 100 km, and winter ranges of different breeding populations were relatively isolated (Galbraith et al. Citation1986). Such study also showed marked differences between regions in the dispersal patterns. In addition to interpopulation differences, age-related, annual and longer-term variation also occurred within populations. Some populations, such as the western Scottish and north Irish Sea birds, were comparatively consistent in their year-to-year dispersal patterns and there were no apparent age-related differences. Others, particularly the Forth/Farnes birds, showed marked age-related and annual variation. These interpopulation and annual differences could be determined by the availability of sheltered feeding conditions, while possible causes of long-term variations might include changing weather patterns or competition between age groups (Galbraith et al. Citation1986). Conversely, north Norwegian Shags undertook the most extensive movements of any of the studied populations. Dispersal distances of over 500 km suggested that these populations might be migratory rather than dispersive. However, preliminary results from recent investigations suggest that the majority of the north Norwegian Shags equipped with miniaturised data loggers (Geolocators) wintered north of the Arctic Circle (Daunt et al. Citation2010).
Despite the assumption that the North Sea provided an effective barrier to Shag movements (Galbraith et al. Citation1986), the most recent genetic studies, even if really detecting an isolation-by-distance, have shown that microsatellite variation provided no evidence that open sea formed a complete barrier to effective dispersal (Barlow et al. Citation2011). According to UK ringing recoveries, even if adult Shags do not disperse widely from the colony in the post-breeding season (Harris & Swann Citation2002; Daunt et al. Citation2006), 2% of the juveniles have been recovered in mainland Europe (Wanless & Harris Citation2004). Moreover, ring recoveries have demonstrated that juvenile Shags perform long-distance movements, on the order of several hundred kilometres, from their natal colony prior to colony recruitment (Harris & Swann Citation2002; Alvarez Citation2009).
Although the movements of the Atlantic subspecies populations are well documented, very little is known about the possible movements of the Mediterranean subspecies Phalacrocorax aristotelis desmarestii Payraudeau, 1826, which is endemic to the Mediterranean and Black Seas (Nelson Citation2005). This subspecies is not thought to move outside its recognised breeding range (Wanless & Harris Citation2004). Birds ringed in Corsica have been recovered in northwestern Italy; 77% of the recoveries over 100 km from breeding colonies were juvenile birds (Brichetti et al. Citation1992).
In the Adriatic Sea, Shags breed in Croatia but many of them spend the post-breeding period in the Gulf of Trieste (Sponza et al. Citation2010; Cosolo et al. Citation2011). This behaviour was shown to be driven by dietary requirements (Cosolo et al. Citation2011). These movements within the Adriatic Sea have not always been a habitual pattern, since they increased consistently from the 1980s (Utmar Citation1999) to become a migratory movement at the present time (Sponza et al. Citation2010), in the sense of a regular back-and-forth seasonal movement of a population between two areas (Berthold Citation2001). Moreover, Shag movements and distribution during the post-breeding period have expanded westwards over the years, actually including the Venice Lagoon (Fracasso et al. Citation2000; Bon et al. Citation2005; Sighele et al. Citation2010). At least 1300–1500 pairs of the Mediterranean Shag breed in the north and central Adriatic Sea (IOO et al. 2013). The species breeds also in the southern Adriatic; however, colonies are smaller and less numerous. Furthermore, up to 4000 individuals of all age classes were counted on the communal roosts far from the colonies in the north Adriatic during late summer and autumn (Škornik et al. Citation2011). This means that the post-breeding migration actually involves the majority of the population breeding in the northern Adriatic.
In this paper we characterise the patterns of such migration and the behaviour of first-year birds, immature birds and adults, on the basis of seven years of colour-ring sightings and recoveries.
Materials and methods
Breeding colonies
The study area covered the northern Adriatic Sea, namely the Istrian coast, the Quarner archipelago and the northern part of the Zadar archipelago. Shags were colour-ringed at the most important Croatian breeding colonies in the northern Adriatic Sea ().
Figure 1. Map of the study area (modified from http://it.bing.com/maps/, © Microsoft Corporation 2012). Shown on the map are: the five breeding colonies (Vrsar, Rovinj, Brijuni archipelago, Oruda, Silbanski grebeni), the three main post-breeding areas (Gulf of Trieste, Venice Lagoon and Slovenian coast) and some locations (Pesaro, Ancona and Pelješac) which are interesting with respect to date of sighting, direction taken and distances covered.
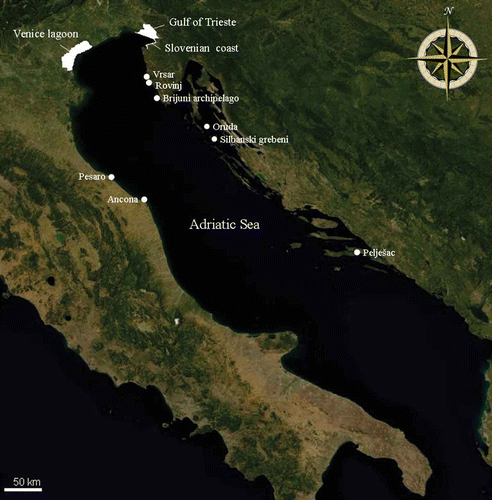
The five colonies are composed of a total of 14 islands. Three colonies are located off the western coast of the Istria peninsula near Vrsar (45°09′N, 13°35′E), Rovinj (between 45°03′–45°04′N and 13°37′–13°38′E) and on islands of the Brijuni archipelago (between 44°53′–44°56′N and 13°42′–13°47′E). Of these colonies, Vrsar is certainly the smallest and least important. The other two colonies are located on Oruda Island (facing Lošinj Island) (44°33′N, 14°34′E) and on Silbanski Grebeni islands (between 44°19′–44°20′N and 14°41′–14°43′E) ().
Ringing activity
A total of 812 Shags were ringed between 2005 and 2011 (seven years) (). Of these, five birds were ringed at Vrsar colony (0.6%), 64 birds at Rovinj colony (7.9%), 391 birds at Brijuni archipelago (48.2%), 120 birds at Oruda Island (14.8%) and 232 birds at Silbanski Grebeni islands (28.6%). 701 Shags (86.3%) were ringed as chicks at the nest, while 111 (13.7%) were ringed as adults. Shags place their nests under dense bushes (mostly Pistacia lentiscus Linnaeus, 1753, and Phillyrea latifolia Linnaeus, 1753) close to the sea. All captures were made by walking along the shore and quietly surrounding the bushes used for nesting. Chicks were captured gently by hand on the nests, ringed and immediately released back to the nests. Adults were captured by hand on the nest or by mist-nets located near the bush entrances and released on the water near their nests (10 m). Adults were observed to return to their breeding sites within 20 min.
Table I. Numbers of Mediterranean Shags ringed in the five Croatian breeding colonies between 2005 and 2011
Birds were individually marked with both metal rings and plastic colour rings, one on each tarsus. Shags were aged and handled by ringers with ringing permits issued by the Institute of Ornithology in Zagreb (Croatia). The colour rings were large and conspicuous; the colour used was orange with a black alphanumeric code. The code was composed of a single letter followed by a two-digit number.
Shags typically do not breed until aged three years (Potts et al. Citation1980). Moreover, the breeding season in the Adriatic Sea is prolonged (from late November to May). The first chick was ringed on 3 February, although the majority were ringed during April (83.0%). Most of the adults were ringed in March (52.6%).
All sightings of colour-rings were entered into a database upon receipt. For each sighting, the date, the observer and the location were logged, while direct linear distance (km) from the colony was calculated for each location. As Shags often move along the coast, this value indicates the extent of the movement, not the length of the actual route passed by an individual bird. Regarding the most distant locations, the rough distance travelled along the coast was calculated using the measuring distance tool of the Google Earth software to estimate the length of the possible route passed by an individual bird.
In order to verify the effect of age in the timing of migration and direct linear distance covered, we separately analysed the following groups: first-year birds at their first migration, immature birds (from the second to the third year of life) and adults (from the fourth year of life). Such distinctions were made according to the date of the sighting. When comparing the different colonies, we excluded the colony of Vrsar, due to the low number of Shags ringed there.
As discussed by Camphuysen et al. (Citation2011), colour-ringing programmes have a clear advantage given the multiple sightings of individual birds (without the need to re-trap) over a large number of years and generally provide large sample sizes (number of individual birds monitored). On the other hand, a disadvantage of this method is caused by the fact that the colour-ringing data could be influenced by spatial and temporal patterns in observer efforts. We believe that the coastal and strictly marine ecology, their gregariousness also outside the breeding period, the habit of forming common roosts and the easy recognisability of Shags as well as a widespread interest for the species and for colour-ring reading by ornithologists, bird-watchers and amateurs reduced the effect of this limit. Moreover, the project over the years was advertised on the “CR-Birding” website, at several ornithological meetings and through the National Ringing Scheme. Up to 29 observers contributed to the data collection from different areas of the Adriatic coast.
Contingency tables tested by the chi-square test (χ2) were used to compare the numerical frequencies. Other statistical differences were assessed with the Kruskal-Wallis test. The significance threshold was set at P < 0.05 and the analysis was performed using SPSS 13.0 and STATISTICA 7.1 software.
Results
Until the end of 2011, 234 Shags (28.8%) had been re-sighted on at least one occasion. The majority (212 birds, 90.6%) were ringed as chicks, while 22 birds (9.4%) as adults. Generally, 150 birds were reported at least once during their first year, which led to 216 sightings.
Moreover, 214 Shags (91.4%) were reported alive, 17 individuals (7.3%) were recovered dead, while three birds (1.3%) were observed first alive and subsequently found dead. Eight out of the 20 recoveries (40.0%) belonged to birds drowned in fishing nets or fish traps.
A total of 568 ring-readings from 45 locations were received and processed (). All sightings came from coastal areas.
Table II. Numbers of sightings and number of Shags sighted for each colony. Data have been categorised according to both (A) the year of ringing and (B) the year of sighting
Origin of the north Adriatic population in the post-breeding period
Most sightings (81.9%) came from the Gulf of Trieste (43.0%) and from Venice Lagoon (38.9%). These localities indicate that Shags could regularly travel up to 300 km along the coast. The Slovenian coast follows with 13.9% of sightings. All other locations amounted to 4.2%. Of these, the three sightings from Pesaro (central Italy, cardinal direction: W–SW; 43°55′N, 12°54′E, coastal distance ca. 540 km) and Ancona (central Italy, SW; 43°31′N, 13°37′E, ca. 580 km), together with the sighting at Pelješac (Croatia, SE; 42°58′N, 17°07′E, ca. 370 km) showed atypical long-distance movements, i.e. direct linear distance >100 km ().
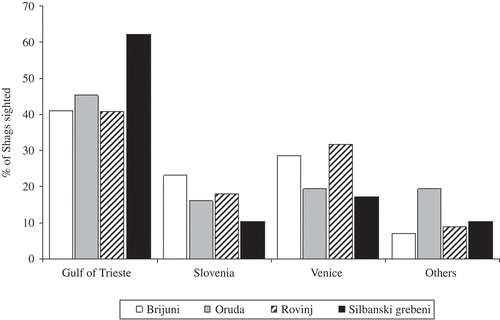
The number of Shags sighted, split by the colony of origin, did not vary significantly between the post-breeding areas (χ2 = 14.62; df = 9; P > 0.10; ). At Venice Lagoon, we recorded a higher percentage of Shags from Rovinj and Brijuni colonies, while Shags from Silbanski Grebeni colonies showed a slight preference towards the Gulf of Trieste ().
In this respect, when we compared the number of sightings from the main post-breeding areas, partitioned by the colony of origin, the preference of Shags from Silbanski Grebeni towards the Gulf of Trieste contributed significantly to the difference (χ2 = 35.62; df = 6; P < 0.0001; Silbanski Grebeni contribution/total chi-square contribution = 22.08/35.62).
Links between the post-breeding areas
The same colour-ringed birds were never observed in both Venice Lagoon and in the Gulf of Trieste, neither in the same nor in different years. Irrespective of the routes taken by individual Shags, on the basis of sightings and recoveries processed, currently there are no links between the two most important post-breeding areas in the north Adriatic Sea.
Just five Shags were recorded at different areas within the same year: four birds were observed initially in the Gulf of Trieste (location: Isonzo river mouth; 45°49′N, 13°31′E) and then along the Slovenian coast (location: Sečovlje Salina; 45°28′N, 13°35′E) and one bird was recorded initially in Venice Lagoon (location: Chioggia; 45°12′N, 12°15′E) and then in Slovenia (location: Sečovlje Salina; 45°29′N, 13°35′E).
Fidelity to post-breeding areas
As regards the Shags sighted several times (118 out of 234 birds), 48.3% were recorded in the same post-breeding area during subsequent years. If we consider just the birds observed during successive years (excluding those birds observed several times in the same year), the percentage increased to 93.4%. The comparison of the number of birds sighted in the same area during successive years, split by the colony of origin, with the total number of Shags sighted for each colony showed that all the colonies concurred equally in fidelity to the post-breeding areas (χ2 = 1.71; df = 3; P > 0.60).
Timing of migration
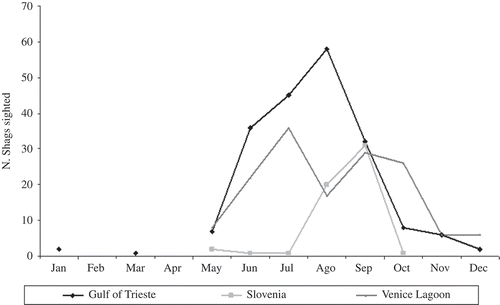
The timing of migration was similar among the different colonies (number of Shags sighted per month, period May–December: χ2 = 16.43; df = 21; P > 0.70), while the seasonal use of the main post-breeding areas differed considerably (number of Shags sighted per month in different post-breeding areas, period May–December: χ2 = 93.93, df = 14, P < 0.0001; ). The main contributions to the difference were: the high number of Shags sighted during September along the Slovenian coast (September contribution/total contribution = 29.93/93.93) and during October in Venice Lagoon (October contribution/total contribution = 20.48/93.93), and the low number of Shags sighted in Slovenia during July (July contribution/total contribution = 11.15/93.93) and during August in Venice Lagoon (August contribution/total contribution = 16.19/93.93) ().
Age-related movements
When we compared Shags from different colonies, both immature birds and adults showed a similar timing of migration (number of Shags sighted, period May–December: immature birds, χ2 = 24.01, df = 21, P > 0.25; adults, χ2 = 12.93, df = 21, P > 0.90). First-year birds behaved similarly, with an exception in June when we found a higher rate of juveniles from Silbanski Grebeni leaving the colony (number of Shags sighted: χ2 = 32.95, df = 18, P < 0.05; Silbanski Grebeni: June contribution/total contribution = 15.21/32.95).
A significant difference was recorded by comparing the three age-groups, i.e. first-year birds, immature birds and adults (number of Shags sighted: χ2 = 27.38, df = 14, P < 0.05). In fact, both immature birds and adults begin to leave the colonies during May or even earlier, whereas first-year Shags depart in June (May contribution/total contribution = 19.14/27.38; ). The contribution of immature birds was particularly high in May (May contribution of immature/total contribution = 11.64/27.38).
Figure 4. Seasonal use of the main post-breeding areas (Gulf of Trieste, Venice Lagoon, Slovenian coast) by (A) first-year, (B) immature and (C) adult birds expressed as numbers of individuals sighted. Lines drawn in order to visualize trends only.
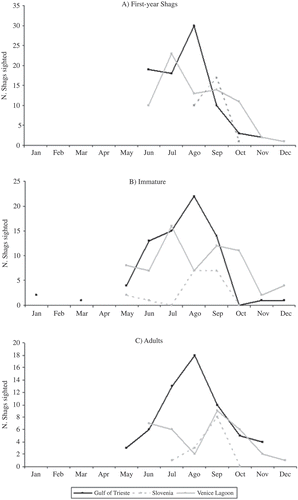
We found no differences in the direct linear distances covered by first-year Shags, immature birds and adults (considering only independent data: Kruskal-Wallis test: H 2,175 = 5.05, P > 0.05) while the seasonal use of the three most important post-breeding areas by first-year Shags, immature birds and adults differed significantly (number of Shags sighted: first-year birds, period June–December, χ2 = 52.52; df = 12; P < 0.0001; immature birds, period May–December, χ2 = 38.52, df = 14, P < 0.001; adults, period May–December, χ2 = 28.62, df = 14, P < 0.05; ). These differences were particularly evident for first-year birds during September (September contribution/total contribution = 22.96/52.52), due to the increased presence along the Slovenian coast (). As regards immature birds (), the main contribution to the total chi-square was recorded during October (October contribution/total contribution = 14.28/38.52), due to the higher number of Shags in Venice Lagoon with respect to the Gulf of Trieste. As regards the adults (), the main contribution to the total chi-square was recorded during September (September contribution/total contribution = 9.52/28.62), due to the increased presence along the Slovenian coast.
Age and migration patterns
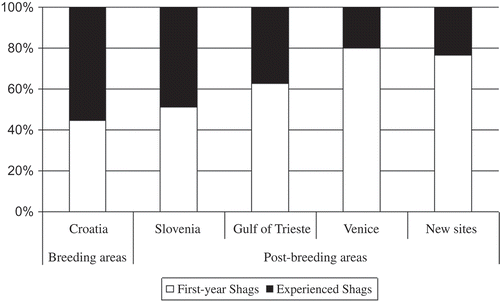
Given the lack of differences in the use of the post-breeding areas between immature birds and adults (number of Shags sighted: χ2 = 0.77, df = 3, P > 0.85), we created the category “experienced Shags”, which included all sightings after the first calendar year. In order to highlight the behaviour of juvenile Shags at their first migration, we analysed just the first sighting of each bird to verify whether the destinations and the behaviour of first-year birds differed from those of experienced Shags. The abundance of first-year and experienced Shags differed significantly between the three main post-breeding areas (χ2 = 9.43; df = 2; P < 0.01). The main contribution to the total chi-square was due to the low presence of experienced Shags in Venice Lagoon, compared to the Gulf of Trieste and the Slovenian coast (Venice Lagoon: experienced Shags contribution/total contribution = 3.63/9.43).
Accordingly, we display in the ratio between first-year birds and experienced Shags recorded at the Croatian breeding area, at each of the post-breeding areas (Slovenian coast, Gulf of Trieste, Venice Lagoon) and at the “new sites”. This latter category takes into account new locations that are interesting if we consider the date, the direction and the distances covered ( and ).
Table III. Sightings of particular interest, given the novelty of the location and the direction and distance covered by birds. *It was considered 2007 only, as the first-year with sightings of colour-rings. **All individuals were immature
Discussion
There are different methodological approaches to study bird migration. Each method has advantages as well as disadvantages and the results are complementary rather than standalone descriptions of migratory pathways and dispersal patterns (Camphuysen et al. Citation2011). We recognise the possible limits of the colour-rings method (Galbraith et al. Citation1986; Camphuysen et al. Citation2011), particularly for possible bias resulting from ring-reading efforts, but we feel confident of the description of the patterns of this migration, given the perspective used and the ecology and behaviour of the Mediterranean Shag (see Materials and methods).
The effective conservation of seabird populations needs the ability to detect adverse changes as quickly as possible to assess their significance and to determine the possible causes (Croxall & Rothery Citation1991). Shag habitual movements between the Croatian breeding colonies and the Gulf of Trieste took place just during the 1980s (Utmar Citation1999; Sponza et al. Citation2010) and were driven by dietary requirements (Cosolo et al. Citation2011). Johansen (Citation1975) identified changes in food distribution as one of the main drives of long-term variations in the dispersal patterns of European Shags. The other causes were changing weather patterns or competition between age groups (Galbraith et al. Citation1986). According to Johansen (Citation1975) and Galbraith et al. (Citation1986), we believe that this migration is probably the later stage in a graded response to deteriorating environmental conditions. In this regard, Sponza et al. (Citation2010) highlighted that the maximum amount of fish landed in the Adriatic Sea (about 220,000 tons) was recorded in 1981, which was followed by a marked decline (Mannini et al. Citation2005). In particular, demersal fisheries decreased by 67% along the eastern Adriatic Sea coastline (Slovenia, Croatia, Bosnia–Herzegovina, Montenegro and Albania), as a consequence of overfishing. We suggest that the Gulf of Trieste was a profitable area to buffer such alterations.
To our knowledge, this is the first Shag population in which it is possible to ascribe specific changes in dispersal patterns to alterations in food accessibility and availability. Both juvenile and adult Shags from the northern Adriatic undertake longer-distance movements than other studied populations, with the exception of the northern Norwegian birds (Galbraith et al. Citation1986).
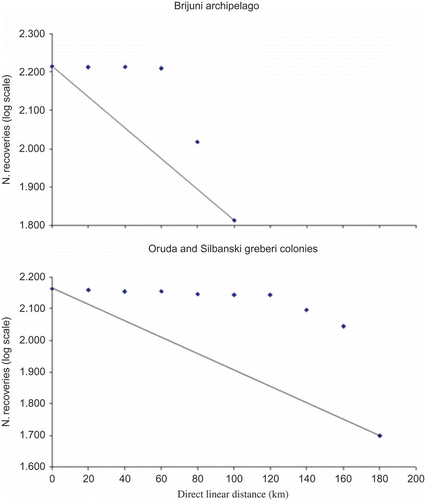
On the other hand, this is an interesting case in which changes in post-breeding dispersal patterns led to a migratory movement within a period of 30 years (Sponza et al. Citation2010). If a species is migratory, a higher-than-expected proportion of birds should move beyond the closer-distance zones, giving a step-shaped distribution when recoveries are plotted against distance (Coulson Citation1961; Coulson & Brazendale Citation1968; Galbraith et al. Citation1986). According to this approach, the analysis of the post-breeding movements of Adriatic Shags, since a rapid shift in distances over 60 km for Brijuni colony and over 160 km for Oruda and Silbanski Grebeni colonies is recorded, shows that Adriatic Shags are migratory rather than dispersive ().
Figure 6. Post-breeding seasonal movements through succeeding distance zones, at intervals of 20 km. All age classes were combined. We analysed just the sightings towards the Gulf of Trieste. On this strictly coastal “route” Shags can stop at any place. However, at the present time we do not know if Shags follow the coast or cross the sea to go to Venice. We also split the analysis for Brijuni archipelago (n sightings = 163) and for Oruda and Silbanski grebeni colonies (n sightings = 146) to reduce the problem of the distance between colonies. The line represents the distribution expected if a purely dispersive movement was taking place.
Actually, Shag movements and distribution during the post-breeding period have expanded westward over the years, now including not only the Gulf of Trieste but also the Venice Lagoon (Fracasso et al. Citation2000). In fact, from 2003–2004 the species was regularly observed in the Venice Lagoon throughout the summer season (Bon et al. Citation2005). Large groups have been reported since 2006 and the first colour-ring was read on 2 July 2007 (Bon et al. Citation2007, Citation2009; Sighele et al. Citation2009, Citation2010). Recently, in the lagoon of Venice, about 300 individuals have been counted (Sighele et al. Citation2010). Moreover, numbers are further increasing in the Gulf of Trieste, where about 3000 individuals were counted during the last summer seasons. Given the high sensitivity of Shags to human disturbance during breeding (Guyot Citation1993), neither the Gulf of Trieste nor the Venice Lagoon offers suitable, low disturbance places for nesting, which are largely present instead on Croatian islets.
All the studied colonies concurred with this migration, regardless of the distance between the colony and the post-breeding areas. Moreover, individuals from different colonies were observed at each of the main post-breeding areas (Gulf of Trieste, Venice Lagoon, Slovenian coast). It is interesting to highlight how Shags from the most distant colony (Silbanski Grebeni) exploit mostly the Gulf of Trieste, which was historically the first post-breeding area (Sponza et al. Citation2010). In contrast, the nearest colonies (Brijuni archipelago and Rovinj colony) are more linked with the Venice Lagoon, a newly discovered area (Fracasso et al. Citation2000; Bon et al. Citation2005; Sighele et al. Citation2010). Given the high fidelity to the post-breeding areas, we assume that this migration originated in the most distant colonies and subsequently it has spread like a wave, involving the northern colonies. These colonies are indeed localised on the coastal route that connects the central Adriatic colonies to the Gulf of Trieste.
The European Shag shows high breeding philopatry, with about 99.0% of adults breeding at the same colony across years (Potts Citation1969; Aebischer Citation1995; Velando & Freire Citation2002). We have few useful sightings to highlight a possible fidelity to the Adriatic breeding sites by the Mediterranean subspecies, while the fidelity to post-breeding areas is quite evident. For example, the Shag “E39” from Silbanski Grebeni has been recorded 17 times in the Gulf of Trieste during four successive years; all sightings originated from the same location (Miramare Castle, 45°42′N, 13°43′E). The lack of ringed birds sighted both in the Gulf of Trieste and in Venice Lagoon is another element in favour of high fidelity to the post-breeding areas.
We believe that the high breeding philopatry (Potts Citation1969; Aebischer Citation1995; Velando & Freire Citation2002), the low occurrence of long-distance or cross-sea movements (Barlow et al. Citation2011) and, actually, a high fidelity to post-breeding sites, reflect the quite sedentary “nature” of this species and, as a consequence, the role of changes in the environmental conditions as presumably decisive elements in these distributional variations.
While there were no Shags observed both in the Gulf of Trieste and Venice Lagoon, four birds were recorded both in the Gulf of Trieste and along the Slovenian coast, and one bird both in the Venice Lagoon and along the Slovenian coast. In all cases, Shags were flying to Slovenia. According to the timing of migration, these observations highlight the role of Slovenia, especially during the return to the breeding colonies. The most important element in comparing the three main post-breeding areas is the largest number of first-year Shags in Venice Lagoon. Moreover, the ratio between first-year birds and experienced Shags increased with the “novelty” of the site (). This highlights the role of young Shags in the discovery of new sites. Such a role could be attributed to “boom and bust” population dynamics, with periodic population crashes followed by rapid population growth (Aebischer Citation1986; Harris & Wanless Citation1996; Frederiksen et al. Citation2008). Following crashes, juvenile Shags have been shown to move about 38% further than during non-crash years (Potts Citation1969). More likely, this behaviour could be identified as an “exploratory migration” (sensu Baker Citation1978) or “intermittent migration” (sensu Berthold Citation2001). According to the exploration model of migratory movements, young birds alternate migration in the standard direction for the population with movements towards unusual directions. Suitable habitats located during this process can be used by birds in future years. This behaviour may help to identify novel feeding areas. Is this the case for the Venice Lagoon? Surely, this could explain why Shags are now restricted in the North Adriatic Sea. From this point of view, the “discovery” of the Gulf of Trieste was sustained by a profitable change in the diet (Cosolo et al. Citation2011).
Conservation perspectives
Twenty colour-ringed Shags (8.6%) were recovered dead. Forty percent of these recoveries belonged to birds drowned in fishing nets or fish traps. Other authors reported high values of mortality caused by fishing gear, possibly affecting the survival rate of birds (Velando & Freire Citation2002). These data are particularly important, given that the Mediterranean Shag is listed in Annex I of the Birds Directive 2009/147/CE and is the focus of an Action Plan (Aguilar & Fernandez Citation2002).
According to Velando and Freire (Citation2002), an effective conservation of the Adriatic population should pass through: (1) the protection of the breeding sites as well as the feeding and roosting areas, and (2) the regulation of demersal fisheries in the feeding areas. Moreover, to better understand the mechanisms underlying this migration we highlight the importance to (1) characterise the feeding areas, in particular the benthic community, and (2) accurately identify the migration routes by the application of miniaturised data loggers.
Acknowledgements
We thank all ringers who took part in Shag ringing generally and in particular T. Blažev, B. Cimador, B. Ende, D. Gatolin, M. Malatestinić, K. Mandić, K. Mikulić, A. Radalj. Moreover, we thank P. Utmar, C. Trani, F. Roppa, N. Ventolini, M. Tofful, R. Kriscjak and M. Kuljerić for help in the field. At Veli Lošinj (Croatia) we thank the Blue World staff, in particular N. Rako and P. Makelworth. At Brijuni archipelago (Croatia), we thank the local authority of the Brijuni National Park. We are thankful to numerous observers who submitted their valuable observations, in particular M. Basso, B. Bašić, R. Benassi, I. Brajnik, S. Candotto, H. Čižmek, D. Degrassi, R. Fae, I. Jambrošić, S. Jović, P. Kanuch, A. Katalinić, K. Kravos, G. Lui, I. Maiorano, M. Mercker, A. Peruško, P. Ronconi, S. Rudolf, L. Sattin, S. Sava, I. Škornik, P. Spadoni, E. Stival, P. Ugo, P. Utmar, G. Vicario, A. Walderstein and E. Zanolini. We are grateful to A. Talamelli and N. Baccetti at the Institute for Environmental Protection and Research (ISPRA), who coordinated the collection of colour-ring recoveries for Italy. We finally thank E.A. Ferrero and the two anonymous referees for valuable criticism.
References
- Aebischer , NJ. 1986 . Retrospective investigation of an ecological disaster in the shag, Phalacrocorax aristotelis – A general method based on long-term marking . Journal of Animal Ecology , 55 : 613 – 629 .
- Aebischer , NJ. 1995 . Philopatry and colony fidelity of Shags Phalacrocorax aristotelis on the east coast of Britain . Ibis , 137 : 11 – 28 .
- Aguilar , JS and Fernandez , G. 2002 . “ Species Action Plan for the Mediterranean Shag (Phalacrocorax aristotelis desmarestii) ” . In Convention on the Conservation of European Wildlife and Natural Habitats, Strasbourg, December 2002 , Cambridge , UK : BirdLife International .
- Alvarez , D. 2009 . Primera cita de un Cormoran moñudo iberico en el norte de Europa . Revista de Anillamiento , 24 : 6 – 9 .
- Baker , RR. 1978 . The evolutionary ecology of animal migration , London : Hodder & Stoughton .
- Barlow , EJ , Daunt , F , Wanless , S , Alvarez , D , Reid , JM and Cavers , S. 2011 . Weak large-scale population genetic structure in a philopatric seabird, the European Shag Phalacrocorax aristotelis . Ibis , 153 : 768 – 778 .
- Berthold , P. 2001 . Bird migration: a general survey , 2nd , Oxford : Oxford University Press . Oxford Ornithology Series
- Bon , M , Sighele , M and Verza , E , eds. 2005 . “ Rapporto ornitologico per la regione Veneto. Anno 2004 ” . In Bollettino del Museo civico di Storia Naturale di Venezia Vol. 56 , 8
- Bon , M , Sighele , M and Verza , E , eds. 2007 . “ Rapporto ornitologico per la regione Veneto. Anno 2006 ” . In Bollettino del Museo civico di Storia Naturale di Venezia Vol. 58 , 274
- Bon , M , Sighele , M and Verza , E , eds. 2009 . “ Rapporto ornitologico per la regione Veneto. Anno 2007 ” . In Bollettino del Museo civico di Storia Naturale di Venezia Vol. 59 , 134
- Brichetti , P , De Franceschi , P and Baccetti , N , eds. 1992 . “ Fauna d'Italia ” . In Aves I , Vol. 29 , Bologna : Calderini, Bologna .
- Camphuysen , CJ , Vercruijsse , HJP and Spaans , AL. 2011 . Colony- and age-specific seasonal dispersal of Herring Gulls Larus argentatus breeding in The Netherlands . Journal of Ornithology , 152 : 849 – 868 .
- Cosolo , M , Privileggi , N , Cimador , B and Sponza , S. 2011 . Dietary changes of Mediterranean Shags Phalacrocorax aristotelis desmarestii between the breeding and post-breeding seasons in the upper Adriatic Sea . Bird Study , 58 : 461 – 472 .
- Coulson , JC. 1961 . Movements and seasonal variation in mortality of Shags and Cormorants ringed on the Farne Islands, Northumberland . British Birds , 54 : 225 – 235 .
- Coulson , JC and Brazendale , MG. 1968 . Movements of Cormorants ringed in the British Isles and evidence of colony-specific dispersal . British Birds , 61 : 1 – 21 .
- Croxall , JP and Rothery , P. 1991 . “ Population regulation of seabirds: Implications of their demography for conservation ” . In Bird population studies: Relevance to conservation and management , Edited by: Perrins , CM , Lebreton , JD and Hirons , GM . 272 – 296 . Oxford : Oxford University Press .
- Daunt , F , Afanasyev , V , Silk , JRD and Wanless , S. 2006 . Extrinsic and intrinsic determinants of winter foraging and breeding phenology in a temperate seabird . Behavioral Ecology and Sociobiology , 59 : 381 – 388 .
- Daunt F, Barrett R, Anker-Nilssen T. 2010. Winter distribution and foraging strategies of European shags. SEAPOP Short Report 3–2010. Available:Accessed Feb 2012 27 http://www.seapop.no (http://www.seapop.no)
- Del Hoyo , J , Elliott , A and Sargatal , J , eds. 1992 . Handbook of the birds of the world , Vol. 1 , Barcelona : Lynx Editions .
- Fracasso , G , Mezzavilla , F and Scarton , F. 2000 . “ Check-list degli uccelli del Veneto (ottobre 2000) ” . In Atti 3° Convegno Faunisti Veneti. Bollettino del Museo civico di Storia Naturale di Venezia Edited by: Bon , M and Scarton , F . 134 editorssuppl. 51
- Frederiksen , M , Daunt , F , Harris , MP and Wanless , S. 2008 . The demographic impact of extreme events: Stochastic weather drives survival and population dynamics in a long-lived seabird . Journal of Animal Ecology , 77 : 1020 – 1029 .
- Galbraith , H , Baillie , R , Furness , RW and Russell , S. 1986 . Regional variations in the dispersal of Shags Phalacrocorax aristotelis in northern Europe . Ornis Scandinavica , 17 : 68 – 74 .
- Gardarsson , A. 1982 . Icelandic Birds . Rit Landverndar , 8 : 15 – 60 .
- Guyot , I. 1993 . “ Breeding distribution and number of Shag Phalacrocorax aristotelis desmarestii in the Mediterranean ” . In Estatus y Conservación de Aves Marinas. Actas del II Simposio MEDMARAVIS Edited by: Aguilar , JS , Monabailliu , X and Paterson , AM . 37 – 45 . Madrid editorspp.
- Harris , MP and Swann , B. 2002 . “ European Shag Phalacrocorax aristotelis ” . In The migration atlas: Movements of the birds of Britain and Ireland , Edited by: Wernham , CV , Toms , MP , Marchant , JH , Clark , JA , Siriwardena , GM and Baillie , SR . 139 – 142 . London : T. & A.D. Poyser .
- Harris , MP and Wanless , S. 1996 . Differential responses of Guillemot Uria aalge and Shag Phalacrocorax aristotelis to a late winter wreck . Bird Study , 43 : 220 – 230 .
- IOO (Institute Of Ornithology: Barišić S, Ćiković D, Kralj J, Sušić G, Tutiš V), Radović D, Budinski I, Crnković R, Delić A, Dumbović V, Grlica ID, Ilić B, Jurinović L, Krnjeta D, Leskovar K, Lisičić D, Lolić I, Lukač G, Mandić K, Mikulić K, Mikuska T, Piasevoli G, Radalj A, Ružanović Z, Šćetarić V, Šetina M, Tomik A . 2013 . Population estimates for SPAs in Croatia. Internal document, State Institute for Nature Protection, Zagreb
- Johansen , O. 1975 . The relation between breeding grounds and wintering grounds in the Shag, Phalacrocorax aristotelis, in Norway as shown by the ringing recoveries . Sterna , 14 : 1 – 21 . in Norwegian with summary in English
- Mannini , P , Massa , F and Milone , N. 2005 . “ Adriatic Sea fisheries: Outline of some main facts ” . In Interactions between aquaculture and capture fisheries: A methodological perspective. General Fisheries Commission for the Mediterranean. No 78. FAO 2005 Edited by: Cataudella , S , Massa , F and Crosetti , D . 124–143 Rome
- Nelson , BJ. 2005 . “ Pelicans, Cormorants, and their relatives ” . In The Pelecaniformes , Oxford : Oxford University Press .
- Petersen , A. 1998 . Islenskir Fuglar , Reykjavik : Vaka-Helgafell .
- Potts , GR. 1969 . The influence of eruptive movements, age, population size and other factors on the survival of the shag (Phalacrocorax aristotelis (L.)) . Journal of Animal Ecology , 38 : 53 – 102 .
- Potts , GR , Coulson , JD and Deans , I. 1980 . Population dynamics and breeding success of the shag, Phalacrocorax aristotelis, on the Farne Islands, Northumberland . Journal of Animal Ecology , 49 : 465 – 484 .
- Sighele , M , Bon , M and Verza , E. , eds. 2009 . “ Rapporto ornitologico per la regione Veneto. Anno 2008 ” . In Bollettino del Museo civico di Storia Naturale di Venezia Vol. 60 , 148
- Sighele , M , Bon , M and Verza , E , eds. 2010 . “ Rapporto ornitologico per la regione Veneto. Anno 2009 ” . In Bollettino del Museo civico di Storia Naturale di Venezia Vol. 61 , 89
- Škornik , I , Utmar , P , Kravos , K , Candotto , S and Crnković , R. 2011 . Important post-breeding roosting area of the Mediterranean Shag Phalacrocorax aristotelis desmarestii in the Gulf of Trieste (N Adriatic). A Poster presented at the 13th MEDMARAVIS Pan-Mediterranean Symposium , Sardinia : Alghero . 14–17 October 2011
- Sponza , S , Cimador , B , Cosolo , M and Ferrero , EA. 2010 . Diving costs and benefits during post-breeding movements of the Mediterranean shag in the North Adriatic Sea . Marine Biology , 157 : 1203 – 1213 .
- Utmar , P. 1999 . “ Marangone dal ciuffo Phalacrocorax aristotelis ” . In Gli uccelli della provincia di Gorizia: Edizioni del Museo Friulano di Storia Naturale, Comune di Udine Edited by: Parodi , R . Vol. 42 , 44 – 45 . editorArti Grafiche Friulane, Tavagnacco (UD)
- Velando , A. 1997 . Ecologıa y Comportamiento del Cormorán Moñudo Phalacrocorax aristotelis en las Islas Cies y Ons , Vigo , Spain : University of Vigo . PhD thesis
- Velando , A and Freire , J. 1999 . Coloniabilidad y conservación de aves marinas: El caso del cormorán moñudo . Etologia , 7 : 55 – 62 .
- Velando , A and Freire , J. 2002 . Population modelling of European Shags (Phalacrocorax aristotelis) at their southern limit: Conservation implications . Biological Conservation , 107 : 59 – 69 .
- Wanless , S and Harris , MP. 2004 . “ European Shag ” . In Seabird populations of Britain and Ireland , Edited by: Mitchell , PI , Newton , SF , Ratcliffe , N and Dunn , TE . 146 – 159 . London : T. & A.D. Poyser .