Abstract
An overview of the reproductive patterns of seven model species of triclads belonging to the genus Dugesia from circum-Mediterranean and Afrotropical freshwater is provided. Populations can reproduce exclusively sexually or exclusively asexually by fissiparity but the coexistence of both reproductive modes is displayed by some lineages. The comparison of data on the life cycles as documented in the literature with new data highlights a wide array of potentialities to shift from an asexual to a sexual state and vice versa in species with fissiparous populations. Life cycles are poorly diversified in species reproducing only sexually. Among fissiparous populations, planarians may sexualise, displaying various grades of reproductive functionality under laboratory conditions. Unexpectedly, asexual reproduction by fission occurred spontaneously in two species during the sexual state of ex-fissiparous individuals. The capacity to develop or reduce the reproductive apparatus during the life cycle either in sexual or in ex-fissiparous individuals illustrates the considerable morphogenetic plasticity in planarians. The Dugesia case contributes to the modelling of reproductive patterns and strategies in basal Metazoa as a continuum from sexual to asexual reproduction and vice versa, rather than a simple clear-cut alternative.
Introduction
In freshwater triclads three main types of life cycle can be distinguished in relation to their reproductive modes: (1) sexual, (2) asexual (fissiparous), and (3) alternating (usually seasonal) between sexual and asexual modes (Ball & Reynoldson Citation1981; Benazzi & Gremigni Citation1982). Freshwater planarians that reproduce sexually are simultaneous hermaphrodites that self rarely. As a rule, planarians exhibit reciprocal cross-fertilization during copulation. In the majority of freshwater triclads exchange of sperm happens by the transfer of a spermatophore from the male apparatus of one copulant to the female apparatus of the partner (cf. Sluys Citation1989). Fertilization may occur a considerable time after copulation due to sperm storage, which may last up to several months (cf. Benazzi & Gremigni Citation1982). Calow and Read (Citation1986) distinguished two types of breeding cycles in triclads: semelparity (breed once and die) and iteroparity (breed repeatedly over several seasons). Iteroparity is dominant in the Dugesiidae and Planariidae, and semelparity in the Dendrocoelidae (cf. Benazzi Citation1992). The development of triclads is direct, without any of those larval stages that may occur in other groups of the Platyhelminthes.
In triclads asexual reproduction occurs, with a few rare exceptions, by transverse fission of the architomic type, i.e. fission is not preceded by differentiation of the new individual (paratomy). Binary fission is the most common mode. The division plane ranges from post-pharyngeal to pre-pharyngeal, with new clones being formed by regeneration of the missing body fragments through recruitment of stem cells, the neoblasts (cf. Baguñà Citation1998). Causes of fissioning have been ascribed to fission-controlling factors (Benazzi Citation1974), chromosomal mutations (De Vries et al. Citation1984), or to aneuploidy (Lepori & Pala Citation1982). Fissioning may be influenced by environmental factors, especially temperature (cf. Kenk Citation1937), and by physiological mechanisms, e.g. by neurosecretory substances (Lender & Zghal Citation1968; Best et al. Citation1969).
The genus Dugesia Girard, Citation1850 is widely distributed in the Palaearctic, Afrotropical, Oriental and Australasian regions. Each species may comprise either a single endemic population or numerous populations that can reproduce sexually and/or asexually.
The present paper provides an overview of the life cycles in the genus Dugesia through examination of seven model species. Comparison of life cycles documented in the literature with new data highlights a great plasticity in reproductive strategies, particularly in fissiparous forms.
Materials and methods
The seven investigated model species are Dugesia hepta Pala, Casu & Vacca, Citation1981, D. benazzii Lepori, Citation1951 , D. etrusca Benazzi, Citation1946, D. maghrebiana Stocchino, Manconi, Corso, Sluys, Casu & Pala, Citation2009 and D. sicula Lepori, 1948, from the Mediterranean sub-region (Western-Palaearctic), and D. aethiopica Stocchino, Corso, Manconi & Pala, Citation2002, and D. afromontana Stocchino & Sluys, Citation2012 from the Afrotropical region.
New data are reported for D. aethiopica, D. benazzii, D. hepta and D. sicula. For D. afromontana, D. etrusca and D. maghrebiana, new observations (Stocchino, pers. obs.) as well as data from the literature (Pala Citation1993; Stocchino et al. Citation2009, Citation2012) have been used. In all cases, the life cycle modalities, sequences of life cycle traits and phases were monitored under long-term laboratory conditions. The specimens collected were reared in glass bowls with dechlorinated tap water under semi-dark conditions, under a natural light cycle, at 18 ± 2°C; worms were fed weekly with fresh beef liver while the bowls were cleaned within 8–12 h after feeding. Observations in the field were performed on D. hepta and D. benazzii, focusing on the presence of cocoons and juveniles.
Species were identified by morphological examination of the diagnostic traits of their copulatory apparatus. Specimens for histological study were fixed for 24 h in Bouin's fluid, dehydrated in a graded ethanol series, cleared in toluene, and embedded in synthetic paraffin. Serial sections were made at intervals of 8 μm and were stained with Harris’ haematoxylin-eosin.
Results
Species with exclusively sexual natural populations: model species Dugesia hepta ( )
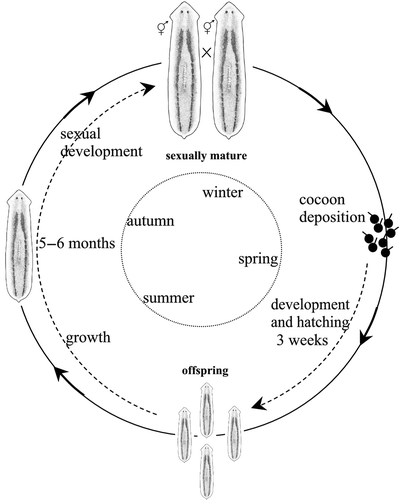
Dugesia hepta is endemic to Sardinia, with a range restricted to four hydrographic basins in the northwestern part of the island (Pala et al. Citation1981; Stocchino et al. Citation2005). The specimens of the studied population (HL lab strain) were collected in June 1995 from the Rio Logulentu. Life cycle monitoring for more than 10 years highlighted that this species is univoltine. The breeding season occurred in autumn when the sexually mature individuals started to mate (). In the following spring, each individual deposited 10–20 cocoons. The cocoon hatching rate was 98%. After about three weeks of development, 8–10 juveniles hatched per cocoon. After a period of 5–6 months these young planarians developed a reproductive apparatus and repeated the cycle (). In the field, abundant cocoons were observed during spring and early summer (June) while juveniles were present in June and July. Following cocoon deposition, specimens sometimes underwent a temporary regression of the reproductive apparatus.
Species with fissiparous natural populations: model species Dugesia aethiopica, D. afromontana, and D. maghrebiana ( , )
All three species are characterized by the development of sexualized individuals under laboratory conditions, representing the so-called ex-fissiparous individuals, showing hyperplasic ovaries and an increased body size. Their taxonomic status was determined on the basis of the diagnostic characters of the copulatory apparatus, which can be observed after the sexualisation process. Natural populations of D. aethiopica and D. afromontana reproduce exclusively by fission in the field. However, individuals belonging to the same strain displayed two reproductive modes in laboratory conditions, i.e. asexual and sexual.
Figure 3. Schematic overview of the life cycle and reproductive patterns of Dugesia afromontana from South Africa.
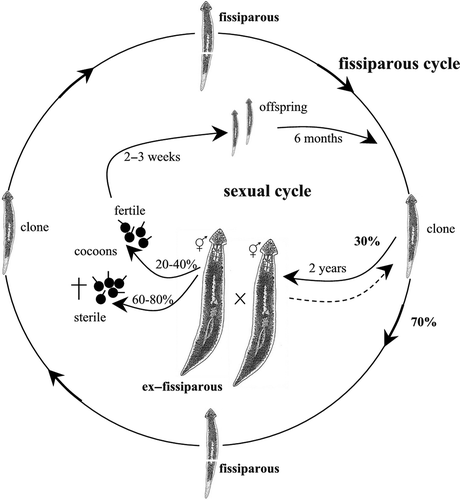
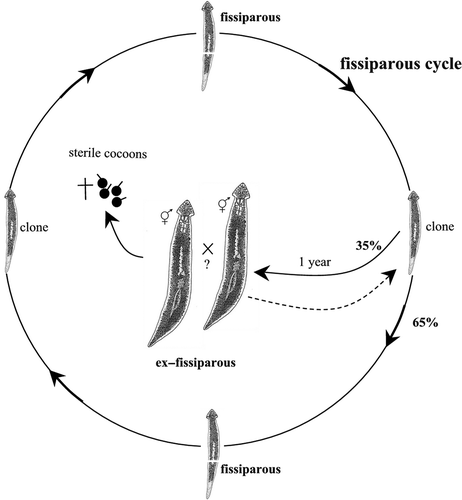
Figure 4. Schematic overview of the life cycle and reproductive pattern of Dugesia maghrebiana from Tunisia.
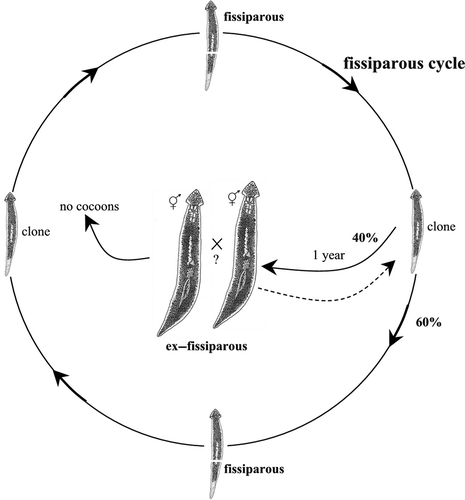
Figure 8. Synoptic scheme of the reproductive patterns in fissiparous lineages of the genus Dugesia. Fissiparity in ex-fissiparous individuals indicated by asterisks (*).
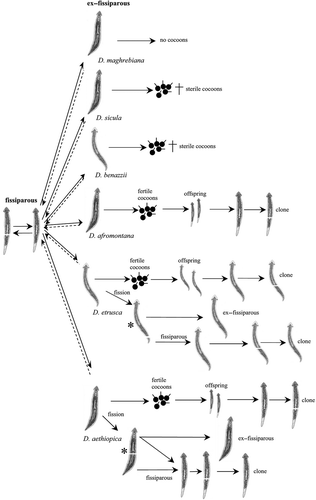
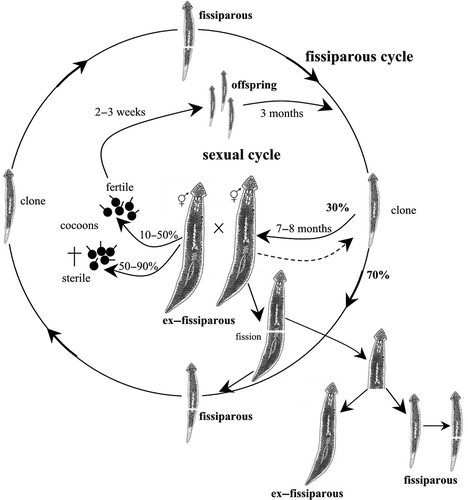
Figure 2. Schematic overview of the life cycle and reproductive patterns of Dugesia aethiopica from Ethiopia.
Dugesia aethiopica
Fissiparous specimens of this species endemic to Lake Tana in Ethiopia were collected in May 1988 (see Stocchino et al. Citation2002). The life cycle was monitored for more than 10 years. Post-pharyngeal transverse fissioning (slightly behind the pharynx) occurred continuously, while sexual reproduction followed by cocoon deposition was less frequent. After about 7–8 months in the laboratory, ca. 30% of the fissiparous specimens displayed a sexualisation process, thus producing ex-fissiparous individuals. Mating occurred in summertime (August–September) followed by cocoon deposition from August to January. Each ex-fissiparous individual laid 5–7 cocoons per reproductive season. The cocoons’ hatching rate was 10–50%. After 2–3 weeks of development, 1–6 juveniles hatched per cocoon. After a growth phase of ca. 3 months, the juveniles divided repeatedly, thus producing new fissiparous clones. After 7–8 months, ca. 30% of these fissiparous specimens displayed a sexualisation process. The asexual and sexual reproductive modes constantly occurred in the laboratory, generation after generation. Ex-fissiparous individuals of D. aethiopica retained the fissioning ability also during their sexual state, i.e. the fully sexualised individuals were able spontaneously to undergo asexual reproduction, which occurred by the typical post-pharyngeal transverse fission (slightly behind the pharynx). However, subsequent regeneration of the two body parts was different. The caudal fragment regenerated the anterior part and, after regression of the reproductive apparatus, produced always a fissiparous individual. In contrast, the cephalic fragment regenerated the post-pharyngeal part, with or without the copulatory apparatus. In the former case, the process was typical of an ex-fissiparous planarian, whereas in the latter the new individual became fissiparous. Moreover, after cocoon deposition, the ex-fissiparous individuals may sometimes resorb the copulatory apparatus and thus return to the fissiparous mode (, ).
Dugesia afromontana
Fissiparous specimens of this species endemic to South Africa were collected from the Afromontane forest in the Amatola Mountains, in April 2006 (see Stocchino et al. Citation2012). The life cycle was monitored during six years. The post-pharyngeal transverse fissioning (slightly behind the pharynx) occurred continuously, while sexual reproduction followed by cocoon deposition was less frequent. After having been kept in the laboratory for about two years, during which the strain notably increased in numbers due to fissioning processes, approximately 30% of the specimens attained the ex-fissiparous sexual state and lost the ability to fission. The sexualisation process occurred in early spring (February–March), followed by mating and deposition of 3–8 cocoons per individual in May, August and September. The cocoons’ hatching rate was 20–40% and, after ca. 2–3 weeks of development, 1–2 juveniles hatched per cocoon. After cocoon deposition, ex-fissiparous planarians may sometimes resorb the copulatory apparatus and return to the fissiparous mode of reproduction (, ).
Dugesia maghrebiana
Fissiparous specimens of the endemic Tunisian species were collected at Oued El Akarit in southeastern Tunisia in May 1990 (see Stocchino et al. Citation2009). The life cycle was monitored for more than 10 years. The division plane during the continuous process of fissioning was post-pharyngeal (slightly behind the pharynx). After one year of rearing, 40% of fissiparous individuals displayed a sexualisation process. Ex-fissiparous sexual individuals of this species, however, did not lay cocoons. Although these planarians lost the fissioning ability, they sometimes reduced the copulatory apparatus and subsequently returned to the fissiparous mode of reproduction (, ).
Species with fissiparous, sexual and mixed natural populations: model species Dugesia sicula ( , )
Dugesia sicula has a Pan-Mediterranean geographic range. This species is mainly composed of fissiparous populations (Pala et al. Citation1995; Charni et al. Citation2004; Lázaro et al. Citation2009) and a few sexual natural populations reported from Mallorca, Algeria and Tunisia (Gourbault Citation1981; Harrath et al. Citation2012; cf. Stocchino et al. Citation2012). Two mixed populations, i.e. including both sexual and fissiparous individuals, were reported from Sicily (Lepori Citation1948a; Benazzi Citation1974). Under laboratory conditions, planarians from fissiparous populations may develop reproductive organs to become sexual individuals. Identification of the specimens was based on characters of the copulatory apparatus, as displayed after the sexualisation process.
Figure 5. Schematic overview of the life cycle and reproductive pattern of a fissiparous population of Dugesia sicula (SA strain from Sardinia).
The specimens of the fissiparous population that we examined (SA lab strain) were collected from Asinara Islet in northwestern Sardinia in May 2001. Observations on the life cycle were performed over more than 10 years. The division plane during the continuous process of fissioning was post-pharyngeal (slightly behind the pharynx). Sexualisation occurred (35%) after the planarians were in the laboratory for about one year. These sexualised planarians were able to lay 3–5 cocoons per individual, which were always sterile. Moreover, during the sexual state these planarians were no longer able to reproduce by fission, although sometimes they reduced the copulatory apparatus and subsequently returned to the fissiparous mode (, ).
Species with sexual and mixed natural populations: model species Dugesia benazzii and D. etrusca ( , , )
Dugesia benazzii is restricted to the islands of Sardinia, Corsica and Capraia (Lepori Citation1951; Benazzi & Benazzii Lentati Citation1976; Vacca et al. Citation1993; Pala et al. Citation1999). Dugesia etrusca is restricted to Tuscany and Sardinia (Benazzi Citation1946; Pala et al. Citation1980).
| a. | Sexual natural populations (, ): The Sardinian populations of D. benazzii and D. etrusca were collected in December 1998 from Su Cantaru Spring, Monte Albo (BA lab strain) (see Stocchino et al. Citation2013) and from the Molara Islet (EM lab strain) in June 1979 respectively (Pala et al. Citation1980). The life cycle of both species was monitored for more than 13 years. In the investigated populations of both species the breeding season occurred in autumn, when sexually mature individuals started to mate. In the following spring, from each individual 10–20 cocoons were laid. The cocoons’ hatching rate was 95–98%. After ca. 3–4 weeks of development, 8–10 young planarians hatched from each cocoon. After a growth phase of 5–6 months, the young worms developed reproductive structures and repeated the cycle (, ). After cocoon deposition, specimens sometimes underwent a phase during which both gonads and copulatory structures appeared to be in a state of regression. In the field, abundant cocoons of D. benazzii were observed during spring and early summer (June), while juveniles occurred in June and July. Figure 6. Schematic overview of the life cycles and reproductive patterns of Dugesia benazzii. A. sexual cycle with seasonal rhythm in both sexual populations and sexual individuals from mixed populations (BA and BL strains from Sardinia); B. life cycle of fissiparous individuals from mixed populations (BL strain). The ex-fissiparous individuals lack the hyperplasic ovaries and increase of body size observed in other species with only fissiparous populations. 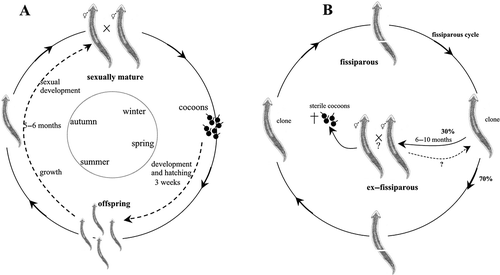 Figure 7. Schematic overview of the life cycles and reproductive patterns of Dugesia etrusca. A. sexual cycle with seasonal rhythm in both sexual populations and sexual individuals from mixed populations (EM strain from Sardinia and ES strain from Tuscany); B. life cycle of fissiparous individuals from mixed populations (ES strain). The ex-fissiparous individuals lack the hyperplasic ovaries and increase of body size observed in other species with only fissiparous populations. 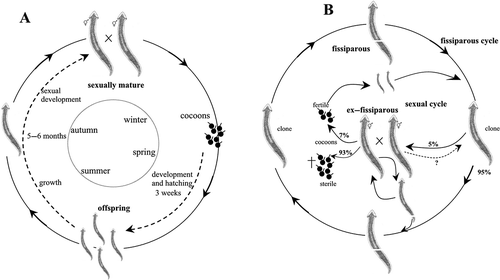 | ||||
| b. | Mixed populations with coexistence of sexual and fissiparous individuals (, , ): The Sardinian population of D. benazzii (BL lab strain) was collected from the Rio Su Lernu (northwestern Sardinia) in 1992. The population of D. etrusca (ES lab strain) is from a small water course at Santaluce in Tuscany (Pala Citation1993). The life cycle was monitored for both species for more than five years. Sexual individuals of mixed populations of both species displayed, in the lab, a reproductive cycle very similar to that of their conspecifics belonging to sexual natural populations (, ). In fissiparous individuals of both species, the division plane during the continuous process of fissioning was post-pharyngeal (slightly behind the pharynx). In both species, fissiparous individuals were able to sexualise under laboratory conditions and developed the reproductive apparatus (gonads and copulatory structures). These sexualised individuals did not differ from fissiparous individuals in morphological traits, and were externally distinguishable from the latter only by the presence of a genital pore. The typical increase of body size and the development of hyperplasic ovaries, as present in species with only fissiparous natural populations (see above), did not occur (, ). In D. benazzii the 30% fissiparous specimens displayed a sexualisation process after having been reared in the lab for ca. 6–10 months. Cocoon production and deposition happened sometimes. However, cocoons were always sterile (, ). Among fissiparous individuals of D. etrusca, sexualisation occurred in only a small fraction of the population (5%). The sexualised planarians were able to lay cocoons but these had only low fertility. The cocoon hatching rate was 7%. Moreover, the juveniles exhibited a markedly delayed growth. In this species, the ex-fissiparous specimens retained the capacity of fissioning, i.e. the fully sexualised individuals were able spontaneously to undergo asexual reproduction, which occurred in a peculiar mode, i.e. by detaching the caudal tip of the body. Subsequent development of the two body parts was different. The caudal fragment regenerated the anterior part and produced a fissiparous individual. The cephalic part, in contrast, regenerated the caudal tip but maintained the ex-fissiparous condition (, ). | ||||
Discussion
Among European dugesiids, D. hepta displays a life cycle comparable, for example, with D. ilvana Lepori, 1948 (Lepori Citation1948b) from Elba Island (Tuscany), and D. gonocephala s.s. (Dugès Citation1830) of Central Europe, which likewise are represented by only sexual populations (Benazzi & Benazzi Lentati Citation1976; De Vries Citation1986). Among the model species in which fission occurs, it is possible to highlight a wide array of potentialities to shift from an asexual to a sexual state and vice versa, with the development of ex-fissiparous individuals showing various grades of reproductive functionality (). The inability of D. maghrebiana to lay cocoons contrasts with the ability to produce fertile cocoons displayed by ex-fissiparous individuals of D. aethiopica, D. afromontana and D. etrusca. In contrast, sterile cocoons were laid by D. sicula and D. benazzii.
The ex-fissiparous specimens of D. maghrebiana, D. afromontana, D. sicula and D. benazzii are no longer able to fission during their sexual state; however, they may resume fissiparous activity after their copulatory apparatus has regressed. In contrast, during their sexual state, the ex-fissiparous individuals of D. etrusca and D. aethiopica may spontaneously reproduce asexually by fission. However, persistence of fission capability in fully sexualized individuals was displayed by D. etrusca and D. aethiopica in different ways, i.e. by distal architomy of the caudal tip in the former species vs. architomy occurring slightly behind the pharynx in the latter (, , ). Until now, spontaneous fission of ex-fissiparous specimens was unknown for any other species of the genus Dugesia. In D. ryukyuensis Kawakatsu, Citation1976 (Kawakatsu et al. Citation1976), sexualisation of asexual strains is accompanied by the stopping of fission in sexualised specimens (called “acquired sexual”) (Kobayashi & Hoshi Citation2002). These authors found that decapitation induced fission during the first stages of sexualisation, while the fully sexualised worms never displayed fission even after decapitation.
Furthermore, Kobayashi et al. (Citation2012) found that these acquired sexuals were more prolific than the specimens reproducing only sexually (the latter called “innate sexuals”) due to the presence in the former of hyperplasic and supernumerary ovaries. These data are in contrast with our results on European and African species in which fertility in ex-fissiparous specimens is always very low and associated, in most of them, to morphological anomalies in the gonads (cf. Stocchino et al. Citation2012). Sexualised specimens of D. ryukyuensis differ from our fertile ex-fissiparous lineages also in that offspring in populations of the former species consisted of both fissiparous and sexual individuals (Kobayashi et al. Citation2009, Citation2012). Fertile cocoons of D. aethiopica, D. afromontana and D. etrusca produce only fissiparous offspring, which in turn may eventually sexualise, as their parents did.
The sexual cycles of species like D. benazzii and D. etrusca, including both exclusively sexual populations and mixed populations, are very similar to those of exclusively sexual species such as D. hepta. In the asexual cycle, the ex-fissiparous individuals of D. benazzii and D. etrusca share the absence of morphological anomalies (such as increase of body size and hyperplasic ovaries) occurring in those species with only fissiparous populations (e.g. D. aethiopica, D. maghrebiana, and D. afromontana).
The coexistence of sexual and asexual individuals in mixed populations rules out the occurrence of alternating stages of sexual and fissiparous reproduction in the same individual. In point of fact, in true alternating reproductive life cycles, only one type of reproduction occurs per season in the population. This type of alternating cycle is not so frequent in the genus Dugesia. Among European species, the only report on the alternation of reproductive modes dates back to Draparnaud (Citation1801) with the description of Dugesia subtentaculata (Draparnaud, Citation1801), from the Montpellier area (France), as “ovipare au printemps, et gemmipare en été”. With respect to D. subtentaculata, De Vries (Citation1986) referred to alternating states of fissiparity and sexual maturity. However, De Vries (Citation1986) focused only on fissiparous populations in which ex-fissiparous specimens, characterized by hyperplasic ovaries, developed spontaneously under lab conditions. Therefore, this case cannot be considered as a real alternation of reproductive modes. Okugawa and Kawakatsu (Citation1956) reported an alternation of sexual and asexual reproduction in three populations of D. gonocephala (presently D. japonica Ichikawa & Kawakatsu, Citation1964) from Japan. In this case, the planarians developed the copulatory apparatus from November to March, and then laid cocoons from April to June. After the breeding season, they reduced the reproductive apparatus and underwent a phase of reproduction by fission. This kind of cycle was observed both in the field and in lab cultures. Aditya and Mahapatra (Citation1991) reported a similar seasonal alternation of reproductive modes on another oriental species, D. bengalensis Kawakatsu, Citation1983 (Kawakatsu et al. Citation1983), with sexual reproduction occurring between October and March, after which the planarians reproduced asexually by fission.
Reduction of the reproductive apparatus in sexual (D. hepta, D. benazzii and D. etrusca) or in ex-fissiparous (D. aethiopica, D. afromontana, D. maghrebiana and D. sicula) individuals signals the considerable morphological plasticity of these flatworms. The reproductive system reduction after cocoon deposition was also observed by Benazzi (Citation1938) in sexual individuals from northern Sardinian populations of Dugesia benazzii. A similar behaviour has been reported for sexual populations of D. sicula from Tunisia and Algeria (Harrath et al. Citation2012), and for dendrocoelid planarians (Stephan-Dubois & Gusse Citation1974; Stocchino et al. Citation2013).
Among the species with fissiparous populations the most plastic life cycle is undoubtedly that of D. aethiopica; indeed (1) ex-fissiparous planarians may lay fertile cocoons from which only fissiparous strains originate; (2) ex-fissiparous planarians may retain the fissioning ability, with a division plane occurring slightly behind the pharynx; (3) both ex-fissiparous and fissiparous planarians may regenerate from the cephalic fragment, while the caudal fragment only gives rise to fissiparous individuals.
To summarise, among species of the genus Dugesia, at least five types of life cycle can be recognized on the basis of their reproductive modes:
| 1. | species with an exclusively sexual strategy, e.g. D. hepta, D. ilvana and D. gonocephala s.s.; | ||||
| 2. | species with fissiparous strategy in the field, but with sexualisation processes under laboratory conditions, e.g. D. aethiopica, D. afromontana and D. maghrebiana; | ||||
| 3. | species with seasonal alternation of sexual and fissiparous reproduction during the year, e.g. D. bengalensis, D. japonica and D. subtentaculata; | ||||
| 4. | species characterized by (a) populations with an exclusively sexual strategy, (b) populations with an exclusively fissiparous cycle in the field, but with sexualisation processes in laboratory conditions and (c) mixed populations with coexistence of sexual and fissiparous individuals, e.g. D. sicula; | ||||
| 5. | species characterized by (a) populations with an exclusively sexual cycle and (b) mixed populations with coexistence of sexual and fissiparous individuals, e.g. D. benazzii and D. etrusca. | ||||
This overview highlights that, under the same standard lab conditions, species reproducing only sexually and the sexual individuals of the mixed populations have a life cycle less diversified than those of the fissiparous populations. The persistence in lab strains of D. hepta and D. benazzii of a seasonal reproductive pattern similar to that observed in the field suggests the presence of an endogenous biological rhythm. In contrast, a spontaneous sexualisation trend, displaying various grades of reproductive functionality, is shared by all fissiparous populations, although the causes determining this process under our standard lab conditions are at present unclear.
The seven Dugesia model species represent an example of transition states between sexual and asexual reproduction. The Dugesia case contributes to the modelling of reproductive patterns and strategies in basal Metazoa as a continuum from sexual to asexual reproduction and vice versa, rather than a simple clear-cut alternative.
Acknowledgements
G.A. Stocchino is grateful to Prof. M. Pala for financial support in memory of her husband, Prof. N.G. Lepori. This research was funded in part by a grant from Fondazione Banco di Sardegna to G.A. Stocchino, the Italian Ministero dell'Università e della Ricerca Scientifica e Tecnologica (MIUR-PRIN 2008 “L'endemismo nella fauna italiana: dalla conoscenza sistematica e biogeografica alla conservazione”) and by the Regione Autonoma Sardegna (RAS2012/CRP-60215 “Conservazione e valorizzazione delle grotte sarde: biodiversità e ruolo socio-economico-culturale”). We are grateful to R. Sluys (University of Leiden) and G. Sella (University of Torino), and to the anonymous reviewers for their very useful suggestions that helped improve the manuscript.
References
- Aditya , AK and Mahapatra , S. 1991 . Notes on the biology of the freshwater planarian Dugesia bengalensis (Platyhelminthes Turbellaria Tricladida) . Hydrobiologia , 227 : 145
- Baguñà , J. 1998 . “ Planarians ” . In Cellular and molecular basis of regeneration: From invertebrates to humans , Edited by: Ferretti , P and Géraudie , J . 135 – 165 . New York : John Wiley & Sons Ltd . editors
- Ball , IR and Reynoldson , TB. 1981 . British planarians , Cambridge : Cambridge University Press .
- Benazzi , M. 1938 . Ricerche sulla riproduzione delle planarie tricladi paludicole con particolare riguardo alla moltiplicazione asessuale . Memorie Accademia Nazionale dei Lincei , 7 : 31 – 89 .
- Benazzi , M. 1946 . Sopra una nuova planaria d'acqua dolce . Archivio Zoologico Italiano , 31 : 93 – 102 .
- Benazzi , M. 1974 . “ Fissioning in planarians from a genetic standpoint ” . In Biology of the Turbellaria , Edited by: Riser , NW and Morse , MP . 476 – 492 . New York : McGraw Hill . editors
- Benazzi , M. 1992 . “ Platyhelminthes – Turbellaria ” . In Reproductive biology of invertebrates. Sexual differentiation and behaviour , Edited by: Adiyodi , KG and Adiyodi , RG . Vol. 5 , 53 – 74 . Chichester : John Wiley & Sons . editors
- Benazzi , M and Benazzi Lentati , G. 1976 . “ Platyhelminthes ” . In Animal cytogenetics , Edited by: John , B . Vol. 1 , 1 – 182 . Berlin–Stuttgart : Gebrüder Borntraeger . editor
- Benazzi , M and Gremigni , V. 1982 . “ Developmental biology of triclad turbellarians (Planaria) ” . In Developmental biology of freshwater invertebrates , Edited by: Harrison , FW and Cowden , RR . 151 – 211 . New York : Alan R. Liss Inc . editors
- Best , JB , Goodman , AB and Pigon , A. 1969 . Fissioning in planarians: Control by the brain . Science , 164 : 565 – 566 .
- Calow , P and Read , DA. 1986 . Ontogenetic patterns and phylogenetic trends in freshwater flatworms (Tricladida): Constraints or selection? . Hydrobiologia , 132 : 263 – 272 .
- Charni , M , Harrath , AH , Sluys , R , Tekaya , S and Zghal , F. 2004 . The freshwater planarian Dugesia sicula Lepori, 1948 (Platyhelminthes, Tricladida) in Tunisia: Ecology, karyology, and morphology . Hydrobiologia , 517 : 161 – 170 .
- De Vries , EJ. 1986 . On the taxonomic status of Dugesia gonocephala and Dugesia subtentaculata (Turbellaria, Tricladida, Paludicola) . Journal of Zoology London , 209 : 43 – 59 .
- De Vries , EJ , Baguñà , J and Ball , IR. 1984 . Chromosomal polymorphism in planarians and the plate tectonics of the western Mediterranean . Genetica , 62 : 187 – 191 .
- Draparnaud , JPR. 1801 . Tableau des Mollusques terrestres et fluviatiles de la France , Montpellier & Paris : Renaud; Bossange, Masson & Besson .
- Dugès , A. 1830 . Aperçu de quelques observations nouvelles sur les Planaires et plusieurs genres voisins . Annales des Sciences Naturelles , 21 : 72 – 91 .
- Girard , C. 1850 . A brief account of the fresh water species of Planariae inhabiting the United States . Proceedings of the Boston Society of Natural History , 3 : 264 – 265 .
- Gourbault , N. 1981 . The karyotypes of Dugesia species from Spain (Turbellaria, Tricladida) . Hydrobiologia , 84 : 45 – 52 .
- Harrath , AH , Sluys , R , Merzoug , D , Yacoubi-Khbiza , M , Alwasel , S and Riutort , M. 2012 . Freshwater planarians (Platyhelminthes, Tricladida) from the Palearctic section of the African continent: New records, with a description of a new species . Zootaxa , 3182 : 1 – 15 .
- Ichikawa , A and Kawakatsu , M. 1964 . A new freshwater planarian, Dugesia japonica, commonly but erroneously known as Dugesia gonocephala (Dugès) . Annotationes Zoologicae Japonenses , 37 : 185 – 194 .
- Kawakatsu , M , Oki , I , Tamura , S and Sugino , H. 1976 . Studies on the morphology, karyology and taxonomy of the Japanese freshwater planarian Dugesia japonica Ichikawa & Kawakatsu, with a description of a new subspecies, Dugesia japonica ryukyensis subspec . nov. Bulletin of Fuji Women's College , 14 : 81 – 126 .
- Kawakatsu , M , Oki , I , Tamura , S , Yamayoshi , T and Aditya , AK. 1983 . A new freshwater planarian from West Bengal . Bulletin of the Biogeographical Society of Japan , 38 : 1 – 10 .
- Kenk , R. 1937 . Sexual and asexual reproduction in Euplanaria tigrina (Girard) . The Biological Bulletin , 73 : 280 – 294 .
- Kobayashi , K , Arioka , S , Hoshi , M and Matsumoto , M. 2009 . Production of asexual and sexual offspring in the triploid planarian Dugesia ryukyuensis . Integrative Zoology , 4 : 265 – 271 .
- Kobayashi , K and Hoshi , M. 2002 . Switching from asexual to sexual reproduction in the planarian Dugesia ryukyuensis: Change of the fissiparous capacity along with the sexualizing process . Zoological Science , 19 : 661 – 666 .
- Kobayashi , K , Maezawa , T , Nakagawa , H and Hoshi , M. 2012 . Existence of two sexual races in the planarian species switching between asexual and sexual reproduction . Zoological Science , 29 : 265 – 272 .
- Lázaro , EM , Sluys , R , Pala , M , Stocchino , GA , Baguñà , J and Riutort , M. 2009 . Molecular barcoding and phylogeography of sexual and asexual freshwater planarians of the genus Dugesia in the Western Mediterranean (Platyhelminthes, Tricladida, Dugesiidae) . Molecular Phylogenetics and Evolution , 52 : 835 – 845 .
- Lender , T and Zghal , F. 1968 . Influence du cerveau et de la neurosécrétion sur la scissiparité de la Planaire Dugesia gonocephala . Comptes Rendus de l'Académie des Sciences de Paris , 267 : 2008 – 2009 .
- Lepori , NG. 1948a . Descrizione di Dugesia sicula, nuova specie di Triclade d'acqua dolce dei dintorni di Catania . Archivio Zoologico Italiano , 33 : 461 – 472 .
- Lepori , NG. 1948b . Descrizione di Dugesia ilvana Benazzi n. sp. di Planaria d'acqua dolce dell'Isola d'Elba . Archivio Zoologico Italiano , 33 : 183 – 193 .
- Lepori , NG. 1951 . Sulle caratteristiche morfologiche e sulla posizione sistematica della planaria di Sardegna e Corsica già ascritta a Dugesia gonocephala (Dugès) . Atti Società Toscana di Scienze Naturali , 58 ( B ) : 1 – 22 .
- Lepori , NG and Pala , M. 1982 . Fissioning in planarians. 1. Karyological analysis of fissiparous strains of Dugesia gonocephala s.l. (Turbellaria Tricladida) collected in Sardinia, in order to determine the factors responsible for fissioning . Monitore Zoologico Italiano , 16 : 105 – 131 .
- Okugawa , KI and Kawakatsu , M. 1956 . Studies on the fission of Japanese fresh-water planaria, Dugesia gonocephala (Dugès). IV. Comparative studies on breeding and fission frequencies of sexual and assumed asexual races which were observed in laboratory cultures and natural habitats . Bulletin of the Kyoto Gakugei University , 8 : 23 – 42 .
- Pala , M. 1993 . Correlation between chromosome complement and reproduction in a population of a freshwater triclad . Animal Biology 39th Annual Meeting of the Italian Embryology Group , 2 : 131
- Pala , M , Casu , S and Stocchino , GA. 1999 . Karyology and karyotype analysis of diploid freshwater planarian populations of the Dugesia gonocephala group (Platyhelminthes, Tricladida) found in Sardinia . Hydrobiologia , 392 : 113 – 119 .
- Pala , M , Casu , S and Vacca , RA. 1980 . Rinvenimento di una planaria ascrivibile a Dugesia etrusca monoadenodactyla Lepori (Turbellaria, Tricladida) nell'isola di Molara (Sardegna) . Bollettino della Società Sarda di Scienze Naturali , 19 : 177 – 181 .
- Pala , M , Casu , S and Vacca , RA. 1981 . Dugesia hepta, nuova specie di planaria d'acqua dolce di Sardegna appartenente alla superspecie Dugesia gonocephala (Dugès) (Turbellaria, Tricladida) . Bollettino della Società Sarda di Scienze Naturali , 20 : 97 – 107 .
- Pala , M , Vacca , RA , Casu , S and Stocchino , GA. 1995 . The freshwater planarian Dugesia sicula Lepori from Sardinia (Platyhelminthes, Tricladida) . Hydrobiologia , 310 : 151 – 156 .
- Sluys , R. 1989 . Phylogenetic relationships of the triclads (Platyhelminthes, Seriata, Tricladida) . Bijdragen Tot de Dierkunde , 59 : 3 – 25 .
- Stephan-Dubois , F and Gusse , M. 1974 . Origine et différenciation des cellules vitellines lors de la régénération saisonnière des vitellogènes chez la planaire Dendrocoelum lacteum . Wilhelm Roux’ Archiv fur Entwicklungsmechanik der Organismen , 174 : 181 – 194 .
- Stocchino , GA , Corso , G , Manconi , R , Casu , S and Pala , M. 2005 . Endemic freshwater planarians of Sardinia: Redescription of Dugesia hepta (Platyhelminthes, Tricladida) with a comparison of the Mediterranean species of the genus . Journal of Natural History , 39 : 1947 – 1960 .
- Stocchino , GA , Corso , G , Manconi , R and Pala , M. 2002 . African planarians: Dugesia aethiopica sp. n. (Platyhelminthes, Tricladida) from Lake Tana (NW Ethiopia) . Italian Journal of Zoology , 69 : 45 – 51 .
- Stocchino , GA , Manconi , R , Corso , G , Sluys , R , Casu , S and Pala , M. 2009 . African planarians: Morphology and karyology of Dugesia maghrebiana sp. n. (Platyhelminthes, Tricladida) from Tunisia . Italian Journal of Zoology , 76 : 83 – 91 .
- Stocchino , GA , Sluys , R and Manconi , R. 2012 . A new species of Dugesia (Platyhelminthes, Tricladida, Dugesiidae) from the Afromontane forest in South Africa, with an overview of freshwater planarians from the African continent . Zootaxa , 3551 : 43 – 58 .
- Stocchino , GA , Sluys , R , Marcia , P and Manconi , R. 2013 . Subterranean aquatic planarians from Sardinia, with a discussion on the penial flagellum and the bursal canal sphincter in the genus Dendrocoelum (Platyhelminthes, Tricladida, Dendrocoelidae) . Journal of Cave and Karst Studies. , In press
- Vacca , RA , Casu , S and Pala , M. 1993 . Popolamento planariologico dei fiumi del Nord Sardegna. 2. I cariotipi dei Tricladi d'acqua dolce rinvenuti nel bacino idrografico del fiume Coghinas . Bollettino della Società Sarda di Scienze Naturali , 29 : 59 – 73 .