Abstract
Black coral communities in the Mediterranean Sea are now recognized to be far more common than what was expected a few years ago; however, among the species reported for this basin, Parantipathes larix is one of the least well known. This species is characterized by tall, monopodial or sparsely branched colonies showing a distinctive bottle-brush pinnulation pattern and fairly elongated whitish polyps. Rarely reported in the literature, this species, despite frequently occurring as fishing bycatch, has never been observed in dense populations and all available information mainly concerns its morphological features. Two forests of P. larix were discovered during a remotely operated vehicle (ROV) survey conducted in July 2012 on board the R/V Astrea between 100 and 200 m on two continental rocky shoals located about 10 miles southeast of the Island of Montecristo (Tuscan Archipelago, Tyrrhenian Sea). Video footage was analyzed using the ROV imaging technique, and the coral communities were described in terms of abundance, size-frequency distribution and associated fauna. P. larix is the dominant component of both coral communities showing a maximal abundance of 4 colonies m−2. Sixteen different patches of P. larix, each covering an approximate area of 30 m2 and spaced on average 60 m apart, were counted on the rocky shoals, representing, with an average height of the colonies of about 1 m, the only true three-dimensional habitat of the study areas. The forests of P. larix of Montecristo represent, as far as we know, a unique case for the exceptional abundance of the colonies and their distribution in discrete patches, which is likely a result of the limited dispersal ability of the larvae. Moreover, their occurrence on isolated banks may suggest a preference for topographic structures subjected to local turbulent hydrographic regimes which are known to support filter-feeding communities.
Introduction
Antipatharians have always been considered to be among the rarest coral species of the Mediterranean Sea (Opresko & Försterra Citation2004); however, today, we have a considerable amount of information about the distribution of these large benthic anthozoans (Bo et al. Citation2008, Citation2009, Citation2011a, 2011b, Citation2012a, Citation2013). These new findings indicate that black corals are among the most conspicuous and widely distributed components of the Mediterranean deep-circalittoral coral communities at depths from 60 to about 300 m. Recorded on both rocky slopes and isolated shoals (Bo et al. Citation2009, Citation2012b, Citation2013), these organisms may reach impressive abundances and sizes, forming important facies in the deep-sea realm. Despite the many new records, much is still to be learned regarding their population structure, associated fauna and functional role in deep-sea ecosystems.
Of the six known Mediterranean species (Opresko & Försterra Citation2004; Bo et al. Citation2008, Citation2009, Citation2011a), Antipathella subpinnata (Ellis & Solander, 1786) is the most widespread (Bo et al. Citation2008). It is able to form dense meadows starting at 60 m and is also occasionally recorded at up to 500 m associated with white coral mounds (Mastrototaro et al. Citation2010). The cogeneric Antipathella wollastoni (Johnson, 1899) forms conspicuous populations in both shallow and deep Atlantic waters, but has also recently been recorded in the Mediterranean basin in the vicinity of the Gibraltar Strait (Ocaña et al. Citation2007). Leiopathes glaberrima (Esper, 1792) is among the most common black corals of the Mediterranean basin: occasionally found at 100 m, but forming dense forests only from 200 m, it is a frequent bycatch of long-line fishermen or trawlers (Deidun et al. Citation2010). In ancient times, this species was used in the Mediterranean basin for jewellery thanks to its smooth skeleton which is almost completely lacking in spines (Opresko Citation1998). Of the two species belonging to the Family Antipathidae, Antipathes dichotoma Pallas, 1766, found at 100 m and deeper, is the type species of the Order Antipatharia (Opresko Citation1972) and is characterized by arborescent colonies with very large polyps (Bo et al. Citation2011a). Antipathes fragilis Gravier, 1918 is a doubtful species, described over 100 years after the other Mediterranean species. It has never been found again with certainty and, since the type specimen is lost, there is no possibility to verify, at present, its taxonomic status.
Parantipathes larix (Esper Citation1790) is a monopodial or sparsely branched species showing a characteristic bottle-brush corallum, up to 2 m tall, and occasionally bearing long lateral ramifications. This is probably one of the least well-known species from an ecological point of view. Rarely reported in the literature for the Mediterranean basin (Esper Citation1790; Pax & Müller Citation1955; Vaissiere & Carpine Citation1964; Vafidis et al. Citation1997; Fabri et al. Citation2011; Bo et al. Citation2012b), remotely operated vehicle (ROV) surveys have never found dense populations of this species, but only sparsely distributed colonies (Bo et al. Citation2012b), both along the continental shelf and in very deep waters (up to 2300 m) (Fabri et al. Citation2011). As for the other Mediterranean black corals, for P. larix there were also no available in vivo photographs, although the morphological characteristics of the skeleton have been well described (Opresko & Baron-Szabo Citation2001; Opresko Citation2002; Bo et al. Citation2012a).
The aim of this paper is to describe, for the first time and by ROV imaging, two forests of P. larix recorded off the coasts of Montecristo Island (Tuscan Archipelago, Tyrrhenian Sea), and to characterize them in terms of population structure, morphology of the colonies and associated fauna. Data about the structure and distribution of the black coral forests are important elements to define a more complete picture of the status of these species in the Mediterranean Sea, and to establish sound conservation programs.
Material and methods
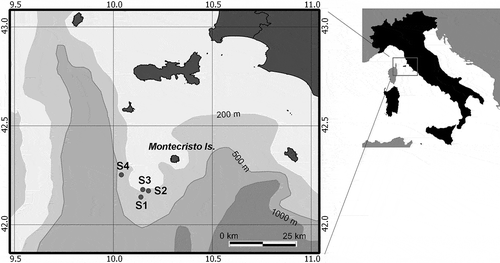
The video and photo material used in this work was collected by an ROV (“Pollux III”) during a survey conducted in July 2012 on board the Research Vessel (R/V) Astrea. Surveys were carried out about 10–12 nautical miles off the coasts of Montecristo Island on four rocky shoals (S1, S2, S3, S4) () located southwest from the island at depths between 100 and 200 m. The shoals are 4–10 nautical miles apart from each other.
Figure 1. Sampling sites. Location of the studied shoals off the coasts of Montecristo Island (Tuscan Archipelago, Tyrrhenian Sea, Italy).
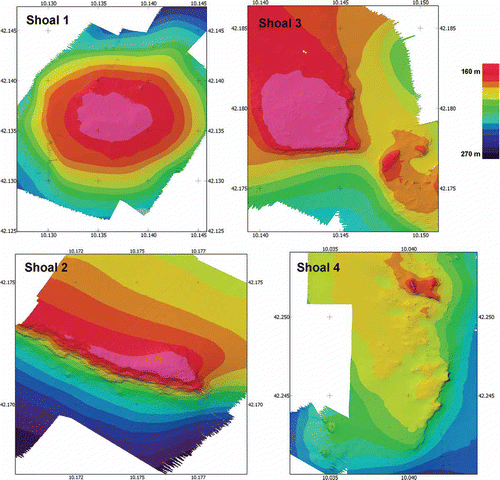
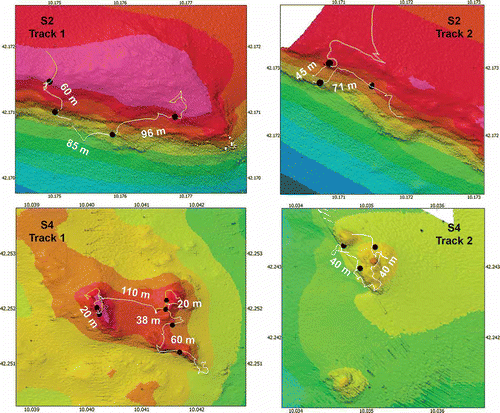
The ROV was equipped with a digital camera (Nikon D80, 10 megapixel), a strobe (Nikon SB 400), a high definition video camera (Sony HDR-HC7), and three jaw grabbers. The ROV hosted also a depth sensor, a compass and three parallel laser beams providing a 10-cm scale for the measurement of the frame area and size of organisms. The ROV was also equipped with an Ultra Short Base Line (USBL) underwater acoustic positioning system providing every second the ROV’s geographic position. Before exploring the areas, each shoal was mapped using a Multibeam in order to obtain a three-dimensional topographic map of the sea bottom ().
The Island of Montecristo is a National Natural Reserve and is part of the National Park of the Tuscan Archipelago. The whole island is subjected to strict protection including the entire marine bottom up to three nautical miles from the island’s coasts. The deep rocky benthic communities of the Tuscan Archipelago, however, have not been subjected to significant protective measures, despite the fact that recent ROV surveys conducted in the coastal deep waters of the island have revealed interesting biocoenoses such as dense facies of the crinoid Leptometra phalangium (Müller, 1841) (Angeletti et al. Citation2010), and sub-fossil deposits of Madrepora oculata Linnaeus, 1758 (Taviani et al. Citation2005).
All four study areas are characterized by accidental rough topography with flat rocky areas or sparse rocky boulders alternating with wide sandy patches. Depending on the local current regime, the rocky areas may be completely unsilted (S1, S3) or covered with a thick layer of of fine sediment (S2, S4).
One colony of P. larix, sampled from S2 at about 190 m, was preserved in 4% formaldehyde and 95% ethanol for morphological analysis. The cnidome of the samples was examined by squeezing polyps or their portions (mouth, tentacles and interpolypar coenenchyme) on a slide and measuring the cnidocysts (length and width in µm) with the optical microscope (100× oil immersion). For the scanning electron microscope (SEM) analysis, fragments of stem and pinnules were coated with gold-palladium in a Balzer Union evaporator and examined with a Philips EM 515 SEM.
One or two ROV dives were conducted in each study area, resulting in a total of 6 hours of video material and 290 high-resolution photographs (). Each ROV video was divided into video frames (selected randomly by the free software DVDVideoSoft) in order to obtain a significant set of video images. A total of 371 video frames (each covering about 3–4 m2 for a final explored rocky area of approximately 1300 m2) were then analyzed with the software ImageJ to calculate the abundance of each target coral species (expressed in terms of number of colonies or individuals m−2 ± SE) and to obtain morphometric data on the colonies of P. larix (height, number and length of the ramifications). Data on the occurrence and type of anthropogenic impact were also considered and expressed as number of frames showing traces of lost fishing gear and typology of abandoned gear.
Table I. Summary of the sampling data for the four study areas.
A one-way Non-Parametric MANOVA was carried out to test for differences in species relative abundance among the target megabenthic assemblages of the four investigated areas (S1, S2, S3, S4) considering the entire set of data [transformed sqrt(x) data, Bray Curtis similarity measure, data distributed homogenously with n = 117, 82, 50 and 122, respectively for the four shoals]. The non-parametric Mann-Whitney analysis was applied to test for significant differences in abundance and height of P. larix between the two principal studied populations, while the Kolmogorov-Smirnov analysis was used to test for differences between the two size-frequency distribution data. Statistical tests were performed using PAST for Windows, version 1.91 (Hammer et al. Citation2001).
Results
Morphology of Parantipathes larix
Parantipathes larix belongs to the Family Schizopathidae, one of the first established families of the Order Antipatharia (Brook Citation1889). The family includes mainly bathyal species characterized by greatly transversally elongated polyps and pinnulated colonies with a typical feather-like or bottle-brush-like morphology (Opresko Citation2002). P. larix is included in the Subfamily Parantipathinae, generally considered to be a phylogenetic link among the families Antipathidae and Schizopathidae on the basis of intermediate anatomical features such as only slightly elongated polyps (Opresko Citation2002).
P. larix was originally described in 1790, but references to the species occur in ancient records and the first illustration of a colony appeared in Citation1617 (Pona, as reported by Pax Citation1940). Nevertheless, a detailed morphological description was given only much later on the basis of the existing museum material (Opresko & Baron-Szabo Citation2001; Opresko Citation2002). P. larix is considered an Atlantic-Mediterranean species living on rocky bottoms at depths from 120 to over 2000 m (Opresko & Försterra Citation2004; Fabri et al. Citation2011; Bo et al. Citation2012b).
On the basis of the ROV images and the studied specimen, P. larix from Montecristo is characterized by monopodial or sparsely branched colonies (basal diameter of the stem from 0.4 to 1.7 cm) with a bottle-brush morphology ( and ) in which the simple pinnules, from 5 to 12 cm long, are arranged in 5–6 rows along the stem and branches, slightly shifted in a spiral disposition (). Occasionally, dense aggregations of pinnules are visible along the ramifications (). On the main stem the spines, 0.05 mm high, are irregularly distributed in small clusters (). The spines on the pinnules (basal diameter on average 0.3 mm) are triangular, simple and smooth, perpendicular and with a pointed tip ( and ). They are arranged in 3–5 longitudinal rows, are 4–5 mm apart in each row, and show an increasing size from the basal (0.03 mm high) to the distal portion (0.11 mm high) of the pinnule.
Polyps are white, slightly elongated in the transverse direction (diameter from 1.6 to 2.2 mm), 0.3–0.4 mm apart, and with tentacles nearly the same length (0.8–1.1 mm long) (H–J). Polyps are arranged monoserially along the pinnules, mainly on their upper side, but this arrangement may vary slightly among different pinnules. Polyps are irregularly distributed along the distal part of the stem (), while they are absent on the basal part and the anchorage. Fertile female colonies may be identified by portions of pinnules with slightly swelled, pinkish polyps. Mature polyps contains about 6–7 eggs (170–290 µm in diameter) located in one or both reproductive mesenteries ().
Figure 3. Parantipathes larix. A, specimen with a greatly ramified corallum showing branches directed upwards and downwards. B, monopodial specimen with no lateral branches. C, pluriserial arrangement of the pinnules around the main stem. D, knots of pinnules around the central stem. E, groups of small spines on the stem. F, spines in three portions of a pinnule, from base to apex. G, close-up view of the pinnular spines. H–I, photographs of living polyps showing the typical white coloration. J, fertile female polyp (white arrow indicates eggs). K, spirocyst. L, basitrich isorhiza. M, mastigophore. Scale bars: A, B, 10 cm. C, D, H, 1 cm. I, 0.5 cm. E, F, J, 1 mm. G, 0.2 mm. K–M, 5 μm.

The cnidome of P. larix consists of spirocysts, 14–17 × 2 μm, present in all examined portions of the polyps, but much more abundant in the tentacular epidermis (); basitrich isorhizas, 17–22 × 3 μm, present in all examined portions, but numerous especially in the tentacles and the mouth (); and microbasic p-mastigophores 15 × 5 μm, found mainly in the mouth and interpolypar coenenchyme ().
Apart from P. larix, five other species are assigned to this genus [Parantipathes tetrasticha (Pourtalès, 1868), Parantipathes helicosticha (Opresko Citation1999), Parantipathes laricides (van Pesch, 1914), Parantipathes wolffi (Pasternak, 1977), and Parantipathes hirondelle Molodtsova, Citation2006]. While the latter three are reported for the Pacific Ocean, P. tetrasticha and P. hirondelle occur in the Atlantic Ocean. P. tetrasticha, known from the western Atlantic basin, is similar to P. larix in the semi-spiral grouping of the pinnules (Opresko Citation2002), which, however, are organized in eight rows and are only 4 cm long (Opresko Citation1999; Opresko & Baron-Szabo Citation2001). Height of spines for P. tetrasticha is reported to be slightly smaller (0.04–0.08 mm) while the transverse diameter of the polyps is known to be slightly larger (2.5 mm) than what was observed for P. larix. P. hirondelle, known from the western Atlantic basin at depths between 300–1500 m, resembles P. larix in the general appearance of the corallum; however, it shows much shorter and more crowded pinnules. Spines on the pinnules of P. hirondelle never exceed 0.05 mm in height, but may be more densely arranged than in P. larix (up to 10 spines per mm) (Molodtsova Citation2006). The transverse diameter of the polyps is slightly smaller (up to 1.7 mm) (Molodtsova Citation2006) than what was observed for P. larix.
The colonies of Montecristo are up to 240 cm high (on average 88 ± 2.7 cm) with a maximum observed number of ramifications of 19 (). Branches may arise from either the apex or the base of the stem and, in the greatly branched specimens, they may form dense aggregations departing from one central knot, giving the colony a distinct three-dimensionality (, and ). The colonies develop vertically even if, in densely branched specimens, the most lateral ramifications are inclined horizontally or downwards (). In these specimens, the branches may reach the third order of ramification. On the basis of 316 measurements, the average branch length is 44 ± 1.5 cm (range 5–160 cm).
Figure 4. Shape of the corallum and associated fauna of Parantipathes larix. A–B, branched specimens of P. larix showing distinct knots of lateral ramifications. C, pair of Anamathia rissoana covered by unidentified athecate hydroids. D, unidentified species of ostracod living among the coral ramifications. E, dead basal portion of the stem covered by hydroids and Filograna spp. F, school of Macroramphosus scolopax swimming among the coral colonies. Scale bars: A, B, E, F, 10 cm. C, 5 cm. D, 0.5 cm.
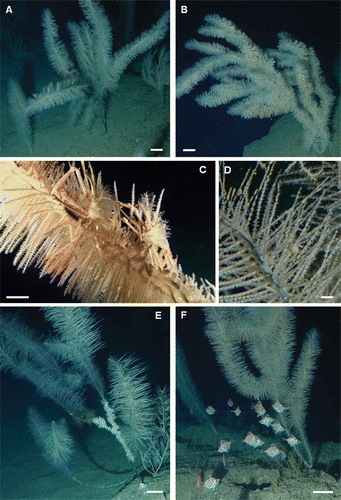
The observed colonies do not host a rich associated fauna on the living portions. Only two specimens of the decapod Anamathia rissoana (Roux, 1828) (hosting unidentified athecate hydroids on the carapace) were found on one colony (), while very common is an unidentified species of a yellow ostracod (). The dead portions of the stem may be overgrown by various hydroid species and by colonies of Filograna spp. (). The most ramified colonies may be used as occasional refuge by some fish species such as Lappanella fasciata (Cocco, 1833) and Macroramphosus scolopax (Linnaeus, 1758) ().
The communities of the Montecristo shoals
In the analysis of the 371 video frames, nine species of gorgonians and antipatharians were selected as target species as well as the sponge Poecillastra compressa (Bowerbank, 1866), the only recognizable massive sponge species of the study areas.
Despite the relative geographic proximity and the similar bathymetric depth range of the four shoals, the video frame analysis revealed significant differences in the relative abundance of species among the four rocky benthic biocoenoses () (P < 0.001, NPMANOVA, F = 30.89).
Figure 5. Species composition of the four studied target communities (number of colonies or specimens m−2 ± SE).
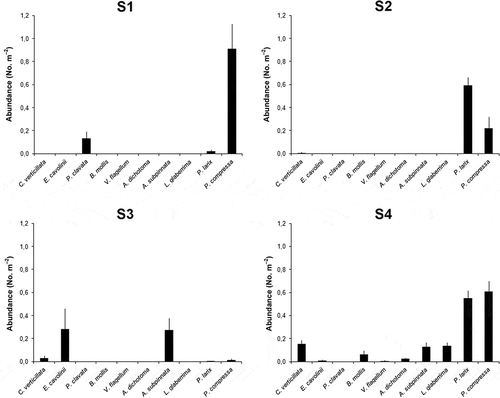
Shoal S1 is characterized by sparse rocky boulders, scarcely silted, on a detritic bottom. The environment is poorly inhabited with a few colonies of Paramuricea clavata (Risso, 1826) (0.13 ± 0.06 colonies m−2) and a dense population of P. compressa (0.91 ± 0.21 specimens m−2, corresponding to 85% of the target community). Parantipathes larix is present only as sparse colonies (average abundance 0.02 ± 0.01 colonies m−2, corresponding to 3% of the target community).
The rocky, silted slopes of shoal S2 are dominated by dense monospecific populations of P. larix (average abundance of 0.59 ± 0.07 colonies m−2, maximum abundances of 2.3 colonies m2, corresponding to 69% of the target community), alternating with wide detritic or rocky patches, that are bare or are characterized by small sponge gardens of P. compressa (0.22 ± 0.10 specimens m−2, corresponding to 30% of the target community) and few colonies of Callogorgia verticillata (Pallas, 1766). The remaining megabenthic community mainly includes encrusting sponges, cidarid sea urchins and sparse living colonies of the scleractinian Dendrophyllia cornigera (Lamarck, 1816).
Shoal S3 is characterized by a dense forest of Antipathella subpinnata (0.27 ± 0.10 colonies m−2, corresponding to 48% of the target community) located on the least silted rocky areas. Several colonies of Eunicella cavolinii (Koch, 1887) were also observed (0.28 ± 0.18 colonies m−2), while C. verticillata, P. larix and P. compressa together represented less than 10% of the community. The rocky boulders hosted coralline algae, encrusting sponges, various echinoderms and some fish species, mainly Conger conger (Linnaeus, 1758).
Shoal S4 occupies a relatively vast rocky area hosting the richest megabenthic community composed of various plurispecific coral forests. P. larix showed an average abundance of 0.55 ± 0.07 colonies m−2 (corresponding to 34% of the target community, and maximum abundances of 4.0 colonies m−2) followed by C. verticillata (0.15 ± 0.03 colonies m−2, corresponding to 8% of the community), A. subpinnata (0.13 ± 0.04 colonies m−2, corresponding to 8% of the community) and Leiopathes glaberrima (0.14 ± 0.10 colonies m−2, corresponding to 7% of the community). About 5% of the community is represented by other species, such as Viminella flagellum (Johnson, 1863), E. cavolinii, Antipathes dichotoma and few colonies of the small, yellow gorgonian Bebryce mollis Philippi, 1841, which was found under the largest species, in rocky crevices or along rocky ridges. Various patches of P. compressa were observed on the rocky boulders (0.61 ± 0.09 specimens m−2, corresponding to 37% of the target community) some of which were evidently damaged by cidarid grazing. The remaining megabenthic community included a few colonies of Corallium rubrum (Linnaeus, 1758), various fish species, crustaceans and echinoderms.
Fishing impacts on the four shoals, expressed as percentage of video frames showing lost gear, are considered to be moderate: S4 had the fewest impacts (4%), followed by S1 (7%), S2 (10%) and S3 (18%). The recorded lost gear included nets and long-lines. Of all the coral colonies observed, only two (one E. cavolinii and one P. larix) were clearly entangled in lost lines.
Population structure of Parantipathes larix from Montecristo
P. larix is present on all four shoals, but only on S2 and S4 does this species form dense forests at depths between 127 and 188 m (). The 433 counted colonies of this species on the two banks showed a typical patchy distribution mainly characterized by small groups with very high abundances (–J). In total, 16 patches were counted on S2 (–C) and S4 (D–J) (7 and 9 respectively). On the basis of their geographic position, it was estimated that the average linear distance between the patches was 58 m (ranging from 20 m to 110 m) (). Along the ROV tracks, patches were found on rocky outcrops and maximal distances occur when detritic areas are present among hardgrounds.
Figure 6. Patches of Parantipathes larix. A–C, patches of tall, poorly branched colonies on S2 bent towards the inclined silted bottom. D–E, dense forests of P. larix on flat rocky elevations in S4. F–J, examples of coral patches on the Montecristo shoals. K, branched specimen from the Gulf of St. Eufemia (130 m depth). L, colony from a rocky shoal off Carloforte (Sardinia), 140 m depth. M, small colonies from Vedove Shoal (Liguria), 200 m depth. N, small colonies from Candelieri Shoal (Campania), 130 m depth. Scale bars: A–J, M, 50 cm. K, L, N, 10 cm.
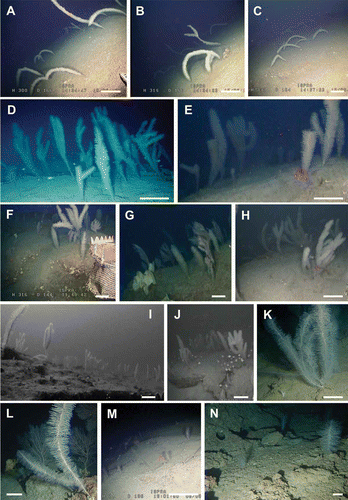
Figure 7. Localization of the patches of Parantipathes larix along the remotely operated vehicle (ROV) tracks. Minimum linear distances among the patches are indicated.
The average abundance of P. larix in a patch is 0.85 ± 0.07 colonies m−2 (ranging 0.3–1.5 colonies m−2) for an average covering area of 31 ± 7 m2 (ranging from 7 to 106 m2), without a significant difference between the two shoals (Mann-Whitney, P = ns).
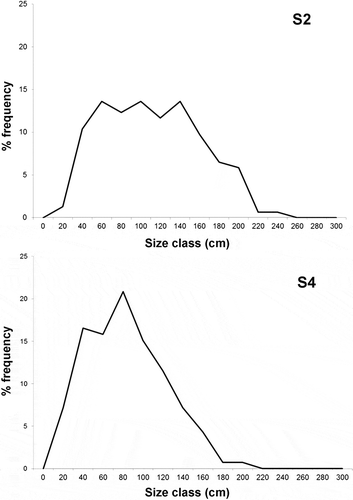
The average height of the colonies in the two areas is significantly different, 103 ± 3.9 cm and 73 ± 3.4 cm, respectively for S2 and S4 (Mann-Whitney, P < 0.001). While in S4 all the colonies have a vertical development (), 45% of the colonies of S2, distributed along a silted inclined slope, are bent horizontally towards the sea bottom in the same direction (–C).
The size-frequency distributions of the colonies in the two forests (S2 and S4) are significantly different (Kolmogorov-Smirnov, P < 0.001), with the modal class of S2 covering a wider range of sizes ().
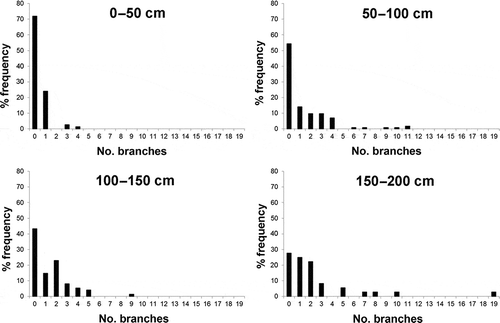
Forty-seven percent of all the studied colonies in the Montecristo area are branched; however, there is no significant correlation among the height and the number of branches in either population (r = 0.009, n = 154 and r = 0.233, n = 139 for S2 and S4, respectively). In S2, 57% of the studied colonies show no branches, while in S4 the percentage decreases to 46%. In terms of branch-frequency distribution, the percentage of specimens in the whole study area with no branches decreases from the smaller-sized colonies (70% in colonies 0–50 cm high) to the larger-sized ones (55%, 43%, 28% respectively for colonies 50–100, 100–150 and 150–200 cm high) ().
Discussion
It is intriguing how a species such as Parantipathes larix, known for almost four centuries and representing a conspicuous structure-forming component of relatively deep coral ecosystems in a well-known basin such as the Mediterranean Sea, has avoided attention for such a long time. This is a common situation for many species living at depths below 100 m, especially branched corals, whose existence was known in early times from occasional fishery by-catches. In comparison, recent explorations are progressively unveiling the characteristics of Mediterranean black coral forests, such as those formed by Antipathella subpinnata in primis, but also by Antipathes dichotoma and Leiopathes glaberrima (Bo et al. Citation2009, Citation2011a; Deidun et al. Citation2010).
Differently from other large antipatharians species, all observations, including the most recent ones (Fabri et al. Citation2011; Bo et al. Citation2012b), indicate that P. larix is usually very sparsely distributed (on average 0.18 ± 0.07 colonies m−2 in Gulf of St. Eufemia; Bo et al. Citation2011a, Citation2012b) ( and ). The forests of Montecristo represent, so far, a unique case both for the exceptional density of the colonies (on average 0.85 ± 0.06 colonies m−2 and a maximum of 4 colonies m−2), but also for the distinct aggregation in patches. This pattern has never been reported before for this species, although small populations of possibly juvenile colonies (less than 30 cm high) have been observed in other localities, such as the Gulf of Salerno and the Ligurian Sea (Bo, pers. obs.) ( and N). Unlike these areas, in Montecristo, juveniles are usually found among tall adult colonies, supporting the idea that the population is in optimal condition being composed of various age cohorts.
At Montecristo, P. larix forms dense forests only in two of the four explored sites, despite the relative proximity of the four areas. This situation may suggest a preference of this species for high topographic structures subjected to complex hydrographic regimes such as those found on seamounts, rocky ridges and rocky shoals that enhance the re-suspension of nutrients and the settling of big filter-feeders such as gorgonians, antipatharians and sponges (Genin et al. Citation1986, Citation1992; Bo et al. Citation2012b).
Within each forest, P. larix is found in small patches. This distribution supports the hypothesis that large benthic anthozoans corals, such as black corals, may have a very limited larval dispersal ability (Mistri & Ceccherelli Citation1994; Coma & Lasker Citation1997; Miller Citation1997, Citation1998; Gori et al. Citation2011; Bo et al. Citation2012b), enhancing the formation of small aggregations. For P. larix, on the basis of the linear distance among discrete patches, we may hypothesize around 60 m of dispersal ability. This value is in accordance with previous literature data on larval dispersal obtained with modelling or genetic techniques for various Mediterranean anthozoan species such as Corallium rubrum (10 m; Costantini et al. Citation2007), Paramuricea clavata and Eunicella singularis (Esper, 1794) (a few meters; Weinberg & Weinberg Citation1979; Coma et al. Citation1995). The only datum available for black corals refers to the fjord species Antipathella fiordensis (Grange, 1990) from New Zealand, for which has been estimated a larval dispersion ranging from less than 5 m to about 50 m (Miller Citation1998). Like this species, for Mediterranean black corals, the restricted larval dispersal ability may be an adaptation to colonize scattered habitats such as the isolated continental rocky outcrops on which they usually live.
The differences between S2 and S4, in terms of both population structure and morphology of the colonies, support the idea that the local oceanographic, topographic and sedimentologic conditions in the two areas are different. The monospecific patches of S2 are distributed on smaller areas than those of S4 and almost half of them, found on a silted inclined slope, are flexed in the same direction and, even if considerably tall, are scarcely branched. Probably this must be attributed to both an inclined bottom and an intense prevailing bottom current. This may also explain the absence of other branched coral species potentially less resistant to marked hydrodynamic regimes due to a greater surface exposed to water friction (Bo et al. Citation2013). The populations of S4, instead, are found on almost flat surfaces: on average the colonies are more ramified and none of them is flexed, probably indicating moderate current conditions. This hypothesis is supported also by the presence of plurispecific patches in this site and a richer associated community.
Unlike the forests of A. subpinnata and A. dichotoma (Bo et al. Citation2008, Citation2009, Citation2011a, Citation2012b), those formed by P. larix do not host a very rich associated fauna. The preference of this species for silted environment may have an influence on the presence of encrusting epibionts; nevertheless, it is only in the plurispecific patches that the fish fauna was observed moving among the ramifications also of P. larix colonies. In comparison to the arborescent colonies of A. subpinnata, the colonies of P. larix are less branched, and thus potentially offer fewer refuges and less protection (Bo et al. Citation2009). Among the species associated with A. subpinnata, only the colonial polychaete Filograna spp. and some hydroid species are present also on the other black coral species, and always on the dead portions of the skeleton. With regard to the species found on living portions of the colony (and considered more strictly specific symbionts), black corals are able to host numerous species of crustaceans, echinoderms, mollusks, polychates and other cnidarians (Tazioli et al. Citation2007; Wagner et al. Citation2012; Gaino et al. Citation2013). An Ectopleura hydroid lives in association with A. subpinnata (Bo et al. Citation2011c), but no such symbiotic relationship has been reported for the other Mediterranean species including P. larix, which, however, may form a stable association with an ostracod, as observed also in various other localities (unpub. obs.).
In ecological terms, large benthic anthozoans corals are generally considered ecosystem engineers, structure-forming species able to create complex three-dimensional habitats, characterized by peculiar physico-chemical features promoting high levels of biodiversity and ecosystem functioning (Linares et al. Citation2008; Scinto et al. Citation2009; Buhl-Mortensen et al. Citation2010; Cerrano et al. Citation2010; Bo et al. Citation2012b). In this sense, the dense aggregations of P. larix may also be considered ecological forests, even if, unlike A. subpinnata, commonly observed in meadows, P. larix is only occasionally forming true facies.
The size-frequency distribution of this species recalls that of other deep-water Mediterranean coral species previously studied, such as Eunicella cavolinii and A. subpinnata (Bo et al. Citation2009, Citation2012b). However, the modal peak does not include, at least in S2, the smaller size-classes, but tends towards the intermediate classes. This pattern may suggest the existence of a stable population of adults in which larval settlement is limited or mortality of young colonies is high, but the growth of settled larvae is fast and their survival is high. The branch-frequency distribution data suggest that not only a larger size, but also a greater branching complexity may be associated with adult colonies of P. larix. Therefore, the final shape of a colony is molded by both ecological constraints and intrinsic morphological factors.
It is interesting to note that the population structure of this species in Montecristo, characterized by a localized large number of individuals (apparently unusual on the basis of the observations made in other localities) and a large number of very tall (hence probably old) specimens, is found in an area which is characterized by very low anthropogenic impacts, indicating that these forests may therefore represent a pristine habitat. However, large collections of P. larix were made in the Tuscan Archipelago in the 1990s by trawling nets working on deep bottoms at about 600 m depth (Mori, pers. comm.), suggesting an even wider distribution areal of this species in the area. The patchy distribution and limited larval dispersal described for P. larix emphasize the fragility of the black coral populations and highlight the potential impacts that localized anthropogenic events may have on these unique Mediterranean assemblages.
Acknowledgements
The authors would like to thank the crew of R/V Astrea for their extraordinary support in the field activities, and the reviewers for providing helpful suggestions and critiques. This work was financed by the Italian Ministry of Agricultural, Food and Forestry Policies. The submitting author declares, on behalf of all the authors, that there are no financial, personal or professional interests that could be construed to have influenced the paper.
References
- Angeletti L, Ceregato A, Ghirelli M, Gualandi B, Lipparini E, Malatesta D, Sperotti A, Taviani M. 2010. ROV-SCUBA integrated survey of the Montecristo Island nature reserve (Tuscan Archipelago National Park, Mediterranean Sea). International Journal of the Society for Underwater Technology 29:1–4.
- Bo M, Bavestrello G, Canese S, Giusti M, Angiolillo M, Cerrano C, Salvati E, Greco S. 2011a. Coral assemblages off the Calabrian Coast (South Italy) with new observations on living colonies of Antipathes dichotoma. Italian Journal of Zoology 78:231–242.
- Bo M, Bavestrello G, Canese S, Giusti M, Salvati E, Angiolillo M, Greco S. 2009. Characteristics of a black coral meadow in the twilight zone of the central Mediterranean Sea. Marine Ecology Progress Series 397:53–61.
- Bo M, Bavestrello G, Kurek D, Paasch S, Brunner E, Born R, Galli R, Stelling AL, Sivkov VN, Petrova O, Vyalikh D, Kummer K, Molodtsov SL, Nowak D, Nowak J, Ehrlich H. 2012a. Isolation and identification of chitin in the black coral Parantipathes larix (Anthozoa: Cnidaria). International Journal of Biological Macromolecules 51:129–137.
- Bo M, Bertolino M, Borghini M, Castellano M, Covazzi Harriague A, Di Camillo CG, Gasparini GP, Misic C, Povero P, Schroeder K, Bavestrello G. 2011b. Characteristics of the mesophotic megabenthic assemblage of the Vercelli Seamount (North Tyrrhenian Sea). PLoS-One 6:e16357.
- Bo M, Canese S, Spaggiari C, Pusceddu A, Bertolino M, Angiolillo M, Giusti M, Loreto MF, Salvati E, Greco S, Bavestrello G. 2012b. Deep coral oases in the South Tyrrhenian Sea. PLoS-One 7:e49870.
- Bo M, Cerrano C, Canese S, Salvati E, Angiolillo M, Santangelo G, Bavestrello G. 2013. The deep coral assemblages of an off-shore deep Mediterranean rocky bank. NW Sicily, Italy: Marine Ecology (in press). DOI: 10.1111/maec.12089.
- Bo M, Di Camillo CG, Puce S, Canese S, Giusti M, Bavestrello G. 2011c. A tubulariid hydroid associated with anthozoans from the Mediterranean Sea. Italian Journal of Zoology 78:487–496.
- Bo M, Tazioli S, Spanò N, Bavestrello G. 2008. Antipathella subpinnata (Antipatharia, Myriopathidae) in Italian seas. Italian Journal of Zoology 75:185–195.
- Brook G. 1889. Report on the Antipatharia collected by H. M. S. Challenger during the years 1873–1876. Reports of the Scientific Results of the Voyage of H. M. S. Challenger 32:1–222.
- Buhl-Mortensen L, Vanreusel A, Gooday AJ, Levin LA, Priede IG, Buhl-Mortensen P, Gheerardyn H, King NJ, Raes M. 2010. Biological structures as a source of habitat heterogeneity and biodiversity on the deep ocean margins. Marine Ecology 31:21–50.
- Cerrano C, Danovaro R, Gambi C, Pusceddu A, Riva A, Schiaparelli S. 2010. Gold coral (Savalia savaglia) and gorgonian forests enhance benthic biodiversity and ecosystem functioning in the mesophotic zone. Biodiversity Conservation 19:153–16.
- Coma R, Lasker HR. 1997. Small-scale heterogeneity of fertilization success in a broadcast spawning octocoral. Journal of Experimental Marine Biology and Ecology 214:107–120.
- Coma R, Ribes M, Zabala M, Gili JM. 1995. Reproduction and cycle of gonadal development in the Mediterranean gorgonian Paramuricea clavata. Marine Ecology Progress Series 117:173–183.
- Costantini F, Fauvelot C, Abbiati M. 2007. Fine-scale genetic structuring in Corallium rubrum: Evidence of inbreeding and limited effective larval dispersal. Marine Ecology Progress Series 340:109–119.
- Deidun A, Tsounis G, Balzan F, Micallef A. 2010. Records of black coral (Antipatharia) and red coral (Corallium rubrum) fishing activities in the Maltese Islands. Marine Biodiversity Records 3:e90.
- Esper EJC. 1790. Fortsetzungen der Pflanzenthiere. Die Planzentiere in Abbildungen nach der Natur mit Farben erleuchtet nebst Beschreibungen. Kaspeschen, Nürberg 2:303 pp.
- Fabri MC, Pedel L, Freiwald A, Madurell T. 2011. Habitats particuliers des étages bathyal et abyssal (Med). In: Fabri MC, Pedel L, editors. Biocénoses des fonds durs du bathyal et de l’abyssal/SRM MO. Initial Assessment for the Water Marine Framework Strategy.
- Gaino E, Bavestrello G, Boyer M, Scoccia F, Bo M. 2013. Biological and ecological relevance of black corals (Antipatharia) in the benthic environment. In: Liñán-Cabello MA, editor. Corals: Classification, habitat and ecological significance. New York: NOVA Science Publishers, Inc. pp. 37–74.
- Genin A, Dayton PK, Lonsdale PF, Spiess FN. 1986. Corals on seamount peaks provide evidence of current acceleration over deep-sea topography. Nature 322:59–61.
- Genin A, Paull CK, Dillon WP. 1992. Anomalous abundances of deep-sea fauna on a rocky bottom exposed to strong currents. Deep-Sea Research I 39:293–302.
- Gori A, Rossi S, Berganzo E, Pretus JL, Dale MRT, Gili JM. 2011. Spatial distribution patterns of the gorgonians Eunicella singularis, Paramuricea clavata, and Leptogorgia sarmentosa (Cape of Creus, Northwestern Mediterranean Sea). Marine Biology 158:143–158.
- Hammer Ø, Harper DAT, Ryan PD. 2001. PAST: paleontological statistics software package for education and data analysis. Palaeo Electronica 4:1–9.
- Linares C, Coma R, Garrabou J, Diaz D, Zabala M. 2008. Size distribution, density and disturbance in two Mediterranean gorgonians: Paramuricea clavata and Eunicella singularis. Journal of Applied Ecology 45:688–699.
- Mastrototaro F, D’Onghia G, Corriero G, Matarrese A, Maiorano P, Panetta P, Gherardi M, Longo C, Rosso A, Sciuto F, Sanfilippo R, Gravili C, Boero F, Taviani M, Tursi A. 2010. Biodiversity of the white coral and sponge community off Cape Santa Maria di Leuca (Mediterranean Sea): An update. Deep Sea Research II 57:412–430.
- Miller KJ. 1997. Genetic structure of black coral populations in New Zealand’s fiords. Marine Ecology Progress Series 161:123–132.
- Miller KJ. 1998. Short-distance dispersal of black coral larvae: Inference from spatial analysis of colony genotypes. Marine Ecology Progress Series 163:225–233.
- Mistri M, Ceccherelli VU. 1994. Growth and secondary production of the Mediterranean gorgonian Paramuricea clavata. Marine Ecology Progress Series 103:291–296.
- Molodtsova TN. 2006. Black corals (Antipatharia: Anthozoa: Cnidaria) of the northeastern Atlantic. In: Mironov AN, Gebruck AV, Southward AJ, editors. Biogeography of the North Atlantic Seamounts. Moscow: KMK Scientific Press. pp. 141–151.
- Ocaña O, Opresko DM, Brito A. 2007. First record of the black coral Antipathella wollastoni (Anthozoa: Antipatharia) outside of Macaronesian Waters. Revista de la Academia Canaria de Ciencias 18:125–138.
- Opresko DM. 1972. Redescriptions and re-evaluations of the Antipatharians described by L. F. de Pourtales. Bulletin of Marine Science 22:950–1017.
- Opresko DM. 1998. Three new species of Leiopathes (Cnidaria: Anthozoa: Antipatharia) from Southern Australia. Records of the South Australian Museum 31:99–111.
- Opresko DM. 1999. New species of Antipathes and Parantipathes (Cnidaria: Anthozoa: Antipatharia) from coastal waters of South Australia and Tasmania. Records of the South Australian Museum 32:143–154.
- Opresko DM. 2002. Revision of the Antipatharia (Cnidaria: Anthozoa). Part II. Schizopathidae. Zoologische Mededelingen, Leiden 76:411–442.
- Opresko DM, Baron-Szabo RC. 2001. Re-descriptions of the antipatharians corals described by E.J.C. Esper with selected English translations of the original German text. (Cnidaria, Anthozoa, Antipatharia). Senckenbergiana Biologica 81:1–21.
- Opresko DM, Försterra G. 2004. Orden Antipatharia (corales negros o espinosos). In: Hofrichter R, editor. El Mar Mediterraneo: fauna, flora, ecologia. Barcelona: Omega 2. pp. 506–509.
- Pax F. 1940. Anthozoa. In: Bronn HG, editor. Klassen und Ordnungen des Tierreich 2. Leipzig: Winter CF Publisher. pp. 267–280.
- Pax F, Müller I. 1955. Gli Antozoi del Museo Civico di Storia Naturale di Trieste. Parte I. Antipatharia, Ceriantharia, Zoantharia, Actinaria, Alcyonaria e Pennatularia. Atti del Museo Civico di Storia Naturale di Trieste 20:103–110.
- Pona G. 1617. Monte Baldo descritto in cui si figurano et descrivono molte rare Piante de gli Antichi, da' Moderni sin' hora non conosciute. Et due Commenti da N. Marogna sopra I‘Amomo de gli Antichi, R Meietti, Venetia. 411 pp.
- Scinto A, Bertolino M, Calcinai B, Huete-Stauffer C, Previati M, Romagnoli T, Cerrano C. 2009. Role of a Paramuricea clavata forest in modifying the coralligenous assemblages. In: Proceedings of the 1st Symposium on the Coralligenous and other calcareous bio-concretions of the Mediterranean Sea. UNEP-MAP-RAC/SPA Tabarka. pp. 137–141.
- Taviani M, Freiwald A, Zibrowius H. 2005. Deep coral growth in the Mediterranean Sea: an overview. In: Freiwald A, Roberts JM, editors. Cold-water corals and ecosystems. Berlin: Springer. Erlangen Earth Conference Series. pp. 137–156.
- Tazioli S, Bo M, Boyer M, Rotinsulu H, Bavestrello G. 2007. Ecology of some common antipatharians from the Marine Park of Bunaken (North Sulawesi, Indonesia). Zoological Studies 46:227–241.
- Vafidis D, Koukouras A, Voultsiadou-Koukoura E. 1997. Actiniaria, Corallimorpharia, and Scleractinia (Hexacorallia, Anthozoa) of the Aegean Sea, with a checklist of the Eastern Mediterranean and Black Sea species. Israel Journal of Zoology 43:55–70.
- Vaissiere R, Carpine C. 1964. Contributions à l’étude bionomique de la Méditerranée occidentale (Côte du Var et des Alpes maritimes – côte occidentale de Corse) Fasc. 4: Compte rendu de plongées en soucoupe plongeante SP 300 (région A1). Bulletin de l‘Institut Océanographique de Monaco 63:1–36.
- Wagner D, Luck DG, Toonen RJ. 2012. The biology and ecology of black corals (Cnidaria: Anthozoa: Hexacorallia: Antipatharia). Advances in Marine Biology 63:67–132.
- Weinberg S, Weinberg F. 1979. The life cycle of a gorgonian: Eunicella singularis (Esper, 1794). Bijdragen tot de Dierkunde 8:127–140.