Abstract
The calanoid copepod Eurytemora affinis Poppe, 1880 is the most abundant species of the mesozooplanktonic community of the Seine estuary (France) in the lower salinity zone. Diapausing eggs of this species have been found in the sediment of this estuary but they are few in comparison with those found for other calanoid species. We thus decided to test the effect of photoperiod on diapausing egg production in E. affinis from the Seine estuary. For this, an experimental device has been developed. A combination of five photoperiods and two temperatures was used and the production of eggs was studied for the first and the second clutch. The production of diapausing eggs was demonstrated under photoperiodic conditions less than or equal to 12 h of light either at 12 or 16°C with a maximum under constant darkness (6%). A significant increase in the number of non-viable eggs was also observed between the first and the second clutch for all tested conditions and this may be due to the non-fertilization of these eggs. We also verified that the late naupliar instars are sensitive to the photoperiodic signal.
The experimental approach has revealed that the production of diapausing eggs in the key species to the downstream Seine estuary, E. affinis, could be induced by short days, but photoperiod was probably not the only factor responsible for the induction of diapausing egg production. The percentage of diapausing eggs obtained under the induction experiment is similar to that obtained from the eggs extracted from the sediment of the Seine estuary (7%). The strategy of E. affinis in this estuary would be to constitute an egg bank, acting as long-term survival or in the case of adverse conditions. The role of geographical position (mainly latitude) and local conditions in the reproductive strategy of E. affinis is discussed.
Introduction
The calanoid copepod Eurytemora affinis Poppe, 1880 is one of the most common species in the northern hemisphere (Mauchline Citation1998; Gasparini et al. Citation1999; Lee Citation1999). This species dominates the zooplankton community in many European estuaries, such as the Gironde, the Elbe and the Schelde (Castel Citation1995; Escaravage & Soetaert Citation1995; Gasparini et al. Citation1999; Peitsch et al. Citation2000). In the Seine estuary, E. affinis reproduces throughout the year but dominates the downstream zooplankton community between April and June (Mouny & Dauvin Citation2002; Devreker et al. Citation2008). All instars combined, the maximum density of this species observed in the bottom was 700,000 individuals (ind) m−3 when water temperatures were around 15°C (Devreker et al. Citation2008). During the summer, when temperatures are close to 20°C, the E. affinis population is gradually replaced by Acartiidae (Acartia bifolosa Giesbrecht, 1881, then Acartia tonsa Dana, 1849), as observed in other estuaries (Escaravage & Soetaert Citation1995; David et al. Citation2007). E. affinis generally plays also an important role in the food web of the estuarine zones it inhabits, because it is one of the major consumers of phytoplankton and detritus, and one of the most abundant preys for predators such as mysids, shrimps and fishes (Tackx et al. Citation2003; Dauvin & Desroy Citation2005).
In the Seine estuary, many studies have been made of the planktonic phase of the life cycle of this species, especially in the framework of the Seine-Aval program (Mouny & Dauvin Citation2002; Beyrend-Dur et al. Citation2009; Devreker et al. Citation2009; Dur et al. Citation2009; Souissi et al. Citation2010). Recently, we focused on the possible role of the dormancy strategy in the calanoid copepods of this highly dynamic estuary. An adult female can produce ready-to-hatch eggs, called “subitaneous eggs”. These eggs can become “quiescent” ones when they encounter adverse environmental conditions (anoxia, temperature decrease and other factors), and later will hatch as soon as they are restored. However, an adult female is also able to produce directly “diapausing eggs” in anticipation of adverse conditions. For this type of eggs, hatching is only possible after a refractory phase (normally chilling) and the return of optimal conditions (Grice & Marcus Citation1981; Chen & Marcus Citation1997; Radzikowski Citation2013). The mechanism of diapause induction is well documented for marine and freshwater copepods as well as many species of insects (Watson & Smallman Citation1971; Marcus Citation1982; Tauber et al. Citation1986; Alekseev Citation1990; Hairston et al. Citation1990). In calanoid copepod species, these parameters can be temperature, photoperiod, crowding, or predation (Marcus Citation1980, Citation1982; Uye Citation1985; Walton Citation1985; Hairston et al. Citation1990; Ban Citation1992; Piercey & Maly Citation2000; Avery Citation2005a, Citation2005b; Wu et al. Citation2007; Boyer & Bonnet Citation2013). Among others, it seems that the temperature and photoperiod as good predictors of season are the two key factors involved in the induction of diapausing egg production (Hairston & Kearns Citation1995; Gyllström & Hansson Citation2004).
Several studies showed that E. affinis from various geographical locations is able to produce subitaneous and diapausing eggs (Ban & Minoda Citation1991, Citation1992, Citation1994; Ban Citation1992; Katajisto Citation1996; Madhupratap et al. Citation1996; Albertsson & Leonardsson Citation2000, Citation2001). Ban (Citation1992) showed that the type of eggs (subitaneous or diapause) produced by females of E. affinis from Lake Ohnuma in Japan was determined in response to environmental conditions in which they lived during their naupliar instars. The author also showed that a short photoperiod 10:14 (light:dark, L:D) induced the production of diapausing eggs, and low temperature (10°C) enhances this effect.
In the Seine estuary, a previous study showed for the first time the high density of calanoid copepod resting eggs in the sediment of the Seine estuary (Glippa et al. Citation2011). It also revealed that the proportion of the E. affinis eggs in the sediment was much lower than expected, and the presence of diapausing eggs was confirmed (Glippa Citation2011; Glippa et al. Citation2011). Indeed, different incubations performed after 1 month or several months at low temperature allowed the emergence of nauplii with the maximum coming from a sediment sample in mid-fall 2008 (Glippa Citation2011), suggesting that females produce diapausing eggs in response to a decrease in day length and that the population can overwinter as dormant eggs in sediments. Moreover, in this estuary, particularly in the maximum turbidity zone where the population of E. affinis is abundant, turbidity is so strong (values ranging from 0.04 to 2.30 g L−1 suspended particulate matter, SPM) that the light signal attenuates rapidly with depth (Glippa Citation2011). In order to better understand the mechanism leading to the production of diapausing eggs in E. affinis from the Seine estuary, an experimental approach under controlled laboratory conditions was used. The main objective of the study was to investigate the effects of different photoperiods on diapausing egg production in E. affinis from the Seine estuary. This was done at two moments corresponding to different seasonal phases (and hence two different temperatures): late fall and the onset of spring. Moreover, we wanted to understand the possible causes of the low proportion of diapausing eggs produced by the E. affinis population in the Seine estuary.
Materials and methods
Sampling and acclimation of Eurytemora affinis
E. affinis was collected in November 2009 (Experiment 1 at 16°C) when the species is supposed to produce diapausing eggs in the field, and in February 2010 (Experiment 2 at 12°C) when the diapause is supposed to be achieved. Zooplankton was collected with a 200-µm mesh size net suspended near the Tancarville bridge at low salinity (1–5 psu), the in situ salinity of E. affinis at maximum abundance (Mouny & Dauvin Citation2002). The collected zooplankton (mainly E. affinis) was stored in 30-L isotherm tanks and transported to the laboratory, where it was placed in a 45-L aquarium containing diluted sea water (adjusted to 15 psu) either at 16°C and 9:15 h L:D photoperiod (natural photoperiod) for Experiment 1 or at 12°C and 11:13 L:D photoperiod for Experiment 2 (natural photoperiod).
Production of nauplii
After 48 h of acclimation to laboratory conditions, about 200 ovigerous females were individually sorted and dispatched in six beakers of 2 L of diluted seawater (adjusted to 15 psu) in the same conditions of temperature and photoperiod described above (40 ovigerous females per beaker). After 2 days, nauplii (N1–N3) were mixed and about 30 nauplii were dispatched in each of 21 beakers containing 200 mL of diluted seawater (adjusted to 15 psu) and then placed in the experimental device ().
Description of the experimental device
An experimental device was designed in order to create different photoperiod conditions. This system was composed of six “boxes” (, C), equipped with two white light-emitting diodes (LED) controlled by a time switch (, D). The light intensity during experimentation was about 0.4 ± 0.2 µE m−2 s−1.
Description of the experimental procedure
The effect of photoperiod on E. affinis diapausing egg production was investigated at 12 ± 1°C and 16 ± 1°C using experimental L:D photoperiods as follows: 0:24, 8:16, 12:12, 16:8, 24:0 h. An additional photoperiod (11L:13D) was used for the second experiment at 12°C. Three replicates were conducted for each incubation regime. Three additional replicates were performed for conditions 8:16 and 16:8 in order to achieve a change in conditions when the nauplii reached the instar N6–C1 and to verify the sensitivity of the naupliar instars to the photoperiod experienced during their development, as shown mainly in cyclopoid species (Alekseev Citation1990) or in E. affinis (Ban Citation1992). Food was provided immediately and then at 2-day intervals in the form of 6 mL of Rhodomonas marina (P.A. Dangeard) Lemmermann, 1899 culture with a concentration (~0.7–1 million cells mL−1) similar to those used in other feeding studies (Souissi et al. Citation2010). Water in the culture was changed only every 2 weeks in order to minimize the effect of stress.
Hatching experiments
After about 30 days (when the copepods had reached adulthood), egg sacs were observed daily under a binocular microscope to determine hatching success. One week of observation was sufficient to determine whether subitaneous (S) or dormant eggs had been produced, as the former hatch in 3–4 days. This procedure was repeated on the first two clutches.
If we did not observe hatching within 3–4 days, eggs were suspected to be dormant and placed in optimal conditions (16°C, water changed every 2 days) to be sure that no hatching occurred. After about 3 weeks of observation, the dormant eggs were placed at low temperature (4–5°C) for 3 months and then again in optimal conditions. This period of 3 months at low temperature, simulating winter conditions (Cáceres & Schwalbach Citation2001; Vandekerkhove et al. Citation2005), usually allows the breaking of the dormancy of diapausing eggs. If hatching occurred after this refractory phase, the eggs were categorised as diapausing (D). The remaining eggs that contain lipidic material were considered full but non-hatched (Fnh). In contrast, empty ones were considered non-viable (Non).
Statistical analysis
The number of diapausing eggs produced during the experiments was very low. Consequently, we pooled all data obtained from different females incubated at each experimental condition (see results in ). Then, we used Chi-square to test the differences between the first and second clutches for each experimental condition. We first compared the differences in egg distribution by using three classes of eggs: “subitaneous”, “diapausing” and “unhatched”. The last class contains non-viable (Non) and full but non-hatched (Fnh) eggs, shown in . Then, we considered two classes of eggs: “non-diapausing” (“subitaneous” + “unhatched”) and “diapausing”. The use of two classes allowed us to reduce the weight of unhatched eggs that were apparently different between clutches.
Table I. Number of subitaneous (S), diapausing (D), non-viable (Non) and full but non-hatched (Fnh) eggs produced by females raised from naupliar instars (N1–N3) under six photoperiods at 12 and 16°C. The number of eggs was counted for the first and the second clutch. For each clutch, the number of females (N) obtained from naupliar instars and producing the different kind of eggs was indicated. (–) missing data. The correlation between photoperiod and clutch size (# subitaneous eggs/female/clutch) was only observed for the first clutch at 16°C (Spearman rho = 1, p < 0.001).
Finally for each temperature and each clutch, a non-parametric Spearman’s rank correlation coefficient was calculated between photoperiod (expressed as the light duration) and the mean number of eggs produced per female.
All statistical analyses were performed with Matlab Software (Mathworks Inc., version 7.5).
Results
Proportions of types of eggs produced by the females of Eurytemora affinis reared from naupliar instars under different combinations of photoperiod at both 16 and 12°C are presented in and .
Figure 2. Percentage of different kind of eggs (subitaneous eggs, diapausing eggs, full but non-hatched eggs and non viable eggs) produced by Eurytemora affinis under 5–6 photoperiods at (A) 12°C and (B) 16°C. For each photoperiod condition, the first histogram corresponds to the first clutch, and the second histogram to the second clutch.
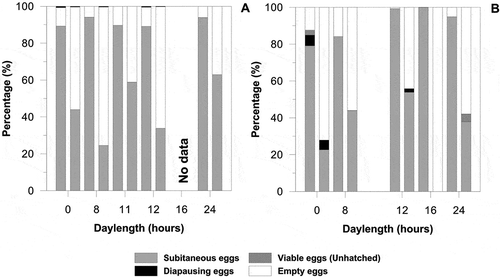
The proportion of subitaneous eggs was very high irrespective of photoperiod and temperature conditions (, ). The percentage of subitaneous eggs decreased systematically between the first and second clutch while the number of non-viable (empty) eggs increased. For the first clutch, the percentage of non-viable eggs did not exceed 15% of total eggs produced, while during the second cltuch, values were never below 40% of total eggs.
The use of the Chi-square test and three classes of eggs, “subitaneous”, “diapausing” and “unhatched”, confirmed that the differences between the first and second clutches were systematically highly significant (p < 0.001). In contrast, when olny two classes of eggs were used (“non-diapausing” and “diapausing”), only a single experimental condition (T = 16°C and photoperiod 12:12) showed a statistically significant difference between clutches (p = 0.019). In general, diapausing eggs were only found for short-day photoperiodic conditions ranging from 0 to 12 h of light. At 12°C (mothers caught in the field in February), the percentage of diapausing eggs did not exceed 0.72% of total eggs produced. In contrast, at 16°C (mothers caught in the field in November), the percentage of diapausing eggs of total eggs produced varied from 1.95 to 5.78%. The maximal value was observed under the condition of constant darkness.
Proportion of subitaneous, diapausing, non-viable and full but non-hatched eggs produced by the females of E. affinis reared from naupliar instars (N1–N3) at two photoperiods (08:16, 16:08), then switched to opposite photoperiod at the instar N6–C1, are summarized in for both experiments at 12 and 16°C.
Table II. Percentage of subitaneous (S), diapausing (D), non-viable (Non) and full but non-hatched (Fnh) eggs produced by females raised from naupliar instars (N1–N3) under two photoperiods at 12 and at 16°C, then switched to the opposite photoperiod during instar (N6-C1). The number of eggs was counted for the first and the second clutch. For each clutch, the number of females (N) obtained from naupliar instars and producing the different kind of eggs was indicated. (–) missing data.
At 12°C, none of the N6–C1 transferred to the 08:16 (L:D) condition reached adulthood. At 16°C, females mainly produced subitaneous eggs during the first clutch (96.1 %) and non-viable eggs in the second clutch (74.1%).
For individuals transferred to a photoperiod of 16:08, mostly subitaneous eggs were observed for the first clutch, either at 12 or 16°C (respectively, 72.9 and 98.4%). At 12°C, 5.9% of diapausing eggs were produced ().
In the second clutch, the majority of eggs produced were non-viable (> 67.1%), although diapausing eggs (1.7%) and full but non-hatched eggs (8.1%) were also observed ().
Discussion
In the Seine estuary, the mesozooplanktonic community is structured along the salinity gradient. Eurytemora afffinis is found in the low-salinity zone (0.5 psu < salinity < 15 psu) where it can represent 90–99% of the mesozooplanktonic species abundance (Mouny & Dauvin Citation2002) and reach up 700,000 ind m−3 near the bottom during the spring (Devreker et al. Citation2008). In contrast to what is observed in Japan, where the species is known to disappear entirely from the water column in winter (Ban & Minoda Citation1989), E. affinis is known to reproduce throughout the year in many estuaries of the northern hemisphere (Cronin et al. Citation1962; Jeffries Citation1962; Haertel et al. Citation1969; De Pauw Citation1973; Soltanpour-Gargari & Wellershaus Citation1985; Baretta & Malschaert Citation1988). For these populations, the production of diapausing eggs was revealed only after extraction of eggs from sediment layers (Madhupratap et al. Citation1996; Katajisto et al. Citation1998; Albertsson & Leonardsson Citation2000, Citation2001). In the Seine estuary, diapausing eggs were also found (Glippa et al. Citation2011) and we assessed here the effect of photoperiod on diapausing egg production in E. affinis.
In this study, we show that the late naupliar instars are sensitive to the photoperiodic signal. Indeed, the same proportions concerning the nature of eggs laid (subitaneous, diapausing) was observed for copepods reared from their naupliar instars under the same photoperiod, and this occurred whether or not they were transferred after the instar N6–C1 to another photoperiod. This suggests, as shown by Ban (Citation1992), that the type of eggs produced (subitaneous or diapausing) by females of E. affinis is determined in response to environmental conditions encountered during their naupliar instars. Similar patterns were observed in cyclopoid copepods and in some insects (Beck Citation1980; Alekseev et al. Citation2007).
In our experiment, we already showed using the Chi-square test (p < 0.001) that the main differences between the first and the second clutch are due to the high proportion of non-viable eggs in the second clutch (). It is possible that the majority of the second clutch eggs are not viable due to the non-fertilization of these eggs. Indeed, females of E. affinis appear to have limited storage capacity of sperm (Barthélémy Citation1998; Souissi Citation2010) and seem to need repeated matings to remain fertile (Heinle Citation1970; Katona Citation1975).
However, production of diapausing eggs has been demonstrated from the incubation of dormant eggs after a chilling period (3 months at low temperature). Diapausing eggs were produced under photoperiodic conditions less than or equal to 12 h of light (, ) either at 12°C or 16°C. These results suggest that the diapausing egg production in E. affinis is triggered by photoperiodic conditions of short days (< 12 h), as has been shown for the same species in Japan in Lake Ohnuma (Ban Citation1992) or for the marine calanoid copepod Labidocera aestiva Wheeler, 1901 in the Atlantic (Marcus Citation1980). It was also shown that a maximum of diapausing eggs was produced under constant darkness (, ). This does not show that the induction of diapausing egg production in this species needs a light signal, but means that life in lightless conditions is accepted by E. affinis as short day length as has been shown many times in other crustaceans (Alekseev et al. Citation2007). In turbid estuarine water, it is therefore possible that light signal is too weak for it to be a trigger for diapause for this species, so it can be substituted by lightless conditions and low temperature. Indeed, it is known that in estuaries, confluence areas between river and marine waters, particularly in macrotidal estuaries, a maximum turbidity zone is formed (Dyer Citation1986). These conditions of turbidity (up to 2.30 g l−1 of SPM in the Seine estuary, Glippa Citation2011) will limit the light penetration and the light signal will attenuate rapidly with depth.
However, it is difficult to define the accurate pattern of light conditions encountered by the population of E. affinis living in the Seine estuary and strongly related to the tidal regime (Devreker et al. Citation2008).
This experimental study failed to show a strong signal of photoperiod on the induction of diapausing egg production, as has been observed in Japan. However, our data show that the experiment conducted with nauplii reared from females caught in the Seine in November (when they are supposed to produce diapausing eggs in the field) produced more eggs than did those reared from females collected in February (12°C). Indeed, a maximum of 1 ± 3 diapausing eggs female−1 was observed in our experiment. This could be due to the fact that E. affinis in Europe and Japan are genetically very different (Lee Citation2000) and that this implies different responses to the tested parameters.
In addition, temperature conditions in the Seine estuary (min. 6°C in winter; up to 22°C in summer) are less extreme than those encountered in the Japanese Lake Ohnuma (surface temperature below 4°C from December to April, up to 28°C in August; Ban & Minoda Citation1989). It has been shown that a drop in temperature (from 15 to 10°C) affects, in the Seine estuary, all reproductive parameters in E. affinis (Devreker et al. Citation2007, Citation2009), reducing the egg production rate from 13 to 4 eggs females−1 day−1. Ovigerous females and nauplii were found in the Seine estuary during a severe winter (5°C in 2005; Devreker et al. Citation2010), indicating that even under low temperatures, this species is able to have successful reproduction.
Nevertheless, our previous study showed that diapausing eggs of this species were present in the Seine sediments (Glippa Citation2011). They represented, in mid-fall 2008, about 7% of the total number of eggs hatched (42,000 nauplii m−3 month−1). In the hatching experiment, a maximum of 6% of diapausing eggs was produced for the total darkness condition. These results suggest that the diapausing egg production of E. affinis in these latitudes is not essential for winter survival since population reproduction with subitaneous eggs is possible in the water column throughout the year. The planktonic E. affinis population could be replaced after the winter period from two sources, as shown in the Baltic Sea (Katajisto et al. Citation1998): (1) hatching of benthic resting eggs; (2) hatching of eggs produced by females that have survived the winter.
However, despite the low production of diapausing eggs, their accumulation in sediments could constitute an egg bank, acting as a long-term survival strategy (beyond 1 year), or to overcome adverse conditions other than seasonal changes in environmental conditions.
The population of E. affinis in the Seine estuary seems to privilege the “plankton” strategy as females remain fertile throughout the year. We believe that the adoption of diapause in E. affinis is flexible and may represent a microevolutionary adaptive strategy of each population.
Glippa (Citation2011) already showed that nauplii of E. affinis can emerge from diapausing eggs, using a sample from the Seine in early summer. Values lower than 10,000 nauplii m−3 month−1 were found in this period. This could constitute a bet-hedging strategy in response to a highly disturbed environment (Menu et al. Citation2000; Meyers & Bull Citation2002). Females of E. affinis produce diapausing eggs in different periods (mid-fall, early summer) in order to give more abilities to the offspring, and thus enable them to find the best conditions (salinity, temperature, food and others) to develop and improve the recruitment of the population. This study represents a first step towards understanding whether the photoperiod (most regular seasonal factor) can induce diapausing egg production in a key species of the low-salinity zone of the Seine estuary. Other factors like the effect of overcrowding, or stress induced by the presence of Acartiidae in the summer, are important to consider in future studies. It is also important to expand the study area to test this hypothesis in another estuary or at other latitudes.
Acknowledgements
This study was carried out within the framework of the Seine-Aval scientific program and it is a contribution to the ZOOSEINE and BIODISEINE projects. Victor Alekseev was supported by Grant No. 11-04-00195-а from the Russian Foundation for Basic Research. The authors are grateful to Dominique Menu, Didier Barras and Laurent Brutier for the design and construction of the induction system.
References
- Albertsson J, Leonardsson K. 2000. Impact of a burrowing deposit-feeder, Monoporeia affinis, on viable zooplankton resting eggs in the northern Baltic Sea. Marine Biology 136:611–619.
- Albertsson J, Leonardsson K. 2001. Deposit-feeding amphipods (Monoporeia affinis) reduce the recruitment of copepod nauplii from benthic resting eggs in the northern Baltic Sea. Marine Biology 138:793–801.
- Alekseev VR. 1990. Diapause in crustaceans: ecological and physiological aspects. Moscow: Nauka Academic Publishers, [in Russian].
- Alekseev VR, De Stasio BT, Gilbert JJ. 2007. Diapause in aquatic invertebrates: theory and human use. Monographiae biologicae. v. 84. Dordrecht: Springer.
- Avery DE. 2005a. Induction of embryonic dormancy in the Calanoid copepod Acartia hudsonica: proximal cues and variation among individuals. Journal of Experimental Marine Biology and Ecology 314:203–214.
- Avery DE. 2005b. Induction of embryonic dormancy in the Calanoid copepod Acartia hudsonica: heritability and phenotypic plasticity in two geographically separated populations. Journal of Experimental Marine Biology and Ecology 314:215–225.
- Ban S. 1992. Effects of photoperiod, temperature, and population density on induction of diapause egg production in Eurytemora affinis (Copepoda:Calanoida) in lake Ohnuma; Hokkaido, Japan. Journal of Crustacean Biology 12:361–367.
- Ban S, Minoda T. 1989. Seasonal distribution of Eurytemora affinis (Poppe, 1880) (Copepoda; Calanoida) in freshwater Lake Ohnuma, Hokkaido. Bulletin of the Faculty of Fisheries Hokkaido University 40:147–153.
- Ban S, Minoda T. 1991. The effect of temperature on the development and hatching of diapause and subitaneous eggs in Eurytemora affinis (Copepoda: Calanoida) in lake Ohnuma, Hokkaido, Japan. Bulletin of the Plankton Society of Japan Special Volume:299–308.
- Ban S, Minoda T. 1992. Hatching of diapause eggs of Eurytemora affinis (Copepoda: Calanoida) collected from lake-bottom sediments. Journal of Crustacean Biology 12:51–56.
- Ban S, Minoda T. 1994. Induction of diapause egg production in Eurytemora affinis by their own metabolites. Hydrobiologia 292–293:185–189.
- Baretta JW, Malschaert JFP. 1988. Distribution and abundance of the zooplankton of the Ems estuary (North Sea). Netherlands Journal of Sea Research 22:69–81.
- Barthélémy RM. 1998. Female genital structures in several families of Centropagoidea (Copepoda: Calanoida). Philosophical Transactions of the Royal Society B: Biological Sciences 353:721–736.
- Beck SD. 1980. Insect photoperiodism. 2nd ed. New York: Academic Press.
- Beyrend-Dur D, Souissi S, Devreker D, Winkler G, Hwang JS. 2009. Life cycle traits of two transatlantic populations of Eurytemora affinis (Copepoda: Calanoida): salinity effects. Journal of Plankton Research 31:713–728.
- Boyer S, Bonnet D. 2013. Triggers for hatching of Paracartia grani (Copepoda: Calanoida) resting eggs: an experimental approach. Journal of Plankton Research 35:668–676.
- Castel J. 1995. Long-term changes in the population of Eurytemora affinis (Copepoda, Calanoida) in the Gironde estuary (1978–1992). Hydrobiologia 311:85–101.
- Chen F, Marcus NH. 1997. Subitaneous, diapause, and delayed-hatching eggs of planktonic copepods from the northern Gulf of Mexico: morphology and hatching success. Marine Biology 127:587–597.
- Cronin LE, Daiber JC, Hulbert EM. 1962. Quantitative seasonal aspects of zooplankton in the Delaware River Estuary. Chesapeake Science 3:63–93.
- Cáceres CE, Schwalbach MS. 2001. How well do laboratory experiments explain field patterns of zooplankton emergence? Freshwater Biology 46:1179–1189.
- Dauvin JC, Desroy N. 2005. The food web in the lower part of the Seine estuary: a synthesis of existing knowledge. Hydrobiologia 540:13–27.
- David V, Sautour B, Chardy P. 2007. Successful colonization of the calanoid copepod Acartia tonsa in the oligo-mesohaline area of the Gironde estuary (SW France)-Natural or anthropogenic forcing? Estuarine. Coastal and Shelf Science 71:429–442.
- De Pauw N. 1973. On the distribution of Eurytemora affinis (POPPE) (Copepoda) in the Western Scheldt Estuary. Verhandlungen des Internationalen Verein Limnologie 18:1462–1472.
- Devreker D, Souissi S, Forget-Leray J, Leboulenger F. 2007. Effects of salinity and temperature on the post-embryonic development of Eurytemora affinis (Copepoda; Calanoida) from the Seine estuary: a laboratory study. Journal of Plankton Research 29:i117–i133.
- Devreker D, Souissi S, Molinero JC, Beyrend-Dur D, Gomez F, Forget-Leray J. 2010. Tidal and annual variability of the population structure of Eurytemora affinis in the middle part of the Seine Estuary during 2005. Estuarine, Coastal and Shelf Science 89:245–255.
- Devreker D, Souissi S, Molinero JC, Nkubito F. 2008. Trade-offs of the copepod Eurytemora affinis in mega-tidal estuaries: insights from high frequency sampling in the Seine estuary. Journal of Plankton Research 30:1329–1342.
- Devreker D, Souissi S, Winkler G, Forget-Leray J, Leboulenger F. 2009. Effects of salinity, temperature and individual variability on the reproduction of Eurytemora affinis (Copepoda; Calanoida) from the Seine estuary: A laboratory study. Journal of Experimental Marine Biology and Ecology 368:113–123.
- Dur G, Souissi S, Devreker D, Ginot V, Schmitt FG, Hwang JS. 2009. An individual-based model to study the reproduction of egg bearing copepods: Application to Eurytemora affinis (Copepoda Calanoida) from the Seine estuary, France. Ecological Modelling 220:1073–1089.
- Dyer K. 1986. Coastal and estuarine sediment dynamics. London: John Wiley & Sons ed.
- Escaravage V, Soetaert K. 1995. Secondary production of the brackish copepod communities and their contribution to the carbon fluxes in the Westerschelde estuary (The Netherlands. Hydrobiologia 311:103–114.
- Gasparini S, Castel J, Irigoien X. 1999. Impact of suspended particulate matter on egg production of the estuarine copepod, Eurytemora affinis. Journal of Marine Systems 22:195–205.
- Glippa O. 2011. Rôle des oeufs de résistance dans la dynamique des populations des principales espèces de copépodes calanoïdes: Cas de l‘estuaire de la Seine. Thèse de Doctorat, Université de Lille 1:200.
- Glippa O, Souissi S, Denis L, Lesourd S. 2011. Calanoid copepod resting egg abundance and hatching success in the sediment of the Seine estuary (France). Estuarine, Coastal and Shelf Science 92:255–262.
- Grice GD, Marcus NH. 1981. Dormant eggs of marine copepods. Oceanography and. Marine Biology - An Annual Review 19:125–140.
- Gyllström M, Hansson LA. 2004. Dormancy in freshwater zooplankton: induction, termination and the importance of benthic-pelagic coupling. Aquatic Sciences 66:274–295.
- Haertel L, Osterberg C, Curl Jr H, Park PK. 1969. Nutrient and plankton ecology of the Columbia River estuary. Ecology 50:962–978.
- Hairston Jr NG, Kearns CM. 1995. The Interaction of photoperiod and temperature in diapause timing: a copepod example. The Biological Bulletin 189:42–48.
- Hairston NG, Dillon TA, De Stasio BT. 1990. A Field test for the cues of diapause in a freshwater copepod. Ecology 71:2218–2223.
- Heinle DR. 1970. Population dynamics of exploited cultures of calanoid copepods. Helgoland Marine Research 20:360–372.
- Jeffries HP. 1962. Salinity space distribution of the estuarine copepod genus Eurytemora. Internationale Revue der gesamten Hydrobiologie und Hydrographie 47:291–300.
- Katajisto T. 1996. Copepod eggs survive a decade in the sediments of the Baltic Sea. Hydrobiologia 320:153–159.
- Katajisto T, Viitasalo M, Koski M. 1998. Seasonal occurence and hatching of calanoid eggs in sediments of the northern Baltic Sea. Marine Ecology Progress Series 163:133–143.
- Katona SK. 1975. Copulation in the copepod Eurytemora affinis (Poppe, 1880). Crustaceana 28:89–95.
- Lee CE. 1999. Rapid and repeated invasions of fresh water by the copepod Eurytemora affinis. Evolution 53:1423–1434.
- Lee CE. 2000. Global phylogeography of a cryptic copepod species complex and reproductive isolation between genetically proximate “populations”. Evolution 54:2014–2027.
- Madhupratap M, Nehring S, Lenz J. 1996. Resting eggs of zooplancton (Copepoda and Cladocera) from the Kiel Bay and adjacent waters (southwestern Baltic). Marine Biology 125:77–87.
- Marcus NH. 1980. Photoperiodic control of diapause in the marine calanoid copepod Labidocera aestiva. The Biological Bulletin 159:311–318.
- Marcus NH. 1982. The reversibility of subitaneous and diapause egg production by individual females of Labidocera aestiva (Copepoda: Calanoida). The Biological Bulletin 162:39–44.
- Mauchline J. 1998. Advances in marine biology - the biology of calanoid copepods. London: Academic Press 33:1–710.
- Menu F, Roebuck JP, Viala M. 2000. Bet-hedging diapause strategies in stochastic environments. The American Naturalist 155:724–734.
- Meyers LA, Bull JJ. 2002. Fighting change with change: adaptive variation in an uncertain world. Trends in Ecology & Evolution 17:551–557.
- Mouny P, Dauvin JC. 2002. Environmental control of mesozooplankton community structure in the Seine estuary (English Channel). Oceanologica Acta 25:13–22.
- Peitsch A, Köpcke B, Bernát N. 2000. Long-term investigation of the distribution of Eurytemora affinis (Calanoida; Copepoda) in the Elbe Estuary. Limnologica - Ecology and Management of Inland Waters 30:175–182.
- Piercey DW, Maly EJ. 2000. Factors influencing the induction of diapausing egg production in the calanoid copepod Diaptomus leptopus. Aquatic Ecology 34:9–17.
- Radzikowski J. 2013. Resistance of dormant stages of planktonic invertebrates to adverse environmental conditions. Journal of Plankton Research. 35:707–723.
- Soltanpour-Gargari A, Wellershaus S. 1985. Eurytemora affinis-one year study of abundance and environmental factors. Veröffentlichungen des Instituts für Meeresforschung in Bremerhaven 20:183–198.
- Souissi A. 2010. Etude de la plasticité et de la morphologie d‘un copépode estuarien: comparaison intercontinentale. Thèse de Doctorat, Université de Lille 1. 233 pp.
- Souissi A, Souissi S, Devreker D, Hwang JS. 2010. Occurence of intersexuality in a laboratory culture of the copepod Eurytemora affinis from the Seine estuary (France). Marine Biology 157:851–861.
- Tackx MLM, Herman PJM, Gasparini S, Irigoien X, Billiones R, Daro MH. 2003. Selective feeding of Eurytemora affinis (Copepoda, Calanoida) in temperate estuaries: model and field observations. Estuarine, Coastal and Shelf Science 56:305–311.
- Tauber MJ, Tauber CA, Masaki S. 1986. Seasonal adaptations of insects. USA: Oxford University Press.
- Uye SI. 1985. Resting egg production as a life history strategy of marine planktonic copepods. Bulletin of Marine Science 37:440–449.
- Vandekerkhove J, Declerck S, Brendonck L, Conde-Porcuna JM, Jeppesen E, Johansson LS, De Meester L. 2005. Uncovering hidden species: Hatching diapausing eggs for the analysis of cladoceran species richness. Limnology and Oceanography: Methods 3:399–407.
- Walton WE. 1985. Factors regulating the reproductive phenology of Onychodiaptomus birgei (Copepoda: Calanoida). Limnology and Oceanography 30:167–169.
- Watson NHF, Smallman BN. 1971. The role of photoperiod and temperature in the induction and termination of an arrested development in two species of freshwater cyclopia copepods. Canadian Journal of Zoology 49:855–862.
- Wu L-S, Wang GZ, Jiang XD, Li SJ. 2007. Seasonal reproductive biology of Centropages tenuiremis (Copepoda) in Xiamen waters, People’s Republic of China. Journal of Plankton Research 29:437–446.
![Figure 1. A, description of the induction system (1, photoperiod of 8:16 light:dark; 2, 12:12; 3, 16:8; 4, 24:0; 5, no condition; 6, 0:24). B, electrical mechanism. C, compartment with beaker and lighting system. D, zoom on the lighting system [two light-emitting diodes (LEDs)].](/cms/asset/cbf412c9-690f-4e58-b727-f09941204370/tizo_a_861024_f0001_oc.jpg)