Abstract
Sparid fishes represent one of the major radiations of predominantly temperate-water benthic fishes. Previous molecular phylogenetic studies suggested that many traditional taxonomic groups, often based on dentition characters, do not correspond to monophyletic groups, suggesting repeated convergence in trophic ecology. In spite of the rich sparid fossil record, no comprehensive, multi-locus timetree based on sparid fossils currently exists for this group. We used a supermatrix approach to assemble a dataset of five loci and 91 sparid species, and time-calibrated this new phylogeny using eight sparid fossils. Our study corroborates the non-monophyly of the traditional sparids without the inclusion of the family Centracanthidae, as well as that of many sparid genera. Based on phylogenetic comparative analyses we find robust support for a scenario of multiple radiations and suggest that these were driven by the invasion of multiple geographic regions by different lineages, as well as by the transition to different trophic ecologies.
Introduction
During the past few years a great deal of attention has been devoted to investigating teleost fish radiations in marine ecosystems. The bulk of the research has focused on scleractinian coral reef-associated lineages such as pufferfishes, triggerfishes and allies (Alfaro et al. Citation2007; Santini et al. Citation2013b, Citation2013c, Citation2013d); wrasses and parrotfishes (Alfaro et al. Citation2009a; Kazancioglu et al. Citation2009; Price et al. Citation2011); surgeonfishes (Klanten et al. Citation2004; Sorenson et al. Citation2013); butterflyfishes (Cowman & Bellwood Citation2011); and damselfishes (Cowman & Bellwood Citation2011; Frédérich et al. Citation2013). Non-reef-associated lineages represent the vast majority of marine fish biodiversity but, with the exception of icefishes and allies (Rutschmann et al. Citation2011; Lautrédou et al. Citation2012; Near et al. Citation2012), have so far received relatively scant attention (e.g., Ruber & Zardoya Citation2005; Santini & Sorenson Citation2013; Santini et al. Citation2013a).
The roughly 130 species of seabreams, porgies, and allies (Sparidae, Acanthomorpha) represent one of the major radiations of demersal percomorph fishes in subtropical and temperate coastal marine and brackish waters. Their rich fossil record, dating to the Early Paleogene, shows that sparids have long been a prominent component of coastal ecosystems (e.g., Day Citation2003). These fishes are a major component of fish communities found over both rocky reefs and sandy habitats in the Northeastern Atlantic and Mediterranean, the Western and Southern African coast, the Western Indian Ocean, the Central Western Atlantic, as well as the Northwestern Pacific. Sparid species are also found in New Zealand waters, Southern Australia, the Red Sea and the Eastern Pacific. Interestingly, within each of these geographic regions, multiple unrelated lineages seem to have converged to occupy similar ecological niches and trophic levels (e.g., Hanel & Sturmbauer Citation2000; Antonucci et al. Citation2009). A number of transitions between durophagous and non-durophagous diets (based on consumption of hard-bodied prey, be these gastropods, bivalves or crustaceans), and repeated evolution of herbivory, piscivory and molluscivory (Antonucci et al. Citation2009; Chiba et al. Citation2009) have occurred, suggesting that this group may have undergone multiple convergent radiations. Under this scenario (also known as iterative radiations) radiating lineages invade similar environments in which they experience comparable selective pressures. As a result, phenotypic characters may be constrained by morphological integration, competition, development or pleiotropic effects (Losos et al. Citation1998; Losos & Ricklefs Citation2009; Losos Citation2011), and this could result in subclades containing similar phenotypic and functional diversity, as observed in a number of studies (e.g., Losos et al. Citation1998; Frédérich et al. Citation2013).
Sparidae includes several economically important species, some of which are extensively cultured in fish farms (e.g., Hempel Citation1993; Basurco et al. Citation2011), and subjects of a number of genome manipulation programs (e.g., Garrido-Ramos et al. Citation1996). Despite their broad economic relevance, Sparidae systematics remains in a state of flux and very little is known about the origin and evolution of this heterogeneous group. Their classification is primarily based on dentition type, meristic features, and squamation patterns livery, leading to the recognition of a variable number of subfamilial groups (Smith Citation1938; Akazaki Citation1962; Smith & Smith Citation1986; Fiedler Citation1991). While a number of heterodontous oral jaw types currently used to identify sparid groups appear to be unique within percomorph fishes (Vandewalle et al. Citation1995), the use of dentition patterns for systematic purposes appears too simplistic according to several recent studies (Hanel & Sturmbauer Citation2000; Day Citation2002; Orrell et al. Citation2002; Orrell & Carpenter Citation2004; Chiba et al. Citation2009). These studies revealed that many of the traditional taxonomic groups (subfamilies and genera) are not monophyletic, suggesting that various dental characters are evolutionarily plastic and are likely the product of convergence among sparid lineages.
A number of previous phylogenetic studies, based on selected mitochondrial markers (Cytb, 16s) and fairly limited sampling (Garrido-Ramos et al. Citation1995; Hanel & Sturmbauer Citation2000; Summerer et al. Citation2001; Orrell et al. Citation2002; Orrell & Carpenter Citation2004; Chiba et al. Citation2009; Hanel & Tsigenopoulos Citation2011), have hinted at paraphyly of the Sparidae, without the inclusion of the family Centracanthidae. To date, only one study has attempted to produce a sparid timetree (Chiba et al. Citation2009), which was calibrated using mostly secondary molecular calibration points from other dating studies and a single sparid fossil (non-sparid calibration points were also used in that study). Furthermore, no study has used phylogenetic comparative methods to formulate and quantitatively test hypotheses of sparid evolution.
In this study we used a supermatrix approach to assemble a dataset containing five loci for 91 species of sparid and centracanthid fishes, plus two outgroups. Our molecular phylogeny was then calibrated using eight fossils and used as a framework to test the hypothesis that sparids underwent multiple convergent evolutionary radiations.
Materials and methods
Phylogenetic analyses
We used the PhyLoTa browser version 1.5 (Sanderson et al. Citation2008) to obtain sequences for five loci for 91 species of sparid and centracanthid fishes: the mitochondrial cox1, Cytb and 16s, and the nuclear Rag1 and Rh (Table S1). A lethrinid and a nemipterid were selected as outgroups on the basis of recent studies of sparoid and percomorph fish relationships (i.e., Carpenter & Johnson Citation2002; Chiba et al. Citation2009; Near et al. Citation2013). We downloaded the alignments generated with MUSCLE (multiple sequence comparison by log expectation) (Edgar Citation2004) from PhyLoTa, checked them by eye in MEGA (Molecular Evolutionary Genetics Analysis) 5 (Tamura et al. Citation2011), and trimmed the 5’ and 3’ ends to minimize the amount of missing data. We ran gene tree analyses using the neighbor-joining method in MEGA 5 (Tamura et al. Citation2011) in order to test for potentially misidentified or contaminated sequences and for major disagreement among the various gene trees. We observed strong congruence among the five trees. We then concatenated the five loci using Mesquite 2.75 (Maddison & Maddison Citation2011). When multiple sequences were available for one species, we retained the longest sequence. Our final data matrix consisted of 600 bp for cox1, 1140 bp for Cytb, 552 bp for 16s, 1428 for Rag1 and 459 for Rh, for a total of 4179 nucleotides.
We used jModelTest (Posada Citation2008) to select the best fitting model of sequence evolution from the candidate pool of models that can be utilized in MrBayes 3.2 (Ronquist et al. Citation2012) using corrected Akaike Information Criterion scores (AICc). We did not include models with the proportion of invariant sites parameter in the candidate pool, as this parameter is already accounted for by the gamma parameter (Yang Citation2006). The HKY + G (Hasegawa, Kishino and Yano + gamma) model was selected as the most appropriate model for Rh, while GTR + G (generalized time-reversible + gamma) was chosen as the best model for cox1, Cytb, 16s, and Rag1.
We performed maximum likelihood analyses using RAxML (Randomized Accelerated Maximum Likelihood) (Stamatakis Citation2006) using the GTR + G model, the closest model to those selected by jModelTest that can be implemented in RAxML. We ran analyses using 1000 fast bootstraps. We ran Bayesian analyses using MrBayes 3.2 (Ronquist et al. Citation2012) after each gene partition had been assigned the model selected by jModelTest (Posada Citation2008). We ran two analyses each for 20 million generations with four chains (three heated, one cold), sampling every 1000 generations. We visually inspected the trace files in Tracer 1.5 (Drummond & Rambaut Citation2007) to verify that the chains had reached convergence. After discarding the first million generations as burnin, we combined the remaining trees to obtain a 50% majority rule consensus tree.
We selected eight fossil calibration points (Appendix I) that identify the minimum age of major sparid fish lineages, and used these in combination with the uncorrelated lognormal priors enforced in BEAST (Bayesian Evolutionary Analysis by Sampling Trees) 1.7.5 (Drummond & Rambaut Citation2007) to infer a relaxed clock molecular timetree. All five loci were assigned the same model selected by jModelTest, and assigned a birth-death prior to the rates of cladogenesis. We ran two sets of analyses with 50 million generations each, and sampling every 1000 generations. We used Tracer 1.5 (Drummond & Rambaut Citation2007) to inspect the chains for convergence, which we interpreted to have occurred when the ESS (effective sample size) for all parameters was over 200. We removed the first 10% of the trees as burnin, used LogCombiner to merge the remaining trees, and TreeAnnotator (Drummond & Rambaut Citation2007) to obtain a timetree.
Rates of lineage diversification, and phenotypic and ecological evolution
We used several comparative methods to investigate patterns of sparid lineage diversification, and phenotypic and ecological evolution. We investigated whether diet, trophic level, habitat or biogeography were correlated with the radiation of sparid subclades. We performed all comparative macroevolutionary analyses using R version 2.15 (R Development Core Team Citation2012), and functions contained in the packages Geiger (Harmon et al. Citation2008), Laser (Rabosky Citation2006), and APE (Analyses of Phylogenetics and Evolution) (Paradis et al. Citation2004).
We assessed the gamma statistic using the Monte Carlo constant rates (MCCR) test (Pybus & Harvey Citation2000). The gamma statistic indicates the extent to which a phylogeny differs from branching events expected under a constant-rates diversification model. We also tested for temporal slowdowns in diversification rates by comparing the fit of a pure birth model; a birth-death model of speciation with exponential and logistic density-dependent decline models of lineage accumulation (DDX and DDL, respectively); an exponentially declining speciation and constant extinction rate (SPVAR); a constant speciation, exponentially increasing extinction rate (EXVAR); and a variable speciation and extinction (BOTHVAR) (Rabosky & Lovette Citation2008) model, using Akaike information criterion (AIC) scores and Akaike weights (Burnham & Anderson Citation2002). The MCCR test and diversification model fitting were performed with the Laser package (Rabosky Citation2006). We also employed MEDUSA (Modeling Evolutionary Diversity Using Stepwise AIC) (Alfaro et al. Citation2009b) to detect potential diversification rate shifts on the timetree using the R package Geiger (Harmon et al. Citation2008). We collapsed branches and assigned 125 taxa (~96% of the total sparid diversity) to 12 terminal clades (Table S1). The remaining diversity was not included in the analysis because it could not be unambiguously assigned to one of the 12 terminal clades. Therefore our analysis is a conservative estimate of sparid diversification rates. AIC score improvement of four units or greater was used as the threshold for retaining rate shifts (Burnham & Anderson Citation2002).
We used Fishbase (Froese & Pauly Citation2013), as well as taxonomic literature (e.g., Smith & Smith Citation1986; Antonucci et al. Citation2009; FAO (Food and Agriculture Organization) identification sheets), to obtain information on maximum body size (total length, TL, the distance between tip of snout and longest lobe of caudal fin), dentition (which is linked to diet, since the presence of well-developed molariform teeth was interpreted as evidence of durophagous lifestyle), trophic level (using the TROPH index, which can be obtained from Fishbase), habitat association (benthopelagic, demersal, or rocky/coral reef association), and biogeography (using the geographic regions identified by Briggs & Bowen Citation2012). We used a maximum likelihood ancestral state reconstruction approach with an MK1 model in APE (Paradis et al. Citation2004) to map the origin and evolution of body size, diet (durophagous vs non-durophagous), trophic level, habitat association and biogeographic distribution () on the timetree. When one species was represented in Fishbase by multiple subspecies, with these distributed across vast geographic areas (e.g., Diplodus sargus), we only used data for the nominal subspecies to avoid using species-complexes as terminal taxa.
Table I. List of sparid species included in this study with data on total length (TL, in cm), habitat type (demersal, reef, benthopelagic), diet (durophagous, non-durophagous), trophic level expressed on the basis of the TROPH index (TROPH index > 4.2: TPs = TP; TROPH index 3.8–4.2: MPs = MP; TROPH index 2.8–3.8: low-predators = LP; TROPH index 2.2–2.8: omnivores = OM; TROPH index < 2.2: herbivore = HE), and biogeographic region. For species found in multiple geographic areas, the area that represents the largest component of their range is indicated in bold. IWP, Indo-West Pacific; WIO, Western Indian Ocean; SAUST, Southern Australia; WATL, Western Atlantic; SAFR, South Africa; EATL + MED, North Eastern Atlantic + Mediterranean; EPAC, Eastern Pacific; WAFR, Western Africa and Central Atlantic.
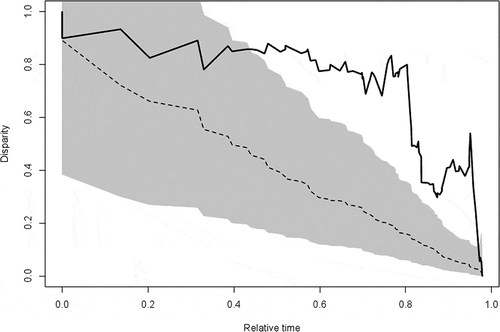
To test whether body size disparity, which we take as a proxy for ecological diversification (see Harmon et al. Citation2010; Slater et al. Citation2010), is distributed among or within subclades, we used APE (Paradis et al. Citation2004) and Geiger (Harmon et al. Citation2008) to calculate the mean subclade disparity through time (Harmon et al. Citation2003). We compared observed body size disparity with disparity expected under Brownian motion (BM) by simulating body size evolution 10,000 times across our tree, then plotting observed and simulated subclade disparity values versus node age. We quantified the difference in relative clade disparity and expectations under the null BM model by calculating the morphological disparity index (MDI) (Harmon et al. Citation2003). A positive MDI value indicates higher subclade disparity than expected.
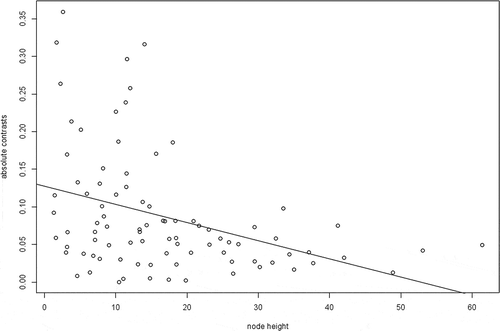
We used the node-height test (Freckleton & Harvey Citation2006) to determine whether the rate of body size evolution has slowed through time by computing the absolute value of the standardized independent contrasts (Felsenstein Citation1985) for body size, which are Brownian rate parameters for the branches over which they are calculated (McPeek Citation1995). We correlated the absolute value of the standardized independent contrasts (Felsenstein Citation1985) for body size with the node height at which they were calculated. A significant positive relationship between node age and absolute independent contrast value would indicate a slow-down in rates of body size evolution through time, which is consistent with a niche-filling scenario (Freckleton & Harvey Citation2006).
Results
Phylogenetic analysis and divergence time estimate
The topologies of the maximum likelihood and Bayesian analyses are highly congruent, so only the RAxML tree with bootstrap proportions (BSP) will be shown (). Sparids appear to be paraphyletic without the inclusion of the centracanthids, but monophyly of this clade is highly supported in both sets of analyses [100 bootstrap proportion (BSP) in the maximum likelihood analyses; posterior probability (PP) of 1.0 in the Bayesian analyses].
Figure 1. Maximum likelihood phylogenetic hypothesis inferred with RAxML (Randomized Accelerated Maximum Likelihood). Black circles indicate bootstrap proportion (BSP) over 80%; grey circles indicate BSP between 50 and 80%; white circles indicate BSP smaller than 50%.
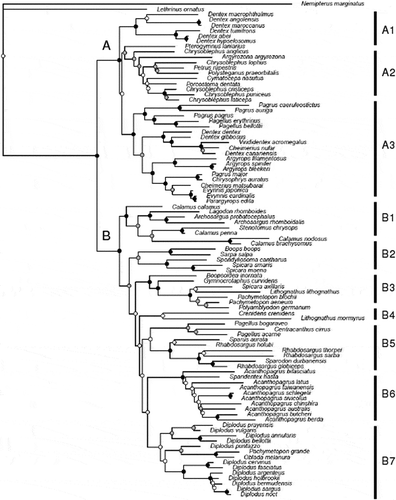
The expanded sparids appear to be composed of two large clades, which we name A and B following Chiba et al. (Citation2009) and Orrell & Carpenter (Citation2004). Clade A, which contains about one third of the sparid diversity and is supported by a 85% BSP and 1.0 PP, includes all species in our sampling from the genera Dentex, Pterogymnus, Chrysoblephus, Argyrozona, Polysteganus, Petrus, Cymatoceps, Porcostoma, Viridentex, Cheimerius, Argyrops, Evynnis, Pagrus and Chrysophrys, as well as two species of Pagellus. Most genera within this clade that are represented in our tree by multiple species (Dentex, Chrysoblephus, Cheimerius, Pagrus) appear to be non-monophyletic. In spite of this, several well-supported subclades can be identified: two Dentex groups; the first, which we labeled A1 (95% BSP, 0.75 PP), contains mostly Indo-Western Pacific and Western Central African species such as D. abei (Iwatsuki, Akazaki & Taniguchi, 2007), D. hypselosomus (Bleeker, 1854), D. tumifrons (Temminck & Schlegel, 1843), D. macrophtalmus (Bloch, 1791), D. maroccanus (Valenciennes, 1830) and D. angolensis (Poll & Maul, 1953); the second, A3 (85% BSP, 1.0 PP), contains several Northeastern Atlantic and Mediterranean species such as D. dentex (Linnaeus, 1758), D. gibbosus (Rafinesque, 1810) and D. canariensis (Steindachner, 1881), as well as Viridentex, Cheimerius, Argyrops, Evynnis, Pagrus, several species of Pagellus and Chrysophrys. The remaining diversity within the sparid clade A appears to belong to a third subclade (A2), poorly supported in both sets of analyses (46% BSP, 0.65 PP), but entirely composed of taxa distributed in or around South African temperate waters.
The second large sparid clade (clade B) appears to be much more diverse in terms of ecology, diet and geographic distribution: its monophyly, as well as that of several subclades within it, is highly supported by BSP > 80% or PP > 0.95 (). The first major subclade, B1, is a large Western Atlantic and Eastern Pacific radiation composed of the genera Calamus, Lagodon, Archosargus and Stenotomus. The second subclade, B2, contains the genera Sarpa and Boops (which appear as sister taxa), Spondyliosoma, as well as some species of the centracanthid genus Spicara. The third subclade, B3, includes Boopsoidea, Gymnocrotaphus, Lithognathus litognathus, Spicara axillaris (Boulenger, 1900), Pachymetopon and Polyamblyodon; a fourth subclade, B4, includes Lithognathus mormyrus (Linnaeus, 1758) and Crenidens. A fifth subclade, B5, includes Pagellus bogaraveo (Brünnich, 1768) and P. acarne (Risso, 1827), the monotypic centracanthid genus Centracanthus, Rhabdosargus, Sparus and Sparodon; a sixth subclade, B6, includes Acanthopagrus plus Sparidentex; and the last subclade, B7, is formed by Diplodus, Pachymetopon and Oblada.
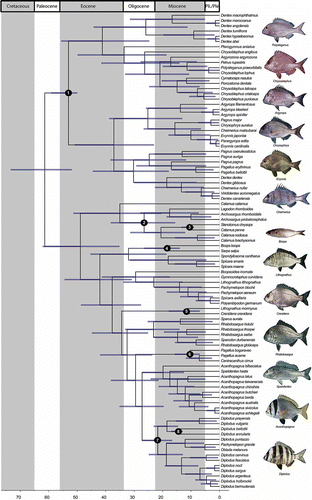
The topology of the BEAST timetree () is largely congruent with that of the RAxML and MrBayes analyses, although some differences exist for nodes that were poorly supported in the likelihood tree (e.g., the position of group A2, which appears sister to subclade A1 instead of A3). Our timetree suggests a Late Cretaceous age for the split between sparids and their close relatives (70 Ma), and a Paleocene origin for the split between sparid clades A and B (61 Ma, 52–73 Ma, 95% highest posterior density, or HPD). Clades A and B originate in the Early Eocene: 53 Ma (50–59, 95% HPD), and 49 Ma (35–62, 95% HPD), respectively. Within clade A, all subclades seem to have appeared in the Middle to Late Eocene and started diversifying during the Oligocene (26 Ma or older), while in the B clade, subclades 4–7 all have crown ages that are of Miocene age (23 Ma or younger).
Figure 2. BEAST chronogram of sparids. Blue bars indicate 95% HPD (highest posterior density); numbered black circles indicate fossil calibration points (see Appendix 1). Fish images modified under Creative Commons license from original photographs by J. E. Randall, A.S. Thorke Østergaard and J.-L. Justine (retrieved from www.fishbase.org).
Analyses of lineage and phenotypic diversification
The MCCR test finds evidence for a slowdown in the diversification rate during the evolution of sparids, a result confirmed by the lineage-through-time plot (Figure S1), even though this result is not statistically significant (γ = –2.680, ρ = 0.059). In order to determine if the pattern of evolution was driven by one of the major sparid subclades, we repeated the MCCR test for clades A and B individually. For clade A, the MCCR test resulted in a negative but not statistically significant value (γ = –1.789, ρ = 0.257), while for the B clade there was strong evidence for a slowdown in diversification (γ = –2.338, ρ = 0.010). Our diversification model-fitting results reveal strong support for a model of speciation with logistic density-dependent decline of lineage accumulation (DDL), with all other models having a ΔAIC of 4.9 or greater (Table S2). MEDUSA failed to detect any rate shifts within the sparid tree (results not shown), as a single rate appears to be the best explanation for the diversity of the entire tree (r = 0.070).
Ancestral state reconstruction of the geographic area (Figure S2) shows that most sparid subclades are geographically restricted: two large subclades (A2, B3) are found predominantly in South African temperate waters (one within each of the two major sparid clades) and one large radiation (B1) is shown to have occurred within the Western Atlantic and Eastern Pacific, while the remaining diversity is spread throughout the Indo-West Pacific (including the Western Indian Ocean) and the Northeastern Atlantic + Mediterranean. The extant diversity of sparids likely originated from an ancestor that inhabited the Central Tethys during the Early Paleocene; this is now reflected in the Northeastern Atlantic and Mediterranean distribution, as well as by the fossil record. From this ancestral region, a number of lineages successfully moved into different geographic regions, often invading the same biogeographic province more than once: the Indo-western Pacific was invaded at least three times, South Africa four times and the Western Atlantic twice. These invasions of new geographic regions show strong temporal correlation: the three Indo-Western Pacific invasions all occurred between the terminal part of the Eocene and the Early Miocene, while the three major invasions of South African waters all date from the Middle to Late Eocene.
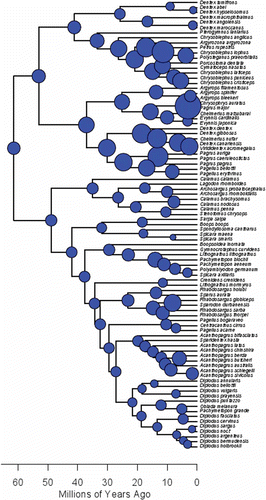
Ancestral state reconstruction of body size on the sparid timetree (expressed as the natural log of the total length) reveals several transitions in body size, with many subclades showing great variation, especially clade A (). The A2 and A3 subclades contain most sparid species reaching over 100 cm in total length, including some of the largest taxa, such as Cymatoceps nasutus (Castelnau, 1861), 150 cm TL; Dentex canariensis, 118 cm; and Petrus rupestris (Valenciennes, 1830), 200 cm. Not all components of clade A, however, reach large size, as several species of Dentex, Evynnis and Pagellus do not surpass lengths of 50 cm TL (e.g., Pagellus bellottii (Steindachner, 1882), 49 cm; Evynnis cardinalis (Lacepède, 1802), 40 cm; Dentex hypselosomus, 31 cm). Within clade B a number of species can also reach very large size (Sparodon durbanensis (Castelnau, 1861), 120 cm TL; Lithognathus lithognathus (Cuvier, 1829), 200 cm), but the vast majority remain below 70 cm in TL. Several lineages, however, seem to have experienced directional selection towards reduced body size, such as the Sarpa + Boops + Spondyliosoma + Spicara maena (Cuvier, 1829) + S. smaris (Cuvier, 1829) clade, and the Diplodus group.
Figure 3. Maximum likelihood reconstruction of ancestral body size on nodes of sparid tree. Circle size proportional to natural log of total length; body size data obtained from www.fishbase.org.
Reconstruction of diet, habitat association and trophic levels reveals multiple transitions between durophagous and non-durophagous diet (Figure S3) among demersal, reef and benthopelagic habitats (Figure S4), and multiple shifts from low-predator to mid- or top-predator (TP) (Figure S5). While durophagy appears to have been the ancestral diet in sparid fishes, at least five transitions to non-durophagy occurred within clade A, and an additional six in clade B. In clade A, many of these transitions mark a shift from a durophagous diet based mostly on shellfish to a diet that includes a substantial component of fishes, cephalopods or sea urchins, and a shift from a status of low-predator (as determined by the TROPH index) to one of mid- or TP (e.g., Petrus, Polysteganus, and the Dentex dentex group). In clade B a number of the transitions from durophagy to non-durophagy are, in contrast, linked to shifts in trophic status from low-predator to omnivore (including foraging on plankton) or herbivore, as seen in Sarpa salpa (Cuvier, 1829) and Crenidens crenidens (Forsskål, 1775).
Subclade disparity through time is significantly higher than expected under a Brownian motion model of body size evolution (MDI = 0.33, p = 0.003; ). The node-height test () resulted in a significantly negative relationship between node age and the absolute values of the standardized independent contrasts (r = –0.0024, d.f. = 86, ρ = 0.0003), indicating that there is an increase in the rate of body size evolution through time.
Figure 4. Mean subclade disparity through time (DTT) for sparid body size (lower solid line). Upper dashed line indicates the median subclade DTT based on 10,000 simulations of character evolution on the phylogeny under Brownian motion. The grey shaded area indicates the 95% DTT range for the simulated data.
Discussion
Phylogeny, fossil record and timescale of sparids
Our phylogenetic analyses infer results that largely agree with previous molecular studies of this family (Hanel & Sturmbauer Citation2000; Orrell et al. Citation2002; Orrell & Carpenter Citation2004; Chiba et al. Citation2009) suggesting paraphyly of the Sparidae without the inclusion of the Centracanthidae, non-monophyly of most of the traditional sparid genera, and the existence of two major, well-supported sparid clades (clades A and B). These results are not unexpected since our matrix includes most of the mitochondrial DNA data on which the previous studies were based, but are further strengthened by the presence of two nuclear loci used in our study. Within this enlarged sparid (including centracanthid) dataset we retrieve three subclades of “Dentex”-like forms (clades A1, A2 and A3), and eight subclades in the second main sparid group (B1 to B8), most of which show a strong biogeographic component. Within clade A, we found two subclades (A1, A3) that contain Eastern Atlantic + Mediterranean species, and Indo-Western Pacific taxa, plus evidence of a major radiation in South African temperate waters (A2). Within the second clade, we identified a Western Atlantic/Eastern Pacific radiation (B1, Calamus + Lagodon) that represents the sister taxon to all remaining members of this clade. Most of the diversity appears to be distributed in the Northeastern Atlantic + Mediterranean and in the Western Indian Ocean + South Africa. This extant distribution is likely a signal of an ancient Tethyan distribution that was fragmented by the closing of the Tethys realm, with the split between the sparid lineages having occurred between ∼ 25 and ∼ 40 Ma (Figure S2). Our topology suggests multiple invasions of temperate waters around the southernmost part of Africa from groups that were originally distributed in more northern latitudes [e.g., clade A3, Rhabdosargus holubi (Steindachner, 1881) vs Sparus; Pachymetopon vs Oblada, Lythognathus mormyrus vs Crenidens].
The molecular timescale that we infer reveals a Late Cretaceous/Early Paleocene age for the crown sparid, and an Eocene origin for almost all the major sparid subclades. This closely matches the oldest records of the family, which consist of isolated conical and subhemispherical teeth from the Thanetian (Late Paleocene) deposits of Ouled Abdoun, Morocco (Arambourg Citation1952), which were erroneously referred to the genus Sparus (see Marsili et al. Citation2007). The Paleocene existence of putative members of the family Sparidae has been also evidenced by the analysis of the otolith assemblages (Schwarzhans Citation2003, Citation2004; Schwarzhans & Bratishko Citation2011). The earliest articulated skeletal remains clearly belonging to the Sparidae date back to the Early Eocene. The oldest skeletal-based record of the family is Sciaenurus bowerbanki (Agassiz Citation1845) from the Ypresian (about 52 Ma) grey silty clay of the London Clay Formation, Isle of Sheppey, Kent, UK (Casier Citation1966; Day Citation2003). The celebrated Late Ypresian (about 50 Ma) fish assemblage from Monte Bolca, Italy, includes at least five sparid species arranged in four genera, Abromasta, Ellaserrata, Pseudosparnodus and Sparnodus (Day Citation2003; Bannikov Citation2006).
According to the phylogenetic analysis proposed by Day (Citation2003), Sciaenurus and the four genera from Monte Bolca are closely related to the extant Dentex dentex and should be included in the crown-group Sparidae. Several articulated skeletal remains assigned to the Sparidae are known from the Oligocene deposits of Europe, including the earliest taxa characterized by molariform teeth (e.g., Bassani Citation1889; Franceschi Citation1922; Switchenska Citation1979; Pharisat Citation1991; Micklich & Parin Citation1996), which date back to the Rupelian (about 31–29 Ma). The otolith record clearly indicates that some of the extant genera appeared early in the Miocene (see Müller Citation1999), exhibiting a vast radiation in the Mediterranean, Paratethys and subtropical Eastern Atlantic (Proto-Mediterranean Atlantic Region of Harzhauser et al. Citation2002). Sparid remains belonging to extant genera (e.g., Boops, Diplodus, Lagodon, Sparus) are relatively common in Middle Miocene deposits (between 15 and 12 Ma) of central and eastern Europe, and the Atlantic Coastal Plain (e.g., Anđelković Citation1989; Bannikov Citation1990, Citation2010; Schmid et al. Citation2001; Gregorová Citation2009; Carnevale & Godfrey, in press). Numerous well-preserved skeletal remains documenting the existence of a structurally heterogeneous sparid assemblage that includes representatives of the extant genera Boops, Crenidens, Dentex, Diplodus, Pagellus, Pagrus, Sparus, plus the extinct genus Paracalamus were described by Arambourg (Citation1927) from the Upper Miocene (Messinian, about 7 Ma) diatomaceous deposits of the Chelif Basin, close to the city of Oran, Algeria, and other localities of Messinian age of the Mediterranean basin (e.g., Bradley & Landini Citation1984; Carnevale Citation2002). Extant species mostly appeared in the record during the Pliocene (e.g., Purdy et al. Citation2001; Landini & Sorbini Citation2005).
Sparid radiations
Our comparative macroevolutionary analyses reveal a very complex scenario for the evolution of sparids, and suggest that this group may have experienced multiple convergent radiations (sensu Frédérich et al. Citation2013). These could have been driven by ecological factors such as transitions from durophagy to non-durophagy, and diet shifts that allowed lineages to move into higher trophic levels, such as in the case of multiple lineages of Dentex-like taxa. Our biogeographic reconstruction shows that sparids likely originated in the Western Central Tethys, with several lineages having been able to colonize other geographical provinces, likely in short periods of time that may have coincided with favorable environmental or climatic conditions. Most geographic provinces were invaded by multiple lineages of sparid fishes, and these repeated invasions, which allowed relatively closely related lineages to experience similar selective pressures while living in the same habitat types, likely resulted in the high level of convergence in many key morphological features (e.g., dentition type) observed in these fishes. This biogeographic scenario is also what we would expect to give rise to a scenario of iterative radiations, an expectation that appears to be at least partly supported by our analyses.
The gamma statistic and the MCCR test failed to statistically support a scenario of slowdown in global diversification rate across the entire sparid clade, a result that would have been consistent with the hypothesis of a single adaptive radiation. The node-height test resulted in a significantly negative relationship between node age and the absolute values of the standardized independent contrasts, suggesting an increase in the rate of of body size evolution through time, and not a slowdown that would have suggested a niche-filling scenario expected in the case of a single adaptive radiation. The significantly positive MDI suggests instead that body size disparity overlaps among subclades, in agreement with a scenario of convergent evolution during parallel radiations. This can also be visually assessed when ancestral body size is reconstructed across the timetree, revealing multiple shifts in body size across several sparid subclades, with several lineages experiencing directional evolution towards enlarged or reduced body size (). Further tests of these preliminary results will likely require a finer investigation of the trophic ecology of sparid species in order to disentangle the mechanisms that may lead to this niche filling.
Repeating the MCCR test for the two sparid subclades (A and B) revealed that only clade B showed a strong signal of slowdown in diversification rate. Interestingly, clade A, which only includes one third of the sparid diversity, is the clade that includes most of the sparid taxa that exceed 100 cm TL, as well as most of the ones that have transitioned from a low-predator trophic level to a mid- or top-predator level. These transitions were often made possible from a transition from a durophagous diet to a non-durophagous diet that incorporates significant amounts of other fishes and cephalopods, and appear to have occurred convergently in different ocean basins (e.g., Northeastern Atlantic, Mediterranean, South Africa). Within clade B, which is shown to have undergone a strong slowdown in diversification rate, the trophic level appears to be more conservative than in clade A. Dietary transitions, however, are still shown to have led to the convergent evolution of omnivory and herbivory in geographically separated lineages (e.g., herbivorous taxa arose in both the Eastern and Western Atlantic; omnivores in Western Atlantic, Eastern Atlantic, South Africa and Western Indian Ocean).
Our results add a phylogenetic and quantitative component to earlier findings of paleoichthyologists. Hanel and Tsigenopoulos (Citation2011) had already suggested that the diversification of the family could be explained by the high adaptability to varying food resources, which led to rapid niche occupation in a range of shallow tropical to temperate marine habitats with consecutive trophic specializations. The analysis of the fossil record clearly revealed that during the first phase of their evolutionary history sparid fishes inhabited tropical paleobiotopes and were characterized by a limited specialization for a durophagous diet. The dentition of most of the Eocene genera consists of large canine, conical, subhemispherical and villiform teeth, with no species characterized by true molariform elements of modern type, the safest indication of a durophagous diet. The alimentary specialization towards durophagy was probably promoted by the increased availability of hard-shelled prey. Molluscs and other hard-shelled invertebrates that constitute the most common prey of sparids characterized by molariform teeth are very abundant in eutrophic settings (see Hallock & Schlager Citation1986). The abrupt global cooling across the Eocene–Oligocene transition that reflected the onset of a major continental glaciation in Antarctica (see Miller et al. Citation1991; Liu et al. Citation2009) led to a marked strengthening of the thermal gradients with the development of the tropical climatic zones. The multiple radiations of durophagous lineages during the Oligocene were probably triggered by enhanced trophic resources associated with a global increase in productivity. The increase of productivity in the oceans since the Late Rupelian (31–30 Ma) resulted in the wide expansion of hard-shelled benthic organisms tolerant to higher levels of trophic resources such as molluscs, bryozoans, echinoids and barnacles, into subtropical and temperate shallow water settings, as evidenced by a long-term shift toward higher carbon isotopic values (see Zachos et al. Citation2001). A prominent pulse of diversification of sparid lineages seems to have occurred also in the Middle Miocene, possibly in response to global environmental changes associated with a general rearrangement of oceanic and atmospheric circulation patterns (Zachos et al. Citation2001). The establishment of the East Antarctic Ice Sheet resulted in the intensification of upwelling and in the increase of weathering rates (e.g., Flower & Kennett Citation1993; John et al. Citation2003). This event produced further global enhancement of trophic resources in the oceans and, as a consequence, an expanded production of conchiferous benthic invertebrates, likely providing the trigger for the diversification of several sparid clades.
Conclusions
Our comparative phylogenetic study of sparid fishes corroborates earlier studies that had inferred non-monophyly of the family without the inclusion of the Centracanthidae, as well as non-monophyly of many of the traditional genera. Sparid fishes are shown to be divided into two large clades, which split from one another in the Late Cretaceous, and started to radiate during the Eocene. Sparid lineages experienced multiple convergent radiations into different geographic regions which were likely driven by diet, both by the appearance of novel shelled invertebrates during the Miocene, and by the multiple transitions from a durophagous to a non-durophagous diet based on either consumption of cephalopods and fishes (in clade A), or soft bodied invertebrates and algae (clade B). Additional studies of sparid macroevolution should focus on the trophic ecology to investigate at a finer level the pattern of niche filling that our analyses suggest.
Fig_S5rev.tif
Download TIFF Image (658 KB)Fig_S4rev.tif
Download TIFF Image (714.6 KB)Fig_S3rev.tif
Download TIFF Image (697.5 KB)Fig_S2rev.tif
Download TIFF Image (871.9 KB)Fig_S1rev.tif
Download TIFF Image (240.3 KB)Appendix 2.R Scripts.txt
Download Text (5.4 KB)Table_S2rev.doc
Download MS Word (27.5 KB)Table_S1rev.doc
Download MS Word (104.5 KB)Acknowledgments
This work was made possible by a Lagrange visiting fellowship to FS by ISI (Istituto per ľInterscambio Scientifico). We thank four anonymous reviewers for comments on an earlier version of this paper.
References
- Agassiz L. 1845. Report on the fossil fishes of the London Clay. Report of the British Association, London 1844, pp. 279–310.
- Akazaki M. 1962. Studies on spariform fishes - anatomy, phylogeny, ecology and taxonomy. Osaka, Japan: 8° Kosugi Co. Ltd.
- Alfaro ME, Santini F, Brock CD. 2007. Do reefs drive diversification in marine teleosts? Evidence from the pufferfishes and their allies (order Tetraodontiformes). Evolution 61:2104–2126.
- Alfaro ME, Brock CD, Banbury BL, Wainwright PC. 2009a. Does evolutionary innovation in pharyngeal jaws lead to rapid lineage diversification in labrid fishes? BMC Evolutionary Biology 9:1.
- Alfaro ME, Santini F, Brock CD, Alamillo H, Dornburg A, Rabosky DL, Carnevale G, Harmong LJ. 2009b. Nine exceptional radiations plus high turnover explain species diversity in jawed vertebrates. Proceedings of the National Academy of Sciences 106:13410–13414.
- Antonucci F, Costa C, Aguzzi J, Cataudella S. 2009. Ecomorphology of morpho-functional relationships in the family of Sparidae: a quantitative statistic approach. Journal of Morphology 270:843–855.
- Anđelković JS. 1989. Tertiary fishes of Yugoslavia. A stratigraphic-paleontologic-paleoecological study. Palaeontologia Jugoslavica 38:1–121.
- Arambourg C. 1927. Les poissons fossiles d’Oran. Matériaux pour la Carte Géologique de l’Algérie, Paléontologie 6:1–293.
- Arambourg C. 1952. Les vertébrés fossiles des gisements de phosphates (Marco – Algérie – Tunisie). Notes et Mémoires du Service Géologique du Maroc 92:1–372.
- Bannikov AF. 1990. A new smarid fish (Perciformes, Centracanthidae) from the Sarmatian of Moldavia. Paleontological Journal 24:99–103.
- Bannikov AF. 2006. Fishes from the Eocene of Bolca, northern Italy, previously classified in the Sparidae, Serranidae and Haemulidae (Perciformes). Geodiversitas 28:249–275.
- Bannikov AF. 2010. Fossil vertebrates of Russia and adjacent countries. Fossil Acanthopterygians Fishes (Teleostei, Acanthopterygii). Moscow: GEOS.
- Bassani F. 1889. Ricerche sui pesci fossili di Chiavon (Strati di Soltzka-Miocene inferiore). Atti della Reale Accademia delle Scienze Fisiche e Matematiche, Napoli 6:1–104.
- Basurco B, Lovatelli A, García B. 2011. Current status of Sparidae aquaculture. In: Pavlidis MA, Mylonas CC, editors. Sparidae: Biology and aquaculture of Gilthead Sea bream and other species. Oxford, NY: Blackwell Publishing Ltd. pp. 1–50.
- Bauchot M-L, Hureau J-C. 1986. Sparidae. In: Whitehead PJP, Bauchot M-L, Hureau J-C, Nielsen J, Tortonese E, editors. Fishes of the Northern-Eastern Atlantic and the Mediterranean. Paris: Unesco. pp. 883–907.
- Bradley F, Landini W. 1984. I fossili del “tripoli” messiniano di Gabbro (Livorno). Palaeontographia Italica 73:5–33.
- Briggs JC, Bowen BW. 2012. A realignment of marine biogeographic provinces with particular reference to fish distributions. Journal of Biogeography 39:12–30.
- Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach. New York: Springer.
- Carnevale G. 2002. Boops roulei Arambourg (Teleostei, Percoidei) in the Messinian of Central Italy, with comments on systematics, paleoecology and zoogeography. Neues Jahrbuch für Geologie und Paläontologie, Monatshefte 12:725–736.
- Carnevale G, Godfrey SJ, Pietsch TW. 2011. Stargazer (Teleostei, Uranoscopidae) cranial remains from the Miocene Calvert Cliffs, Maryland, U.S.A. (St Marys Formation, Chesapeake Group). Journal of Vertebrate Paleontology 31:1200–1209.
- Carnevale G, Godfrey SJ. in press. Miocene bony fishes of the Calvert, Choptank, St. Marys and Eastover Formations, Chesapeake Group, Maryland and Virginia. In: Godfrey SJeditor. The Geology and Vertebrate Paleontology of the Calvert Cliffs. Bloomington, IN: Indiana University Press.
- Carnevale G, Harzhauser M, Schultz O. 2012. The Miocene gadid fish Palimphemus anceps Kner, 1862: A reappraisal. Geodiversitas 34:625–643.
- Carnevale G, Harzhauser M. 2013. Middle Miocene rockling (Teleostei, Gadidae) from the Paratethys (St. Margarethen in Burgenland, Austria). Bulletin of Geosciences 88:609–620.
- Carpenter KE, Johnson GD. 2002. A phylogeny of sparoid fishes (Perciformes, Percoidei) based on morphology. Ichthyological Research 49:114–127.
- Casier E. 1966. Faune ichthyologique du London Clay. London: British Museum (Natural History).
- Chiba SN, Iwatsuki Y, Yoshino T, Hanzawa N. 2009. Comprehensive phylogeny of the family Sparidae (Perciformes: Teleostei) inferred from mitochondrial gene analyses. Genes and Genetic Systems 84:153–170.
- Cowman PF, Bellwood DR. 2011. Coral reefs as drivers of cladogenesis: Expanding coral reefs, cryptic extinction events, and the development of biodiversity hotspots. Journal of Evolutionary Biology 24:2543–2562.
- Day JJ. 2002. Phylogenetic relationships of the Sparidae (Teleostei: Percoidei) and implications for convergent trophic evolution. Biological Journal of the Linnean Society 76:269–301.
- Day JJ. 2003. Evolutionary relationships of the Sparidae (Teleostei: Percoidei): integrating fossil and Recent data. Transactions of the Royal Society of Edinburgh: Earth Sciences 93:333–353.
- Drummond AJ, Rambaut A. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolutionary Biology 7:214.
- Edgar RC. 2004. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic acids research 32:1792–1797.
- Felsenstein J. 1985. Phylogenies and the comparative method. The American Naturalist 26:1–25.
- Fiedler K. 1991. Familie Sparidae. In: Starck D, editor. Lehrbuch der Speziellen Zoologie. Teil 2: Fische. Jena: Gustav Fischer Verlag. pp. 354–355.
- Flower BP, Kennett JP. 1993. Relations between Monterey Formation deposition and middle Miocene global cooling: Naples Beach section, California. Geology 21:877–880.
- Franceschi D. 1922. Pesci fossili nuovi o poco noti del Terziario italiano. Palaeontographia Italica 23:69–84.
- Freckleton RP, Harvey PH. 2006. Detecting non-Brownian trait evolution in adaptive radiations. PLoS Biology 4:e373.
- Froese R, Pauly D. 2013. FishBase. Available: http://www.fishbase.org. Accessed Apr 2013 2.
- Frédérich B, Sorenson L, Santini F, Slater GJ, Alfaro ME. 2013. Iterative ecological radiation and convergence during the evolutionary history of damselfishes (Pomacentridae). The American Naturalist 181:94–113.
- Garrido-Ramos M, Jamilena M, Lozano R, Cárdenas S, Ruiz Rejón C, Ruiz Rejón M. 1995. Phylogenetic relationships of the Sparidae family (Pisces, Perciformes) inferred from satellite-DNA. Hereditas 122:1–6.
- Garrido-Ramos M, de la Herrán R, Lozano R, Cárdenas S, Ruiz Rejón C, Ruiz Rejón M. 1996. Induction of triploidy in offspring of gilthead seabrem (Sparus aurata) by means of heat shock. Journal of Applied Ichthyology 12:53–55.
- Gregorová R. 2009. Diplodus sp. (Sparidae, Perciformes): a new fossil record of an articulated skeleton from Devíska Nová Ves (Upper Badenian, Slovakia). Annalen des Naturhistorishes Museum in Wien 111A:313–322.
- Hallock P, Schlager W. 1986. Nutrient excess and the demise of coral reefs and carbonate platforms. Palaios 1:389–398.
- Hanel R, Sturmbauer C. 2000. Multiple recurrent evolution of trophic types in northeastern Atlantic and Mediterranean seabreams (Sparidae, Percoidei). Journal of Molecular Evolution 50:276–283.
- Hanel R, Tsigenopoulos CS. 2011. Phylogeny, evolution, and taxonomy of sparids with some notes on their ecology and biology. In: Pavlidis MA, Mylonas CCeditors. Sparidae: Biology and aquaculture of gilthead sea bream and other species. Oxford, NY: Blackwell Publishing Ltd. pp. 51–73.
- Harmon LJ, Losos JB, Davies TJ, Gillespie RG, Gittleman JL, Jennings WB, Kozak KH, McPeek MA, Moreno-Roark F, Near TJ, Purvis A, Ricklefs RE, Schluter D, Schulte IIJE, Seehausen O, Sidlauskas BL, Torres-Carvajal O, Weir JT, Mooers AØ. 2010. Early bursts of body size and shape evolution are rare in comparative data. Evolution 64:2385–2396.
- Harmon LJ, Schulte J, Larson A, Losos J. 2003. Tempo and mode of evolutionary radiation in iguanian lizards. Science 301:961–964.
- Harmon LJ, Weir J, Brock C, Glor R, Challenger W. 2008. Geiger: Investigating evolutionary radiations. Bioinformatics 24:129–131.
- Harzhauser M, Piller WE, Steininger FF. 2002. Circum-Mediterranean Oligo-Miocene biogeographic evolution – the gastropods’ point of view. Palaeogeography, Palaeoclimatology, Palaeoecology 183:103–133.
- Hempel E. 1993. Constraints and possibilities for developing aquaculture. Aquaculture International 1:2–19.
- Iakovleva AI. 2011. Palynological reconstruction of the Eocene marine palaeoenvironments in south of Western Siberia. Acta Palaeobotanica 51:229–248.
- John CM, Mutti M, Adatte T. 2003. Mixed carbonate-siliciclastic record on the North African margin (Malta) – Coupling of weathering processes and the mid Miocene climate. Geological Society of America Bulletin 115:217–229.
- Kazancioglu E, Near TJ, Hanel R, Wainwright PC. 2009. Influence of sexual selection and feeding functional morphology on diversification rate of parrotfishes (Scaridae). Proceedings of the Royal Society of London, Series B 276:3439–3446.
- King C. 1981. The stratigraphy of the London Clay and associated deposits. Tertiary Research Special Volume 6:3–158.
- King C. 1984. The stratigraphy of the London Clay Formation and Virginia Water Formation in the coastal sections of the Isle of Sheppey (Kent, England). Tertiary Research 5:121–160.
- Klanten SO, van Herwerden L, Choat JH, Blair D. 2004. Patterns of lineage diversification in the genus Naso (Acanthuridae). Molecular Phylogenetics and Evolution 32:221–235.
- Kováč M, Baráth I, Harzhauser M, Hlavatý I, Hudáčková N. 2004. Miocene depositional systems and sequence stratigraphy of the Vienna Basin. Courier Forschungsinstitut Senckenberg 246:187–212.
- Landini W, Sorbini C. 2005. Evolutionary dynamics in the fish faunas of the Mediterranean basin during the Plio-Pleistocene. Quaternary International 140–141:64–89.
- Lautrédou A-C, Hinsinger DD, Gallut C, Cheng C-H C, Berkani M, Ozouf-Costaz C, Cruaud C, Lecointre G, Dettai A. 2012. Phylogenetic footprints of an Antarctic radiation: The Trematominae (Notothenioidei, Teleostei). Molecular Phylogenetics and Evolution 65:87–101.
- Liu Z, Pagani M, Zinniker D, DeConto R, Huber M, Brinkhuis H, Shah SR, Leckie RM, Pearson A. 2009. Global cooling during the Eocene-Oligocene climate transition. Science 323:1187–1190.
- Losos JB. 2011. Convergence, adaptation, and constraint. Evolution 65:1827–1840.
- Losos JB, Jackman TR, Larson A, De Queiroz K, Rodriguez-Schettino L. 1998. Contingency and determinism in replicated adaptive radiations of island lizards. Science 279:2115–2118.
- Losos JB, Ricklefs RE. 2009. Adaptation and diversification on islands. Nature 457:830–836.
- Maddison WP, Maddison DR. 2011. Mesquite: A modular system for evolutionary analysis, version 2.75. Available: http://www.mesquiteproject.org.
- Marsili S, Carnevale G, Danese E, Bianucci G, Landini W. 2007. Early Miocene vertebrates from Montagna della Maiella, Italy. Annales de Paléontologie 93:27–66.
- McPeek MA. 1995. Testing hypotheses about evolutionary change on single branches of a phylogeny using evolutionary contrasts. The American Naturalist 45:686–703.
- Micklich N, Parin N. 1996. The fishfauna of Frauenweiler (Middle Oligocene, Rupelian; Germany): First results of a review. Publicaciones Especiales de l’Instituto Español de Oceanografía 21:129–148.
- Miller KG, Wright JD, Fairbanks RG. 1991. Unlocking the ice house: Oligocene-Miocene oxygen isotopes, eustasy, and margin erosion. Journal of Geophysical Research 96:6829–6848.
- Müller A. 1999. Ichthyofaunen aus dem atlantischen Tertiär der USA. Leipziger Geowissenschaften. 9:1–360.
- Near TJ, Dornburg A, Kuhn KL, Eastman JT, Pennington JN, Patarnello T, Zane L, Fernández DA, Jones CD. 2012. Ancient climate change, antifreeze, and the evolutionary diversification of Antarctic fishes. Proceedings of the National Academy of Sciences 109:3434–3439.
- Near TJ, Dornburg A, Eytan RI, Keck B, Smith WL, Kuhn KL, Moore JA, Price SA, Burbrink FT, Friedman M, Wainwright PC. 2013. Phylogeny and tempo of diversification in the superradiation of spiny-rayed fishes. 110:12738–12743.
- Orrell TM, Carpenter KE, Musick JA, Graves JE. 2002. Phylogenetic and biogeographic analysis of the Sparidae (Perciformes: Percoidei) from Cytochrome b sequences. Copeia 3:618–631.
- Orrell TM, Carpenter KE. 2004. A phylogeny of the fish family Sparidae (porgies) inferred from mitochondrial sequence data. Molecular Phylogenetics and Evolution 32:425–434.
- Paradis E, Claude J, Strimmer K. 2004. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 20:289–290.
- Pharisat A. 1991. La Paléoichthyofaune du Rupélien marin de Froidefontaine (Territoire de Belfort). Taxinomie et populations, genèse du gisement. Implications paléobiogéographiques. Annales Scientifiques de l’Université de Franche-Comté, Besançon 11:13–98.
- Posada D. 2008. JModelTest: Phylogenetic model averaging. Molecular Biology and Evolution 25:1253–1256.
- Price SA, Holzman R, Near TJ, Wainwright PC. 2011. Coral reefs promote the evolution of morphological diversity and ecological novelty in labrid fishes. Ecology Letters 14:462–469.
- Purdy RW, Schneider VP, Applegate SP, McLellan JH, Meyer RL, Slaughter BH. 2001. The Neogene sharks, rays, and bony fishes from Lee Creek Mine, Aurora, North Carolina. Smithsonian Contributions to Paleobiology 90:71–202.
- Pybus OG, Harvey PH. 2000. Testing macro-evolutionary models using incomplete molecular phylogenies. Proceedings of the Royal Society of London Series B-Biological Sciences 267:2267–2272.
- R Development Core Team. 2012. R: A language and environment for statistical computing. Vienna, Austria. Available: http://www.R-project.org. Accessed Sep 2012 20.
- Rabosky DL. 2006. LASER: A maximum likelihood toolkit for detecting temporal shifts in diversification rates from molecular phylogenies. Evolutionary Bioinformatics 2:257–260.
- Rabosky DL, Lovette IJ. 2008. Density-dependent diversification in North American wood warblers. Proceedings of the Royal Society of London B 275:2363–2371.
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61:539–542.
- Ruber L, Zardoya R. 2005. Rapid cladogenesis in marine fishes revisited. Evolution 59:1119–1127.
- Rutschmann S, Matschiner M, Damerau M, Muschick M, Lehman MF, Hanel R, Salzburger W. 2011. Parallel ecological diversification in Antarctic notothenioid fishes as evidence for adaptive radiation. Molecular Ecology 20:4707–4721.
- Sanderson MJ, Boss D, Chen D, Cranston KA, Wehe A. 2008. The PhyLoTA Browser: processing GenBank for molecular phylogenetics research. Systematic Biology 57:335–346.
- Santini F, Sorenson L. 2013. First molecular timetree of billfishes (Istiophoriformes: Acanthomorpha) shows a Late Miocene radiation of marlins and allies. Italian Journal of Zoology 80:4, 481–489.
- Santini F, Carnevale G, Sorenson L. 2013a. First molecular scombrid timetree (Scombridae, Percomorpha) shows recent radiation of tunas following invasion of pelagic habitat. Italian Journal of Zoology 80:210–221.
- Santini F, Nguyen M, Sorenson L, Waltzek TB, Alfaro JWL, Eastman JM, Alfaro ME. 2013b. Do habitat shifts drive the diversity in teleost fishes? An example from the pufferfishes (Tetraodontidae). Journal of Evolutionary Biology 26:1003–1018.
- Santini F, Sorenson L, Alfaro ME. 2013c. A new multi-locus timescale reveals the evolutionary basis of diversity patterns in triggerfishes and filefishes (Balistidae, Monacanthidae; Tetraodontiformes). Molecular Phylogenetics and Evolution 69:165–176.
- Santini F, Sorenson L, Marcroft T, Dornburg A, Alfaro ME. 2013d. A multilocus molecular phylogeny of boxfishes (Aracanidae, Ostraciidae; Tetraodontiformes). Molecular Phylogenetics and Evolution. 66:153–160.
- Schmid HP, Harzhauser M, Kroh A. 2001. Hypoxic events on a Middle Miocene carbonate platform of the Central Paratethys (Austria, Badenian, 14 Ma), with contributions by Coric S, Rögl F. & Schultz O. Annalen des Naturhistorisches Museum in Wien 102A:1–50.
- Schwarzhans W. 2003. Fish otoliths from the Paleocene of Denmark. Geological Survey of Denmark and Greenland Bulletin 2:1–94.
- Schwarzhans W. 2004. Fish otoliths from the Paleocene (Selandian) of West Greenland. Meddeleser om Grønland 42:1–32.
- Schwarzhans W, Bratishko A. 2011. The otoliths from the middle Paleocene of Luzanivka (Cherkasy district, Ukraine). Neues Jahrbuch fur Geologie und Paläontologie, Abhandlungen 261:83–110.
- Sieber R, Weinfurter E. 1967. Otolithen aus Tiefen Gosauschichten, Osterreichs. Annalen des Naturhistorisches Museum in Wien 71:353–361.
- Slater GJ, Price SA, Santini F, Alfaro ME. 2010. Diversity versus disparity and the radiation of modern cetaceans. Proceedings of the Royal Society B 277:3097–3104.
- Smith JLB. 1938. Sparidae and Denticidae. Transactions of the Royal Society of South Africa 26:255.
- Smith JLB, Smith MM. 1986. Family No. 183: Sparidae. In: Smith MM, Heemstra PC, editors. Smith’s sea fishes. Johannesburg: Macmillan. pp. 580–595.
- Sorenson L, Santini F, Carnevale G, Alfaro ME. 2013. A multi-locus timetree of surgeonfishes (Acanthuridae, Percomorpha), with revised family taxonomy. Molecular Phylogenetics and Evolution 68:150–160.
- Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690.
- Summerer M, Hanel R, Sturmbauer C. 2001. Mitochondrial phylogeny and biogeographic affinities of sea breams of the genus Diplodus (Sparidae). Journal of Fish Biology 59:1638–1652.
- Switchenska AA. 1979. A new species of the genus Sparus (Teleostei) from the Lower Oligocene of Caucasus. Paleontological Journal 3:139–141.
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Biology and Evolution 28:2731–2739.
- Vandewalle P, Saintin P, Chardon M. 1995. Structures and movements of the buccal and pharyngeal jaws in relation to feeding in Diplodus sargus. Journal of Fish Biology 46:623–656.
- Yang Z. 2006. Computational molecular evolution. Oxford, NY: Oxford University Press.
- Zachos J, Pagani M, Sloan L, Thomas E, Billups K. 2001. Trends, thythms, and aberrations in global climate 65 Ma to Present. Science 292:686–693.
Appendix I
The fossils used as calibration points, and the justification for their placement are as follows:
MRCA (most recent common ancestor) of clade A (calibration 1, ). The British fossil Sciaenurus bowerbanki (Agassiz Citation1845) from the Eocene London Clay Formation is the oldest crown Sparidae currently known, and appears as sister to Dentex in Day (Citation2003) morphological phylogeny. Fossil material belonging to this species was collected from the Ypresian grey silty clay of the London Clay Formation cropping out at Sheppey (Kent). According to King (Citation1981, Citation1984), the Sheppey outcrops of the London Clay Formation can be assigned to the lower portion of the Charlesdowniea coleothrypta dinoflagellate cyst assemblage. The first occurrence of Charlesdowniea coleothrypta is correlated to the early part of middle NP12 calcareous nannoplankton Zone (polarity chron C23r), dating back to 52.2–52.1 Ma (Iakovleva Citation2011). The otoliths assigned to the perciform “genus Epigonidarum” weinbergi from the Coniacian of Tiefe Gosau, Ennstaler Alpen, Austria (Late Cretaceous, 89–84 Ma) are used to establish an upper boundary (Sieber & Weinfurter Citation1967). Thus, the prior assumed 52 My as the minimum age, and 84 My as the upper boundary.
MRCA of Lagodon versus Calamus (calibration 2). Incisiviform teeth with bilobate tips and base rounded in cross section from the Miocene Calvert Formation, Chesapeake Group, Maryland, USA. are the oldest fossils assigned to an indeterminate species of the genus Lagodon (Carnevale & Godfrey in press). The fossils were collected from siliciclastic deposits belonging to the Langhian Plum Point Member of the Calvert Formation, dated at about 15 Ma. Due to the uncertainty regarding the correct placement of this fossil within subclade B1, we conservatively consider it to provide a minimum age for the split between Lagodon + Archosargus and Calamus (minus C. calamus) and Stenotomus. Sciaenurus bowerbanki from the Eocene London Clay Formation of Sheppey, UK. (Ypresian, about 52 Ma) is used to establish an upper boundary. Thus, the prior assumed 15 My as the minimum age, and 52 My as the upper boundary.
MRCA of Stenotomus versus Calamus (calibration 3). Isolated premaxillae from the Upper Miocene deposits of the St. Marys Formation, Chesapeake Group, Maryland, USA document the earliest occurrence of the genus Stenotomus in the fossil record (Carnevale & Godfrey in press). The isolated premaxillary remains assigned to Stenotomus were recovered from the Tortonian (about 9 Ma; Carnevale et al. Citation2011) siliciclastic sediments of the Little Cove Point Member of the St. Marys Formation and are characterized by a spatulate alveolar process bearing molariform teeth gradually increasing in size distally. The teeth assigned to Lagodon sp. from the Middle Miocene Plum Point Member of the Calvert Formation, Maryland, USA (Langhian, about 15 Ma) are used to establish an upper boundary. Thus, the prior assumed 9 My as the minimum age, and 15 My as the upper boundary.
MRCA of Boops versus Sarpa (calibration 4). Fossil material assigned to Boops sp. from the Middle Miocene corallinacean limestone of St. Margarethen, Eisenstadt-Sopron Basin, Austria (Carnevale & Harzhauser Citation2013) provides a minimum age estimate for the split between the genera Boops and Sarpa. The material consists of a dozen of specimens assigned to the genus Boops on the basis of the typical multidentate incisiviform teeth, predorsal formula and median-fin formulae (see Bauchot & Hureau Citation1986; Carnevale Citation2002; Day Citation2002). The fossils were collected at the Kummer Quarry, close to St. Margarethen from greenish-whitish calcarenitic marls that contain a rich assemblage of well-preserved teleost fishes as well as representatives of several groups of invertebrates (see Carnevale et al. Citation2012; Carnevale & Harzhauser Citation2013). The productive fossiliferous strata were assigned to the part of the calcareous nannoplankton zone NN5b roughly coincident with the Langhian–Serravallian boundary, around 14 Ma (Schmid et al. Citation2001). Sciaenurus bowerbanki from the Eocene London Clay Formation of Sheppey, UK (Ypresian, about 52 Ma) indicates the earliest appearance of taxa belonging to the Sparidae. Thus, the prior assumed 14 My as the minimum age, and 52 as the upper boundary.
MRCA of Crenidens versus Lithognathus (calibration 5). A single specimen assigned to the extinct species Crenidens intermedius from the Messinian diatomites of Raz-el-Aïn (about 7 Ma), Chelif Basin, Algeria (Arambourg Citation1927) documents the earliest occurrence of the genus Crenidens in the fossil record. Boops sp. from the Middle Miocene corallinacean limestone of St. Margarethen, Austria (around the Langhian–Serravallian boundary, about 14 Ma) is used to establish the upper boundary. Thus, the prior assumed 7 My as the minimum age, and 14 as the upper boundary.
MRCA of Pagellus acarne versus bogaraveo (calibration 6). Because of its remarkable affinities with the extant species Pagellus acarne, the fossil Pagellus leptosomus from the Messinian diatomites of the Chelif Basin, Algeria (about 7 Ma; see Arambourg Citation1927) is used to define the minimum age of the split between Pagellus acarne and Pagellus bogaraveo. Boops sp. from the Middle Miocene corallinacean limestone of St. Margarethen, Austria (around the Langhian–Serravallian boundary, about 14 Ma) is used to establish the upper boundary. We thus assigned a lower bound of 7 My and an upper bound of 14 My to this calibration.
MRCA of Diplodus (calibration 7). A single specimen from the Middle Miocene (Badenian) of Devíska Nová Ves, Vienna Basin, Slovakia is used herein to determine the minimum age of the crown Diplodus (Gregorová Citation2009). Kováč et al. (Citation2004) provided evidence on the age of the fossiliferous deposits of Devíska Nová Ves on the basis of calcareous nannoplankton biostratigraphy, and assigned them to Zone NN6 (Serravallian), with an estimated age of about 13 Ma. The Eocene Sciaenurus bowerbanki from the London Clay Formation of Sheppey, UK (Ypresian, about 52 Ma) is used to establish an upper boundary. Thus, we assigned a lower bound of 13 My and an upper bound of 52 My to this calibration.
MRCA of Diplodus annularis versus bellottii (calibration 8). The extinct species Diplodus oranensis from the Messinian diatomites of the Chelif Basin, Algeria (about 7 Ma) is closely related to the extant Diplodus annularis (Arambourg Citation1927) and is used to determine the minimum age for the split between Diplodus annularis and Diplodus bellottii. Diplodus sp. from the Middle Miocene of Devíska Nová Ves, Vienna Basin, Slovakia (Serravallian, about 13 Ma) is used to establish the upper boundary. Thus, the prior assumed a minimum age of 7 My and an upper boundary of 13 My.