Abstract
Three new species of hyporheic Parastenocarididae from Turkey and Thailand are described in this paper, based on their morphological features. Kinnecaris xanthi sp. nov. and Kinnecaris draconis sp. nov. were collected in Anatolic Turkey, respectively from one and two rivers; Kinnecaris iulianae sp. nov. was collected from several streams on Pha-Ngan Island (Thailand). The two new species from Turkey both lack the typical pitted integument and their P5 fused with the intercoxal sclerite in both sexes; they share a similar shape of the caudal rami in both sexes and of P4 in male. They differ in the ornamentation of the genital double-somite and the armature of A1 in females; in the ornamentation of the anal operculum; in the shape of P2 endopod of both sexes, and P3 endopod of females; in the ornamentation of the first exopodal segment of P3 in males, and in the armature of P4 endopod in females. Kinnecaris iulianae sp. nov. shares with the other species from the Oriental and Australian Region the presence of a pitted cuticle, the size and shape of caudal rami and the shape and ornamentation of P3 in the males, and is characterized by the ventral ornamentation of the genital double-somite and urosomites of the females, and by the lack of ornamentation at the insertion of the P4 endopod of both sexes. The descriptions of these three new Kinnecaris widen the distribution of the genus which, so far, was considered Gondwanian. In this paper, we also re-examine the diagnostic characters of the genus and their presence and/or variability in the known species of Kinnecaris, discuss the taxonomic position of Kinnecaris lyncaea and emend the genus diagnosis.
Introduction
Knowledge of the systematics of Parastenocarididae has recently advanced, thanks to in-depth taxonomic studies and investigation of the phylogenetic relationships within genera. Some of the 25 genera described by Jakobi (Citation1972) were recently redefined (e.g., Corgosinho & Martinez Arbizu Citation2005; Corgosinho et al. Citation2010, Citation2012; Schminke Citation2013). Kinnecaris Jacobi, 1972 was redefined by Schminke (Citation2008) who allocated to this genus the type species Kinnecaris forficulata (Chappuis, 1952), one new species, 12 species of Parastenocaris Kessler, 1913, and three of Cafferocaris Jakobi (Citation1972), synonymizing the latter genus with Kinnecaris. Schminke (Citation2008) identified 11 morphological characters which, according to this author, are shared by all the Kinnecaris known at that time. However, Schminke (Citation2008) noticed that only three species, namely K. quollensis (Cottarelli & Bruno Citation1995), K. eberhardi (Karanovic Citation2005) and K. giselae Schminke (Citation2008), shared all of these 11 characters. The remaining 14 species show only some of the characters, due to the incomplete or incorrect original descriptions, or to the lack of description for the male, as occurs for K. fluviatilis (Wells, 1964) and K. solitaria (Karanovic Citation2004). Later on, Kinnecaris godavari Ranga Reddy and Schminke (Citation2009) was described from India; this species shows all 11 diagnostic characters. Finally, in a recent paper based on morphological and biomolecular data, Karanovic and Cooper (Citation2011) described seven more new species from Australia and, although some of these species do not display all 11 diagnostic characters listed by Schminke (Citation2008), they are nonetheless discussed by the authors as typical members of the genus Kinnecaris (e.g., Karanovic & Cooper Citation2011, p. 29, for K. esbe, Citation2011). The three new Kinnecaris described in this paper also do not share all of Schminke’s 11 diagnostic characters. Since Karanovic and Cooper (Citation2011) already expressed doubts on the certain attribution of some of the 17 species listed by Schminke (Citation2008) to Kinnecaris, we will re-examine in this paper Schminke’s (Citation2008) 11 diagnostic characters and their presence and/or variability in the known species of Kinnecaris. We aim to contribute to a future and detailed revision of this genus, where the primitive and derived character states will be identified, to prevent Kinnecaris from becoming a taxonomic repository, as occurred, for instance, for Parastenocaris or for the minuta-group Lang, 1948.
In this paper, we describe three new species: Kinnecaris xanthi sp. nov., Kinnecaris draconis sp. nov. and Kinnecaris iulianae sp. nov. The first two new species were collected in Anatolian Turkey, respectively from the hyporheic zone of the Karamenderes River, near the ruins of the ancient Troy in the Çanakkale Province, and in the hyporheic zone of the Anamur and Dalaman rivers, in the Mersin and Muğla Provinces. Kinnecaris iulianae sp. nov. was collected from several streams on Pha-Ngan Island (Thailand), a hotspot of diversity for hyporheic harpacticoids (Cottarelli et al. Citation2010). Only one Parastenocarididae was so far recorded for Turkey: Parastenocaris phyllopora Noodt 1954 from Inznik-Gölu, Bursa Province, Anatolia, and we are presently studying two more new species of Fontinalicaridinae from Anatolia. In Thailand, only the Parastenocarididae Asiacaris dispar Cottarelli Bruno and Berera, Citation2010, belonging to a monospecific genus, was recorded from the same Pha-Ngan Island. We are presently studying seven new taxa of Parastenocarididae from Pha-Ngan and the adjacent Samui islands.
The descriptions of these three new Kinnecaris allowed a reconsideration of the biogeography of the genus which, until now, was considered Gondwanian (Ranga Reddy & Schminke Citation2009; Karanovic & Cooper Citation2011). In fact, the species so far allocated to Kinnecaris are distributed in Africa, Madagascar, Papua New Guinea, India and Australia. The record of these new species widens the distribution of the genus, and sheds new light on its biogeography.
Materials and methods
Kinnecaris xanthi sp. nov. was collected in the hyporheic habitat of a lowland, meandering river with low water velocity. The collecting station was located on the sandy, right bank of the Karamenderes River, Çanakkale Province, opposite the archaeological sites of the ancient Troy (Truva in modern Turkish language), in Anatolia, not far from the sea, southwest of the Dardanelles Strait. Samples were collected with the Karaman–Chappuis method (Delamare-Deboutteville Citation1960), by digging holes in the medium-fine sand on the bank, and filtering the interstitial water with a 60 µm mesh. Kinnecaris xanthi sp. nov. was the only harpacticoid present in the samples, together with some Cyclopoida not yet identified. The Anamur River (also called Dragon River) in southeastern Anatolia, Mersin Province, runs underground from its source in the Taurus Mountains near the village of Sigozu as a subterranean river, and surfaces for 35 km to its mouth into the Mediterranean Sea. In its surface reaches, it is mainly torrential, with large boulders alternating with sand and gravel bars and banks. The sampling station is located 18 km from the river mouth, near the historic Alakopru Bridge, on the right bank; holes were dug in the coarse sand and water was collected as described for the previous species. The accompanying crustacean fauna was represented by unidentified Cyclopoida and Harpacticoida Canthocamptidae. The Dalaman River runs in the southwestern coast of Anatolia, in the Muğla Province. It has its source within the Kocas Mountain near the village of Dirmil, and runs for 229 km before reaching the Mediterranean Sea. The sampling site is about 6.5 km upstream from the river mouth, on the right bank; samples were collected as described above from coarse sand deposits, and K. draconis sp. nov. was the only copepod present in the samples.
Pha-Ngan Island is an island of the Samui Archipelago, in the Southern Gulf of Thailand (Suratthani Province); the archipelago comprises over 40 islands, representing old igneous formations; granite is the dominant lithological component. Pha-Ngan Island has an area of 167 km2, over 90% of which is covered by tropical rainforest and jungle. The island is predominantly hilly and is rich in watercourses, mainly creeks and streams which often form waterfalls. The highest elevation is Khao Ra, the highest peak of Pha-Ngan Island [about 630 m above sea level (a.s.l.)] and numerous hills ranging from 415 to 525 m a.s.l. We collected in this island extensively, for three consecutive years from 2006 to 2009, from 16 stations, since the island fresh waters are rich in Parastenocarididae (Cottarelli et al. Citation2010). K. iulianae sp. nov. was collected in four of the 16 sampling stations, which have all been investigated over the 3 years; these stations are located in forested hills (stations 10 and 14), and in the agricultural lowland (stations 1 and 5). All stations are in small watercourses with different flow. pH was generally acidic, ranging from 5.0 to 6.2 in three stations, and measuring 7.2 in the last one (station 5). The sand grain size ranged from medium to medium-coarse sand, while the temperature did not differ greatly with different elevation, but rather followed the daily trend of the atmospheric temperature. The interstitial water temperature never reached the values of the surface water or atmospheric air. In station 14, Kinnecaris iulianae sp. nov. was collected with two Parastenocaris sp. sensu Reid (Citation1995) still under study, and two new taxa of Canthocamptidae. The new species was always abundant except in station 14 (type locality), where the Parastenocaris sp. sensu Reid (Citation1995) was more abundant (this species is also abundant in all of the other stations of the island). In station 10, K. iulianae sp. nov. was collected with a parabathynellid sincarid, representing the second record of sincarids for Thailand.
Specimens were collected using the Karaman–Chappuis method, fixed in 5% buffered formalin solution, sorted and mounted in polyvinyl lactophenol (K. draconis sp. nov.) or Faure’s medium (K. xanthi sp. nov. and K. iulianae sp. nov.). Specimens of K. xanthi sp. nov. and K. draconis sp. nov. were mounted on microscope slides and covered with a coverslip. Specimens of K. iulianae sp. nov. were mounted between two coverslips, to allow observation from two sides, and fragments of human hair were inserted near the non-dissected specimens to avoid deformation (Karanovic Citation2005). Once the medium was dry, the coverslips were fixed to a microscope slide with pieces of adhesive tape. Drawings were made at different magnifications, to a maximum of 1250×, using drawing tubes mounted on a Zeiss Axioskop® phase-contrast microscope connected to a Coolpix® 5000 digital camera with a phototube, and a Polyvar Reichert-Jung® interferential-contrast microscope. Pictures were taken at 40× in phase contrast.
The description of Kinnecaris draconis sp. nov. is preliminary because it was conducted on undissected specimens mounted more than 40 years ago in lateral view; as a consequence, some features and microcharacters were not discernible in all of the specimens (and are reported in the following description as “not visible in slides”). However, all of the main characters and microcharacters are clearly visible and, according to us, adequate to characterize this new species. The undissected specimens in lateral view of K. xanthi sp. nov. and K. draconis sp. nov. are slightly flattened (with the exception of the female of K. draconis sp. nov. illustrated in ), and, as a consequence, the P5 appears to adhere to the urosomite when it is in fact projecting outwards.
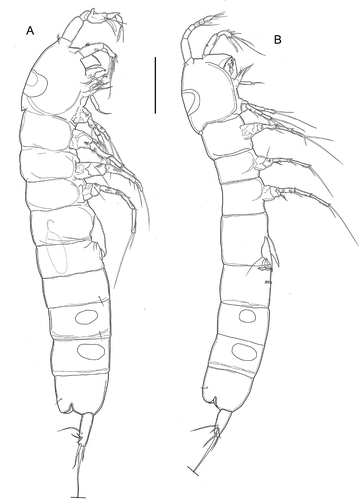
Figure 1. Kinnecaris xanthi sp. nov. A, male, habitus, lateral view. B, female, habitus, lateral view (sensillar pattern omitted). Scale bar: 50 µm.
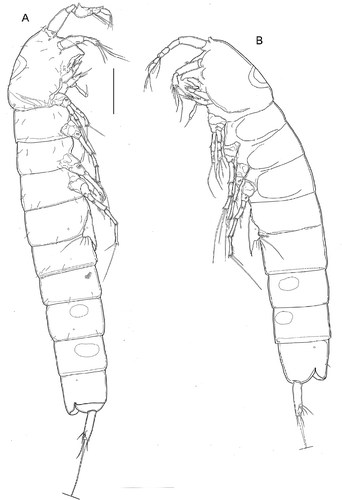
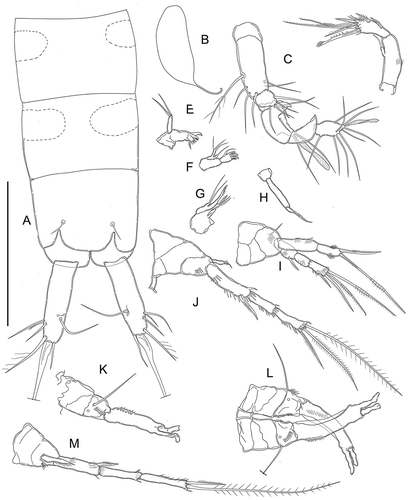
Figure 2. Kinnecaris xanthi sp. nov. A, male, fourth and fifth urosomites, anal somite, anal operculum and caudal ramus, lateral view. B, male, anal somite, anal operculum and caudal rami, dorsal view. C, male, spermatophore. D, male, rostrum and antennule, dorsal view. E, male, antennule, outer view, schematic, main armature omitted. F, male, antennule, ventral view (armature partly omitted, insertion point of setae marked by circles). G, male, antenna. H, male, labrum. I, male, mandible. J, male, maxillule. K, male, maxilla. L, male, maxilliped. Scale bar: 50 µm.
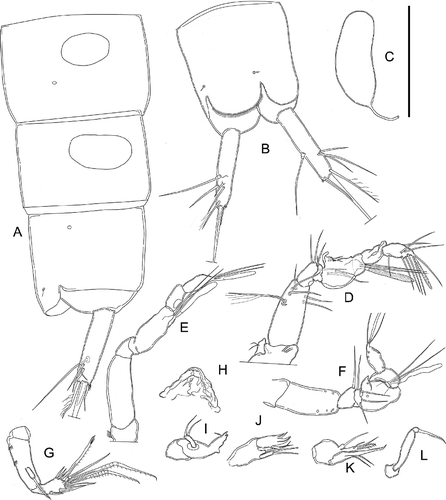
Figure 3. Kinnecaris xanthi sp. nov. A, male, leg 1. B, male, leg 2. C, male, leg 3. D, male, leg 3 (variability). E, male, leg 4, dorsal view. F, male, leg 4, ventral view. G, male, leg 4 (variability). H, male, first and second urosomites, leg 5, leg 6, ventral view. Scale bar: 50 µm.
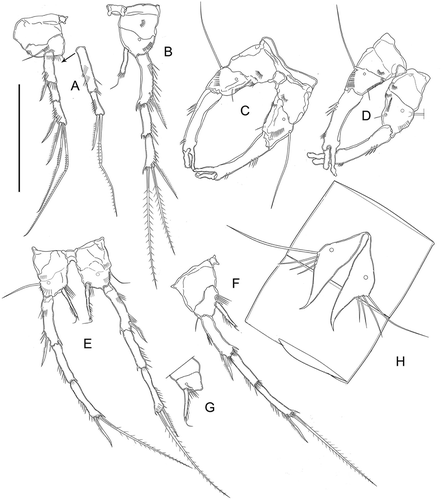
Figure 4. Kinnecaris xanthi sp. nov. A, female, fourth and fifth urosomites, anal somite, anal operculum and caudal ramus, lateral view. B, female, first urosomite, leg 5, genital double-somite and genital field, ventral view. C, female, antennule, dorsal view. D, female, leg 2. E, female, leg 3. F, female, leg 4. Scale bar: 50 µm.
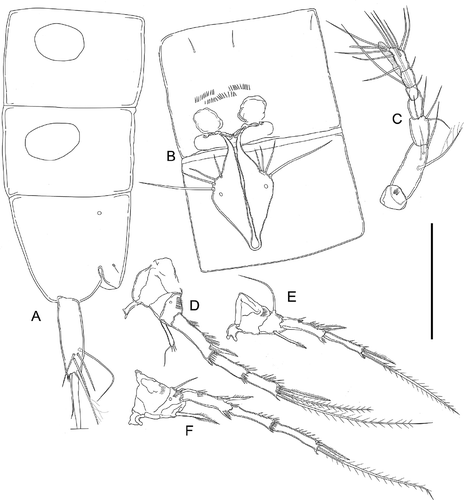
Figure 5. Kinnecaris draconis sp. nov. A, male, habitus, lateral view. B, female, habitus, lateral view. Scale bar: 50 µm.
The following abbreviations are used throughout the text and figures: enp, endopod; exp, exopod; A1, antennule; A2, antenna; P1–P5, first to fifth pereiopod; P6, rudimentary sixth pereiopod. The nomenclature and descriptive terminology follow Huys and Boxshall (Citation1991) except for the caudal ramus, for which we followed Huys et al. (Citation1996), who described the basic setal pattern and defined the terminology for harpacticoids.
Specimens are deposited at the Natural History Museum, London (NHMUK) and at V. Cottarelli’s collection at the Dipartimento per l’Innovazione dei Sistemi Biologici, Agroalimentari e Forestali, of the Tuscia University (DIBAF).
Systematic account
Family Parastenocarididae Chappuis, 1940
Subfamily Parastenocaridinae Chappuis, 1940
Kinnecaris xanthi sp. nov.
Type locality
Hyporheic habitat on the left bank of the Karamenderes River, Turkey, Çanakkale province, west of the archaeological site of ancient Troy (the modern Truva) 39° 57’ 42” N, 26° 13’ 52” E, 21 m a.s.l.
Type material
Holotype: dissected male mounted on one slide labelled: “Kinnecaris xanthi holotype: male, Karamenderes River, Turkey, 16/VIII/1997” (NHMUK 2015. 584). Paratypes: four dissected and two undissected males, mounted each on one slide labelled: “Kinnecaris xanthi paratype male, Karamenderes River, Turkey, 16/VIII/1997” (NHMUK 2015. 585, NHMUK 2015. 586, DIBAF); five dissected and two undissected females mounted each on one slide labelled: “Kinnecaris xanthi paratype female, Karamenderes River, Turkey, 16/VIII/1997” (NHMUK 2015. 587, NHMUK 2015. 588, DIBAF). All material collected by V. Cottarelli and G. Bettini.
Description of male
Body unpigmented, naupliar eye absent. Total body length, measured from tip of rostrum to posterior margin of caudal rami (excluding caudal setae) from 400 to 440 µm, mean 418 μm (n = 7); length of specimen in : 452 μm. Habitus () cylindrical and slender, without any demarcation between prosome and urosome; prosome-to-urosome ratio 1.09. Free pedigerous somites without any lateral or dorsal expansions, all connected by well-developed arthrodial membranes. Integument weakly sclerotized, without cuticular pits, ornamented with sensilla on all somites except preanal one. Double dorsal cuticular window (smaller window with thinner integument inside larger one) on cephalothorax (); fourth and fifth urosomites each with a pair of lateral elliptical windows (), larger on fifth urosomite. Cephalothorax () representing almost 20% of total body length (measured from tip of rostrum to end of caudal ramus). Sensillar pattern as in . First and fourth urosomites with pair of cuticular pores laterally (one pore on each side) in anterior third (). Third urosomite () with ventro-lateral short row of minute spinules. Anal somite (, ) with pair of large dorsal sensilla at base of anal operculum, pair of cuticular pores laterally (one pore on each side) in anterior third. Anal operculum (, ) well developed, with convex smooth distal margin. Spermatophore () about 1.2 times as long as genital somite.
Caudal rami (, ): shorter than last urosomite, approximately cylindrical, slightly divergent; length/width: 4. Anterolateral accessory seta (I), and posterolateral seta (III) subequal, anterolateral seta (II) longer than seta I and III (length seta/length caudal ramus: 0.32), all three setae inserted together distally at ¾ length of the caudal ramus. Outer terminal seta (IV) pinnate (length seta/length caudal ramus: 0.73); inner terminal seta (V) without fracture plane. Terminal accessory seta (VI) short (length seta/length caudal ramus: 0.43) and smooth with spinules near insertion. Dorsal seta (VII) articulate, long (length seta/length caudal ramus: 0.85), inserted distally at 2/3 length of the caudal ramus and proximally to setae I–III.
Rostrum (): small, demarcated at base, triangular, almost reaching distal margin of first antennular segment, ornamented with two dorsal sensilla.
Antennule (): prehensile and strongly digeniculate, eight-segmented, pocket-knife type sensu Schminke (Citation2010) (). First segment very short with transversal row of spinules, second segment longest, with six setae, the longest seta uniplumose and basally biarticulate. Third segment with four distal bare setae; fourth segment reduced to a small sclerite with one short seta. Fifth segment enlarged, with proximal long seta and inner protrusion, which is approximately conical and folded at the tip, carrying two small setae, one longer seta proximally inserted on the segment; distal tubercle with two equal setae and one large aesthetasc, restricted at midlength, almost reaching to the end of eighth segment (). Sixth segment bare, partially fused to previous one. Seventh segment bare, distal anterior corner protruding as a triangular apophysis, bifid in lateral view (, ). Eighth segment with seven setae and apical acrothek represented by two subequal setae and a slender shorter aesthetasc, approximately as long as segment (). Armature formula: 1-[0], 2-[1 uniplumose + 5 bare], 3-[4 bare], 4-[1 bare], 5-[6 + ae], 6-[0], 7-[0], 8-[7 bare + (2 + ae)].
Antenna (): coxa unarmed; allobasis with two transverse rows of spinules on inner margin. Exopod represented by a small segment merged with allobasis, with pinnate apical seta. Endopod bearing on the apex two lateral and five distal elements, two of them geniculate, one transformed, all elements with long spinules near their insertions.
Labrum (): large and approximately triangular, with convex and smooth anterior surface, narrow cutting edge, cutting edge with apical row of slender denticles.
Mandible (): coxal gnathobase bare, cutting edge with apical teeth and small supabical pinnate seta. One-segmented palp, with two distal setae of equal length.
Maxillule (): praecoxal arthrite with four terminal, curved robust spines apically denticulated, one subdistal curved seta. Coxal endite short, with one apical thin seta reaching to the end of arthrite. Basis cylindrical, with two distal bare setae of same length. Endopod and exopod absent (fused to basis without trace).
Maxilla (): syncoxa with two endites, proximal endite short, with one thin seta; distal endite cylindrical, longer, armed apically with two subequal thin bare setae and one enlarged pinnate seta; allobasis prolonged into apical pinnate claw; endopod represented by small segment fused at the base, with two short setae of equal length.
Maxilliped (): prehensile. Syncoxa small and unarmed; basis slim and elongate, unarmed; endopod represented by distally unipinnate claw.
P1 (): with smooth and small intercoxal sclerite; coxa large, unarmed, unornamented; basis large, armed with single slender seta on outer margin; ornamented with transverse row of minute spinules at base of outer seta, transverse spinular row along inner margin at midlength; transverse spinular row along distal margin on anterior surface, between exopod and endopod. Exopod three-segmented, as long as endopod, second segment shortest; exp-1 with thin and slightly curved pinnate seta on outer distal corner; exp-2 unarmed; exp-3 with two geniculate and one normal pinnate apical setae, and one subapical pinnate seta. Endopod two-segmented; enp-1 as long as the first two segments of the corresponding exopod, with two transversal rows of spinules on the outer margin, one spinule at ¾ of the inner margin, and two spinules on the inner distal corner; enp-2 thinner than enp-1 and as long as half of enp-1, with longitudinal row of six spinules on the outer margin, and long, geniculate pinnate seta, and shorter pinnate seta on apex.
P2 (): with smooth and large intercoxal sclerite, twice as wide as long. Coxa unarmed, ornamented with transversal row of spinules at midlength. Basis unarmed, with spinular row on outer margin and one large pore. Exopod three-segmented, ornamented with large spinules along outer margin; exp-1 longest, slightly curved inwards with strong distolateral pinnate spine and transversal spinular row at ¼ of the outer margin; second and third segments of same length, exp-2 unarmed; exp-3 armed with subapical outer spine and two apical setae. Endopod one-segmented, about ½ the length of the corresponding exp-1, represented by cylindrical segment, with apical seta about 0.7 times as long as segment, slightly curved inwards, surrounded by five short spinules.
P3 (): with smooth praecoxa and intercoxal sclerite. Intercoxal sclerite narrow and tall, trapezoidal, unornamented and with slightly concave distal margin. Coxa with transversal spinular row. Basis robust, with long, slender, smooth outer seta, row of transversal spinules; short longitudinal row of small spinules along inner margin below endopod, one transversal spinular row on inner proximal corner, one large basal pore. Endopod reduced to very thin seta, difficult to observe at the microscope. Exp-1 distally slender, length to width ratio: 3.9, slightly curved inwards, ornamented with proximal longitudinal row of four spinules and distal longitudinal row of two spinules along outer margin; inner margin with thin hyaline membrane with smooth margin. Exp-2 fused with exp-1 and prolonged into short apophysis with blunt round tip. Distal thumb represented by leaf-like, apically curved segment, slightly shorter than apophysis.
P4 (, , ): intercoxal sclerite smaller than in second or third legs, and with more deeply concave distal margin, smooth. Coxa ornamented with outer transversal spinular row. Basis with basal pore, armed with single slender seta on outer margin; ornamented with row of spinules at base of outer seta, a row of four thin spinules of same length and one shorter, thicker and apically bent spinule near the endopod insertion (, ). Exopod three-segmented, slender, first and last segments approximately of the same length, middle segment slightly shorter, exp-1 slightly curved inwards. Exp-1 with distolateral pinnate spine; exp-2 unarmed; exp-3 armed with subapical outer pinnate spine and apical pinnate seta, spine length less than 1/3 of seta length, exp-3 ornamented with row of spinules along the distal outer margin as characteristic of the genus. Endopod one-segmented about 0.7 as long as corresponding exp-1, represented by cylindrical segment, narrowing towards the tip, armed with single slender apical seta, ornamented with row of short apical spinules and row of longer spinules along the outer margin.
P5 (): fused to intercoxal sclerite, represented by two triangular cuticular plates with inner-distal corner produced into long and curved spiniform process, with large cuticular pore on anterior surface. Armature on free distal margin, from inner to outer: three bare setae of increasing length, long basipodal seta.
P6 (, ): vestigial, fused into simple cuticular plate, unornamented and unarmed.
Description of female
Body length, excluding caudal setae, from 372 to 454 μm, mean 417 μm (n = 7); length of specimen in : 454 μm. Habitus (), ornamentation of somites, pigmentation and lack of naupliar eye as in male, except genital and first urosomite fused into double-somite. Prosome/urosome ratio 1.03; cephalothorax slightly wider than genital double-somite, double hyaline window as in male; third, fourth (preanal) and fifth (anal) urosomites similar to those in male but apparently without lateral pores (, ), with lateral abdominal windows () subequal. Genital double-somite () without any trace of subdivision, ornamented with four posterior sensilla (two ventral, and two lateral), and three transversal rows of ventral spinules posterior of the genital field. Genital field () occupying anterior ventral half of genital double-somite; single genital aperture covered by fused vestigial sixth legs; median copulatory pore located medially at 1/7th of double-somite length and also covered by sixth legs.
Caudal rami (, ) identical to those of male but slightly shorter in proportion to anal somite, length/width: 3.9. Ornamentation and armature identical to those in male.
Rostrum, antenna, oral appendages, maxilliped as in male.
Antennule (): seven-segmented, aesthetasc on fourth segment shorter than in male, reaching below end of seventh segment. First segment with distal spinular row. Second segment longest. Apical acrothek represented by two setae of same length and slender aesthetasc. Armature formula: 1-[0], 2-[1 pinnate +3 bare], 3-[4 bare], 4-[2 + ae], 5-[1], 6-[1], 7-[7 bare + (2 + ae)].
P1 (): ornamentation and armature as in male, similar in shape, but endopod slightly longer than exopod.
P2 (): intercoxal sclerite longer and narrower than in male, coxa and basis as in male but coxa (apparently) bare. Exp-1 less inwardly curved than in male, ornamentation and armature of exopod as in male. Endopod similar in shape and ornamentation to the male one, apical seta longer, as long as segment.
P3 (): intercoxal sclerite narrow and tall, with concave margin, smooth. Coxa with transversal row of spinules on distal outer margin. Basis with outer seta and spinular row; exopod two-segmented, as normal in Parastenocarididae: segments of same length, ornamented with spinular row along outer margin. Exp-1 with distolateral pinnate spine and transversal spinular row at 1/3 of the outer margin; exp-2 with subapical outer pinnate spine and apical pinnate seta, spine slightly longer than 1/3 of seta. Endopod represented by a very thin and pointed segment, pinnate in the distal ¼, 0.8 times as long as corresponding exp-1.
P4 (): intercoxal sclerite, coxa, basis, exopod as in male except exp-1 not curved inwards, exp-3 subapical spine length 1/3 of seta length. Endopod represented by a thin cylindrical segment, ending in a pointed tip, pinnate in the distal half, slightly shorter than the corresponding exp-1.
P5 (): very similar to that of male.
P6 (): vestigial, fused into simple cuticular plate, covering gonopore, unornamented and unarmed.
Variability
One male paratype with proximal longitudinal row of P3 of three spinules and distal longitudinal row of four spinules along outer margin of exp-1 in both P3 (); a second male paratype with a row of five thin spinules (instead of four) near endopod insertion of both P4 ().
Etymology
The species epithet is the masculine singular genitive of the name Xanthus, a god in the Greek mythology who, according to the Iliad, lived in the Karamenderes River (i.e., the ancient Scamander River), and who tried to drown the Greek warrior Achilles during the Trojan War.
Kinnecaris draconis sp. nov.
Type locality
Hyporheic habitat on the left bank of the Anamur River, Turkey, Mersin Province, 36°10’30”N, 32°53’40”E. Other locality: Dalaman River, Turkey, Muğla Province, 36°43’43”N, 28°46’09”E.
Type material
Holotype: undissected male mounted with one unidissected female on one slide labelled: “Kinnecaris draconis male holotype and female paratype, Anamur River, Turkey, 17/VI/1970” (NHMUK 2015. 589–590). Paratypes: one undissected male mounted with one undissected female on one slide labelled: “Kinnecaris draconis one male and one female paratype, Anamur River, Turkey, 17/VI/1970” (NHMUK 2015. 591–592). One undissected female mounted on a slide labelled: “Kinnecaris draconis female paratype, Anamur River, Turkey, 17/VI/1970” (NHMUK 2015. 593); two undissected females mounted on a slide labelled: “Kinnecaris draconis two female paratypes, Anamur River, Turkey, 17/VI/1970” (DIBAF); three undissected females and one undissected male mounted on one slide labelled: “Kinnecaris draconis three female and one male paratypes, Anamur River, Turkey, 17/VI/1970” (DIBAF). Four undissected males mounted on one slide labelled “Kinnecaris draconis four male paratypes, Dalaman River, Turkey, 17/VI/1970” (NHMUK 2015. 594–597). All material collected by R. Argano, L. Boitani, V. Cottarelli.
Description of male
Body unpigmented, naupliar eye absent. Total body length, measured from tip of rostrum to posterior margin of caudal rami (excluding caudal setae), from 398 to 457 µm, mean 411 μm (n = 7); length of specimen in : 457 μm. Habitus () cylindrical, without any demarcation between prosome and urosome; prosome-to-urosome ratio 1.77. Free pedigerous somites without any lateral or dorsal expansions, all connected by well-developed arthrodial membranes. Integument relatively weakly sclerotized, without cuticular pits, ornamented with sensilla on all somites except preanal one. Double dorsal cuticular window (smaller window with thinner integument inside larger one) on cephalothorax (); fourth and fifth urosomites each with pair of lateral elliptical windows, larger on fifth urosomite. Cephalothorax () representing almost 20% of total body length. Sensillar pattern not discernible. Third urosomite () with ventrolateral short row of minute spinules. Anal somite () with pair of large dorsal sensilla at base of anal operculum. Anal operculum () well developed, with convex and smooth distal margin. Spermatophore () about 1.4 times as long as genital somite.
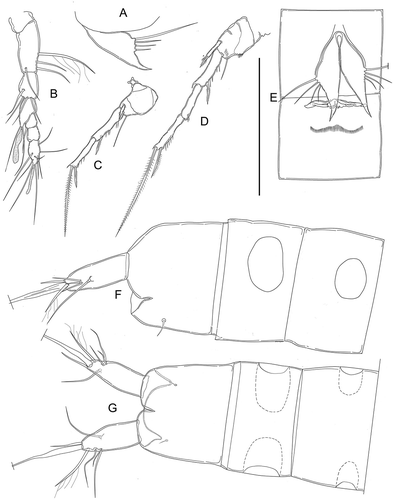
Figure 6. Kinnecaris draconis sp. nov. A, male, fourth and fifth urosomites, anal somite, anal operculum and caudal rami, dorsal view. B, male, spermatophore. C, male, antennule, dorsal view. D, male, antenna. E, male, mandible. F, male, maxillule. G, male, maxilla. H, male, maxilliped. I, male, leg 1. J, male, leg 2. K, male, leg 3, lateral view (variability). L, male, leg 3. M, male, leg 4. Scale bar: 50 µm.
Caudal rami (, ) slightly shorter than last urosomite, approximately cylindrical, slightly enlarged at 2/3 of the length, narrowing distally, slightly divergent; length/width: 3.3. Anterolateral accessory seta (I) longer than posterolateral seta (III), anterolateral seta (II) much shorter than seta I and III (length seta/length caudal ramus: 0.23), all three setae inserted together distally at ¾ length of the caudal ramus. Outer terminal seta (IV) pinnate (length seta/length caudal ramus: 0.7); inner terminal seta (V) without fracture plane. Terminal accessory seta (VI) short (length seta/length caudal ramus: 0.36) and smooth without spinules near insertion. Dorsal seta (VII) articulate, long (length seta/length caudal ramus: 0.64), inserted distally at 2/3 length of the caudal ramus and proximally to setae I–III.
Rostrum () small, not demarcated at base, triangular, almost reaching distal margin of first antennular segment, ornamented with two dorsal sensilla.
Antennule (): prehensile and strongly digeniculate, eight-segmented, pocket-knife type sensu Schminke (Citation2010). First segment very short and bare, second segment longest, with six setae, the longest seta plumose and basally biarticulate. Third segment with four distal bare setae; fourth segment reduced to a small bare sclerite. Fifth segment most enlarged of all segments, with inner protrusion which is approximately conical and folded at the tip, carrying one small seta and one longer seta proximally inserted; one seta distal to the protrusion, distal tubercle with two equal setae and one large aesthetasc, restricted at midlength, almost reaching to the end of eighth segment. Sixth segment bare, partially fused to previous one. Seventh segment bare, distal anterior corner protruding as a triangular apophysis, with pointed tip carrying a small hyaline membrane. Eighth segment with seven setae and apical acrothek represented by two subequal setae and a slender shorter aesthetasc, approximately as long as segment. Armature formula: 1-[0], 2-[1 plumose + 5 bare], 3-[4 bare], 4-[0], 5-[5 + ae], 6-[0], 7-[0], 8-[7 bare + (2 + ae)].
Antenna (): coxa unarmed; allobasis with one transverse row of spinules on inner margin. Exopod represented by a small segment merged with allobasis, with pinnate apical seta. Endopod bearing on the apex two lateral and five distal elements, two of them geniculate, one transformed, all elements with spinules near their insertions.
Mandible (): coxal gnathobase bare, cutting edge with two apical teeth, one small plate with five small apical spinules, one small pinnate seta. One-segmented palp, with two distal setae of equal lengths.
Maxillule (): praecoxal arthrite with three terminal, curved robust spines apically denticulated, interspread by three thin setae and one subdistal composite, curved seta. Coxal endite short, with one apical thin seta reaching to the end of arthrite. Basis cylindrical, with two distal naked setae of same length. Endopod and exopod absent.
Maxilla (): syncoxa with two endites: proximal endite short, with one thin seta; distal endite cylindrical, longer, armed apically with two thin bare setae and one enlarged pinnate seta; allobasis prolonged into apical pinnate claw; endopod represented by a small segment fused at the base, with two setae of subequal length.
Maxilliped (): prehensile. Syncoxa small and unarmed; basis slim and elongate, unarmed; endopod represented by distally unipinnate claw.
P1 (): intercoxal sclerite not observable on slides: coxa large, unarmed, unornamented; basis large, armed with single slender seta on outer margin; ornamented with transverse row of minute spinules at base of outer seta. Exopod three-segmented, slightly shorter than endopod, second segment shortest; exp-1 with thin and slightly curved pinnate seta on outer distal corner; exp-2 unarmed, exp-3 with two geniculate and one normal pinnate apical setae, and one subapical pinnate seta. Endopod two-segmented; enp-1 as long as the first two segments of the corresponding exopod, with two transversal rows of spinules on the outer margin; enp-2 thinner than enp-1 and slightly longer than half of enp-1, with longitudinal row of four spinules on the outer margin, long, geniculate pinnate seta and shorter pinnate seta on apex.
P2 (): with smooth and large intercoxal sclerite, twice as wide as long. Coxa unarmed, basis unarmed, with spinule row on outer margin. Exopod three-segmented, ornamented with large spinules along outer margin; exp-1 longest, slightly curved inwards with strong distolateral pinnate spine and transversal spinular row at ¼ of the outer margin; exp-2 shortest, unarmed; exp-3 armed with subapical outer spine and two apical setae. Endopod one-segmented, very small and thin, less than 1/3 the length of the corresponding exp-1, represented by cylindrical segment, with apical seta about 0.7 times as long as segment, slightly curved inwards, surrounded by three short spinules.
P3 (, ): intercoxal sclerite narrow and tall, trapezoidal, unornamented and with slightly concave distal margin. Praecoxa and coxa unornamented. Basis robust, with long, slender, smooth outer seta, row of long spinules above it and one large basal pore. Endopod missing. Exp-1 length-to-width ratio: 4.5, slightly curved inwards, with distal inner small tubercle, ornamented with proximal longitudinal row of eight spinules; inner margin with hyaline membrane with smooth margin. Exp-2 fused with exp-1 and prolonged into short apophysis turning outwards at 2/3 of the lenght, with blunt round tip. Distal thumb represented by a laminar segment folded along main axis, with bifid and rounded tips; thumb shorter than apophysis.
P4 (, ): intercoxal sclerite not visible on slides. Coxa unornamented. Basis armed with single slender seta on outer margin; ornamented with row of spinules at base of outer seta, a row of few thin spinules of same length and one longer spinule near the endopod insertion. Exopod three-segmented, slender, last segment longest; exopod ornamented with spinular row along outer margin of last two segments. Exp-1 with strong distolateral pinnate spine and transversal spinular row at 1/3 of the outer margin and below the spine; exp-2 and exp-3 with longitudinal spinular row on outer margin as characteristic of the genus (), armed with subapical outer pinnate spine and apical pinnate seta, spine length less than 1/3 of seta length. Endopod one-segmented about 0.8 as long as corresponding exp-1, represented by cylindrical segment with pointed tip, ornamented with longitudinal row of spinules.
P5 (): fused to intercoxal sclerite (?), represented by two triangular cuticular plates with inner-distal corner produced into long and curved spiniform process. Armature on free distal margin, from inner to outer: three bare setae of increasing length, long basipodal seta.
Figure 7. Kinnecaris draconis sp. nov. A, male, leg 5. B, female, antennule, ventral view. C, female, leg 3. D, female, leg 4. E, female, first urosomite, leg 5, genital double-somite and genital field, ventral view. F, female, fourth and fifth urosomites, anal somite, anal operculum and caudal ramus, lateral view. G, female, fourth and fifth urosomites, anal somite, anal operculum and caudal ramus, dorsal view. Scale bar: 50 µm.
P6 (): vestigial, fused into simple cuticular plate, unornamented and unarmed.
Description of female
Body length, excluding caudal setae, from 396 to 415 μm, mean 405 μm (n = 7); length of specimen in : 400 μm. Habitus (), ornamentation of prosomites, pigmentation and lack of naupliar eye as in male, except genital and first urosomite fused into double-somite. Prosome/urosome ratio 0.86; cephalothorax slightly wider than genital double-somite, double hyaline window as in male. Third, fourth (preanal) and fifth (anal) urosomites similar to those in male (, ), with elliptical window on fourth segment smaller than the other one. Genital double-somite () wider than long, without any trace of subdivision, one transversal rows of ventral spinules posterior of the genital field. Genital field () occupying anterior ventral 1/3 of genital double-somite; single genital aperture covered by fused vestigial sixth legs; median copulatory pore located medially at 1/6 of double-somite length and also covered by sixth legs. Seminal receptacles not visible in slides.
Caudal rami (, ): tapering apically and much shorter than those of male, also in proportion to anal somite, length/width: 2.7. Ornamentation and armature similar to those in male, but length seta II/length caudal ramus: 0.40, seta IV/length caudal ramus: 1; length seta VI/length caudal ramus: 0.53: length seta VII/length caudal ramus: 1.17. Seta VII inserted at same level of setae I–III.
Rostrum, antenna, oral appendages, maxilliped, P1, P2 as in male.
Antennule (): seven-segmented, aesthetasc on fourth segment shorter than in male, reaching end of seventh segment. First segment bare. Second segment longest. Fifth segment with long seta; sixth segment bare. Apical acrothek represented by two setae of same length and slender aesthetasc. Armature formula: 1-[0], 2-[1 pinnate +3 bare], 3-[4 bare], 4-[2 + ae], 5-[1], 6-[0], 7-[7 bare + (2 + ae)].
P3 (): intercoxal sclerite narrow and tall, with concave margin, smooth. Coxa bare, basis with outer seta and spinular row; exopod two-segmented, as normal in Parastenocarididae. Exp-1 slightly longer than exp-2, both segments ornamented with spinular row along outer margin. Exp-1 with distolateral pinnate spine and transversal spinular row at 1/3 of the outer margin; exp-2 armed with subapical outer pinnate spine and apical pinnate seta, spine slightly less than 1/3 of seta. Endopod represented by a very thin and pointed segment, pinnate in the distal quarter, 0.4 times as long as corresponding exp-1.
P4 (): intercoxal sclerite, basis, exopod as in male except; coxa with spinular row, exp-3 subapical spine length 1/3 of seta length. Endopod represented by a thin cylindrical segment, as long as half of the corresponding exp-2, ornamented with three distal spinules and ornated with one apical pinnate seta, as long as 0.6 times the segment.
P5 (): fused to intercoxal sclerite, very similar to that of male.
P6 (): vestigial, fused into simple cuticular plate, covering gonopore, unornamented and unarmed.
Variability
One male with spinular row of P3 exp-1 composed of six spinules instead of eight ().
Etymology
The species epithet is the masculine singular genitive of the Latin name draco = dragon. The Anamur River, where the species was collected, is also locally called Dragon River.
Kinnecaris iulianae sp. nov.
Type locality
Hyporheic habitat in several stations of Koh Pha-Ngan, Thailand. Station 1: 13/II/06, 23/I/2009. 09° 45’ 38” N, 99° 59’ 6” E, at sea level. Brook in a coconut grove, medium-fine sand, water level: −15 cm from the surface, clear water, pH 5.7, water temperature 25.0°C. Station 4: 26/I/2009. 9° 47’ 00” N, 99° 59’ 6” E, 30 m a.s.l. Stream in sparse tree field, boulders and medium-coarse sand, water level: −20 cm from the surface, clear water, pH 6.5, water temperature 25.1°C. Station 5: 26/I/2009. 9° 46’ 20” N, 100° 0’ 26” E, 3 m a.s.l. Brook in a meadow, medium-coarse sand, water level: −20 cm from the surface, clear water, pH 7.2, water temperature 25.7°C. Station 14 (near Phaeng Waterfall, Than Sadet Ko Pha-Ngan National Park): 22/II/07, 09/II/2008, 02/II/2009. 9° 44’ 5” N, 100° 1’ 7” E, 195 m a.s.l. Waterfall with plunge pools in pluvial forest, boulders and coarse sand, water level: −15 cm from the surface, clear water, pH 6.2, water temperature 23.2°C.
Type material
Holotype: dissected male mounted on a slide labelled: “Kinnecaris iulianae holotype male, stat. 14, Pha-Ngan Island, Thailand 09/II/2008” (NHMUK 2015. 598). Paratypes: two dissected males each mounted on one slide labelled “Kinnecaris iulianae paratype male, stat. 14, Pha-Ngan Island, Thailand 22/II/2007” (NHMUK 2015. 599, DIBAF); two dissected and five undissected males each mounted on one slide labelled “Kinnecaris iulianae paratype male, stat. 14, Pha-Ngan Island, Thailand 09/II/2008” (NHMUK 2015. 600, DIBAF); one undissected male mounted on a slide labelled “Kinnecaris iulianae paratype male, stat. 1, Pha-Ngan Island, Thailand 09/II/2008” (NHMUK 2015. 601); one undissected male mounted on a slide labelled “Kinnecaris iulianae paratype male, stat. 1, Pha-Ngan Island, Thailand 29/I/2009” (DIBAF); one dissected male mounted on a slide labelled “Kinnecaris iulianae paratype male, stat. 5, Pha-Ngan Island, Thailand 26/I/2009” (DIBAF); two dissected and four undissected females, each mounted on a slide labelled “Kinnecaris iulianae paratype female, stat. 14, Pha-Ngan Island, Thailand 22/II/2007” (NHMUK 2015. 602, NHMUK 2015. 603); two undissected females, each mounted on a slide labelled “Kinnecaris iulianae paratype female, stat. 14, Pha-Ngan Island, Thailand 14/II/2009” (NHMUK 2015. 604, DIBAF); one dissected and two undissected females each mounted on a slide labelled “Kinnecaris iulianae paratype female, stat. 14, Pha-Ngan Island, Thailand 9/II/2008” (DIBAF); three undissected females each mounted on a slide labelled “Kinnecaris iulianae paratype female, stat. 1, Pha-Ngan Island, Thailand 13/II/2006” (DIBAF); one undissected female mounted on a slide labelled “Kinnecaris iulianae paratype female, stat. 4, Pha-Ngan Island, Thailand 26/I/2009” (DIBAF); three undissected females each mounted on a slide labelled “Kinnecaris iulianae paratype female, stat. 5, Pha-Ngan Island, Thailand 26/I/2009” (DIBAF). All material collected by V. Cottarelli and G. Bettini.
Description of male
Body unpigmented, naupliar eye absent. Total body length, measured from tip of rostrum to posterior margin of caudal rami (excluding caudal setae), from 340 to 456 µm, mean 399 μm (n = 7); length of specimen in : 340 μm. Habitus () cylindrical and very slender, without any demarcation between prosome and urosome; prosome-to-urosome ratio 0.90. Free pedigerous somites without any lateral or dorsal expansions, all connected by well-developed arthrodial membranes, second free pedigerour somite with distolateral large pore. Integument relatively weakly sclerotized, with dense and shallow cuticular pits (), ornamented with sensilla on all somites except preanal one. Double dorsal cuticular window (smaller window with thinner integument inside larger one) on cephalothorax (); fourth and fifth urosomites each with pair of lateral elliptical windows, those on the fifth urosomites proportionally larger (, , ). Cephalothorax () representing about 19% of total body length. Sensillar pattern as in . Third urosomite () with ventro-lateral row of short spinules. Anal somite (, , ) with pair of large dorsal sensilla at base of anal operculum. Anal operculum (, ) well developed, with convex and smooth distal margin. Spermatophore () about 1.7 times as long as genital somite.
Figure 8. Kinnecaris iulianae sp. nov. A, male, habitus, lateral view. B, female, habitus, lateral view (sensillar pattern omitted). Scale bar: 50 µm.
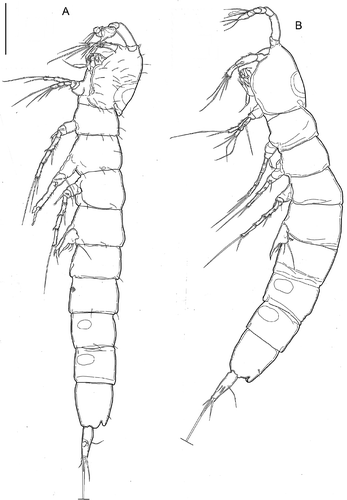
Figure 9. Kinnecaris iulianae sp. nov. A, male, double dorsal cuticular window on cephalothorax, lateral view. B, male, leg 5, leg 6, first to fourth urosomites, ventral view. C, male, fourth and fifth urosomites, anal somite, anal operculum and caudal rami, ventral view (square insert with detail of pitted cuticle). D, male, fourth and fifth urosomites, anal somite, anal operculum and caudal ramus, lateral view. E, male, spermatophore. F, male, antennule, dorsal view. G, male, antenna. H, male, mandible. I, male, maxillule. J, male, maxilla. K, male, maxilliped. Scale bar: 50 µm.
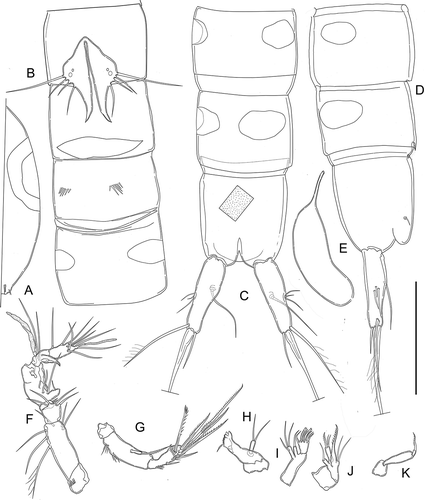
Figure 10. Kinnecaris iulianae sp. nov. A, male, leg 1. B, male, leg 2. C, male, leg 3, D, male, leg 4, lateral view. E, male, leg 4. F, male, leg 5. G, male, first urosomite and P5 (variability), ventral view. H, male, leg 5 (variability). Scale bar: 50 µm.
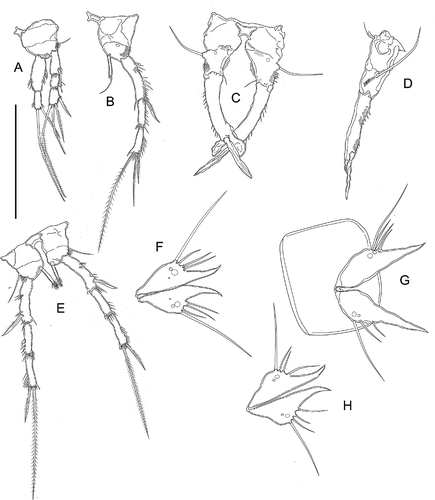
Figure 11. Kinnecaris iulianae sp. nov. A, female, fifth urosomites, anal somite, anal operculum and caudal ramus, lateral view. B, female, fourth and fifth urosomites, anal somite, anal operculum and caudal rami, dorsal view. C, female, genital double-somite, genital field, P5, ventral view. D, female, antennule. E, female, leg 2. F, female, leg 3. G, female, leg 4. H, female, leg 5. I, female, leg 5 (variability). J, female, genital field, ventral view. Scale bar: 50 µm.
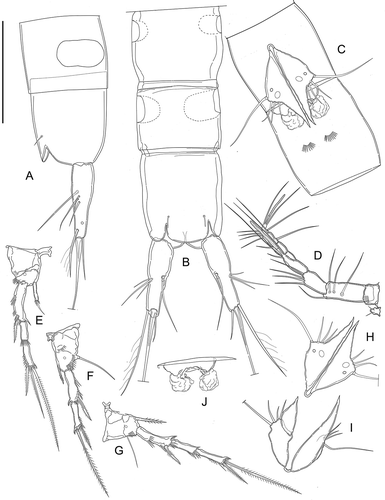
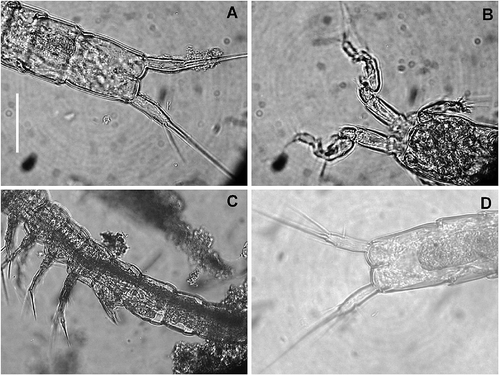
Figure 12. Kinnecaris iulianae sp. nov. Pictures taken with phase-contrast microscope at 40x. A, male, fourth and fifth urosomites, anal somite, anal operculum and caudal rami, ventral view. B, male, antennule, ventral view. C, female, legs 1 to 5, lateral view. D, female, fifth urosomites, anal somite, anal operculum and caudal rami, ventral view. Scale bar: 50 µm.
Caudal rami (, , ): shorter than last urosomite, enlarged dorsally in the first half, narrowing apically, divergent; length/width: 3.6. Anterolateral accessory seta (I) very short, posterolateral seta (III) and anterolateral seta (II) subequal but seta III longest (length seta/length caudal ramus: 0.33), all three setae inserted together exactly at half the length of the caudal ramus. Outer terminal seta (IV) pinnate (length seta/length caudal ramus: 0.5); inner terminal seta (V) without fracture plane. Terminal accessory seta (VI) long (length seta/length caudal ramus: 1.1) and smooth with spinules near insertion. Dorsal seta (VII) articulate (length seta/length caudal ramus: 0.6), inserted proximally at 2/5 length of the caudal ramus and proximally to setae I–III.
Rostrum (): small, demarcated at base, triangular, almost reaching distal margin of first antennular segment, ornamented with two dorsal sensilla.
Antennule (, ): prehensile and strongly digeniculate, eight-segmented pocket-knife type sensu Schminke (Citation2010). First segment very short with transversal row of spinules, second segment longest, with six setae, the longest seta unipinnate and basally biarticulate. Third segment with four distal bare setae; fourth segment reduced to a small sclerite with two short setae of same length. Fifth segment most enlarged of all segments, with proximal long seta and inner conical protrusion, carrying one small seta, one long seta inserted at the base of the protrusion, a shorter seta inserted near the protrusion; distal tubercle with two subequal setae and one large aesthetasc, restricted at midlength, reaching past the end of eighth segment. Sixth segment bare, partially fused to previous one. Seventh segment bare, distal anterior corner protruding as a pointed triangular apophysis that can fold back into the matching the tubercle on fifth segment. Eighth segment with seven setae and apical acrothek represented by two subequal setae and a slender aesthetasc, longer than segment. Armature formula: 1-[0], 2-[1 uniplumose + 5 bare], 3-[4 bare], 4-[2 bare], 5-[5 + ae], 6-[0], 7-[0], 8-[7 bare + (2 + ae)].
Antenna () coxa unarmed; allobasis with two transverse rows of spinules proximally on inner margin. Exopod represented by a small segment merged with allobasis, with pinnate apical seta 3.3 times as long as exopod. Endopod bearing on the apex two lateral and five distal elements, two of them geniculate, one transformed, all elements with spinules near their insertions.
Mandible (): coxal gnathobase bare, cutting edge with two apical teeth, one rectangular small plate, distal denticulated margin, one lateral pinnate seta. One-segmented palp, with two distal setae of equal length.
Maxillule (): praecoxal arthrite with three terminal, curved robust spines, two of which transformed and one is pinnate, and three thin and bare setae: one subdistal composite, curved seta. Coxal endite long, with one apical thin seta reaching past the end of arthrite. Basis cylindrical, with two distal naked setae of same length and one subdistal shorter one. Endopod and exopod absent (fused to basis without trace).
Maxilla (): syncoxa with two endites: proximal endite with thin seta; distal endite cylindrical, longer, armed apically with two thin bare setae and one enlarged pinnate seta; allobasis prolonged into apical pinnate claw; endopod represented by a small segment fused at the base, with two setae of equal length.
Maxilliped (): prehensile. Syncoxa small and unarmed; basis slim and elongate, unarmed; endopod represented by distally unipinnate claw.
P1 (): Intercoxal sclerite small, quadrangular, concave and smooth. Coxa large, unarmed, unornamented; basis large, armed with single slender seta on outer margin; ornamented with transverse row of minute spinules at base of outer seta, and transverse spinular row along distal margin on anterior surface, between exopod and endopod. Exopod three-segmented, as long as endopod, the second segment shortest; exp-1 with one thin and slightly curved pinnate seta on outer distal corner; exp-2 without ornamentation, exp-3 with two geniculate and one normal pinnate apical setae, and subapical pinnate seta. Endopod two-segmented; enp-1 slightly shorter than the first two segments of the corresponding exopod, with two transversal rows of spinules on the outer margin, row of spinules at ¾ of the inner margin; enp-2 thinner than enp-1 and as long as half of it, with longitudinal row of spinules on the outer margin, and long, geniculate pinnate seta, and shorter pinnate seta on apex.
P2 (): with smooth and large intercoxal sclerite, twice as wide as long. Coxa unarmed, ornamented with a row of spinules near distal outer corner. Basis unarmed, with spinular row on outer margin and one large pore. Exopod three-segmented, ornamented with large spinules along outer margin; exp-1 longest, slightly curved inwards with strong distolateral pinnate spine and transversal spinular row at about half of the outer margin; exp-2 unarmed, slightly shorter than exp-3 which is armed with subapical outer spine and two apical setae. Endopod one-segmented, about half the length of the corresponding exp-1, represented by cylindrical and thin segment, with apical seta slightly longer than endopod, slightly curved inwards, surrounded by short spinules.
P3 (, ): with smooth praecoxa, coxa and intercoxal sclerite. Intercoxal sclerite large, trapezoidal, unornamented and with slightly concave distal margin. Coxa with arched transversal spinular row. Basis robust, with long, slender, smooth outer seta, and a row of long spinules above it; short longitudinal row of small denticles along inner margin below endopod. Endopod reduced to a very thin segment, with very small apical seta. Exp-1 distally slender, length-to-width ratio: 5, slightly curved inwards, ornamented with proximal and distal longitudinal rows of spinules along outer margin; inner margin with thin hyaline membrane with smooth margin. Exp-2 fused with exp-1 and prolonged into long, leaf-like, slightly concave apophysis with membranous margins. Distal thumb represented by spear-like, pointed segment, almost twice as long as apophysis.
P4 (): intercoxal sclerite smaller than in second or third legs, and with more deeply concave distal margin, smooth. Coxa bare. Basis with basal pore, armed with single slender seta on outer margin; ornamented with row of spinules at base of outer seta. Exopod three-segmented, slender, second and third segments of approximately the same length, exp-1 longest, slightly curved inwards. Exopod ornamented with few large spinules along outer margin on all segments, more numerous on last segment as typical of the genus. Exp-1 with strong distolateral pinnate spine; exp-2 unarmed; exp-3 armed with subapical outer pinnate spine and apical pinnate seta, spine length less than 1/3 of seta length. Endopod one-segmented, about half as long as corresponding exp-1, represented by cylindrical segment, narrowing towards the tip, ornamented with row of short apical spines and without spinules near its insertion.
P5 (, ): with very small, arch-shaped intercoxal sclerite; represented by two triangular cuticular plates with inner-distal corner produced into long spiniform process slightly curved inwards, with one small and two large cuticular pores on anterior surface. Armature on free distal margin, from inner to outer: three bare setae of similar length but outermost seta shortest, long basipodal seta.
P6 (): vestigial, fused into a transversally elongated cuticular plate, unornamented and unarmed, representing 80% of genital somite width.
Description of female
Body length, excluding caudal setae, from 336 to 476 μm, mean 402 µm (n = 7); length of specimen in : 336 µm. Habitus (, ), ornamentation of prosomites, pigmentation and lack of naupliar eye as in male, except genital and first urosomite fused into double-somite. Prosome/urosome ratio 0.75; cephalothorax slightly wider than genital double-somite, double hyaline window as in male. Genital double-somite () longer than wide, without any trace of subdivision, with two arched rows of ventral spinules at about halfway along the segment. Genital field () occupying anterior ventral third of genital double-somite; single genital aperture covered by fused vestigial sixth legs; median copulatory pore located medially at 1/10th of double-somite length and also covered by sixth legs. Third, fourth (preanal) and fifth (anal) urosomites similar to those in male, with lateral elliptical windows larger than in male (, ).
Caudal rami (, ) similar to those of male length/width: 3.8.
Rostrum, antenna, oral appendages, maxilliped as in male.
Antennule () seven-segmented, aesthetasc on fourth segment slightly longer than in male, reaching past end of seventh segment. First segment with distal spinular row. Second segment longest. Apical acrothek represented by two setae of same length and slender aesthetasc. Armature formula: 1-[0], 2-[1 pinnate +3 bare], 3-[4 bare], 4-[2 + ae], 5-[1], 6-[1], 7-[7 bare + (2 + ae)].
P1: armature and ornamentation as in male, similar in shape, but endopod slightly longer than exopod.
P2 (): intercoxal sclerite longer and narrower than in male, coxa and basis as in male; exp-1 less inwardly curved than in male, ornamentation and armature of exopod as in male. Endopod similar in shape and ornamentation to the male one, apical seta shorter than endopod.
P3 (): intercoxal sclerite much smaller than in male, narrow and tall, with concave margin, smooth. Coxa with transversal row of spinules near distal inner margin. Basis with pore, outer seta and dense spinular row; exopod two-segmented, as normal in Parastenocarididae. Exopod, ornamented with spinular row along outer margin. Exp-1 longer than exp-2, with distolateral pinnate spine and transversal spinular row at 1/3 of the outer margin; exp-2 thinner than exp-1, armed with subapical outer pinnate spine and apical pinnate seta, spine about 1/3 of seta. Endopod represented by a very thin and pointed segment, pinnate in the distal 1/3, 0.5 times as long as corresponding exp-1.
P4 (): intercoxal sclerite smaller than in male; coxa, basis, exopod as in male except exp-1 not curved inwards, exp-3 subapical spine length almost 1/3 of seta length. Endopod represented by a thin cylindrical pointed segment, almost as long as the corresponding exp-1, ornamented with spinules along the distal half.
P5 (, ): very similar to that of male but with only one large and one small pores.
P6 (): vestigial, fused into simple cuticular plate, covering gonopore, unornamented and unarmed.
Variability
One female paratype with a malformed P5 with the pointed tip much shorter and larger, separated from the rest of the P5 by a transversal ridge (two-segmented?) (). Two male paratypes with only one large and one small pore (, ); one of these paratypes also with only two lateral setae on one of the P5 (), the other paratype with lateral setae of different relative lengths ().
Etymology
We would like to dedicate this species to our dear friend, Dr. Giuliana Bettini, for the help often provided during several collecting trips. The species epithet is the feminine singular genitive of the Latin adjective “Iulianus” from which the Italian name was derived.
Affinities
The two new species from Turkey share the following features: (1) lack of pitted integument; (2) caudal rami with similar shape in both sexes (parallel margins, shorter than last urosomite, especially in K. draconis sp. nov.); (3) P5 fused with intercoxal sclerite in both sexes; (4) P4 endopod in male with similar size, shape and ornamentation. The differences between the two species are the following: (1) ventral ornamentation of the genital double-somite in females: K. xanthi sp. nov. has three transversal rows of spinules, whereas K. draconis sp. nov. has only one, much longer row, with a shape suggesting that this condition may be derived from the fusion of the three spinules present in K. xanthi sp. nov. (apomorphy?); (2) A1 in females with one seta on segment 5 and 6 in K. xanthi sp. nov. versus only on segment 5 in K. draconis sp. nov. (apomorphy), and with a spinular row on the first segment in both sexes of the former, lacking in those of the latter; (3) P2 enp in both sexes, and P3 enp in female very small in K. draconis sp. nov. (apomorphy) and longer and larger in K. xanthi sp. nov.; (4) P3 in males with thumb slightly shorter than apophysis, with similar shape in the two species, but K. draconis sp. nov. has only one spinular row on the outer margin of exp-1; the enp is missing on males of K. draconis sp. nov. and is present, albeit reduced to a very thin seta, in K. xanthi sp. nov.; (5) P4 enp in females with distal pinnate seta in K. draconis sp. nov., the seta is fused to the segment to form a pointed, long segment, pinnate in the apical half, in K. xanthi sp. nov. (apomorphy); (6) P5 with one pore in both sexes in K. xanthi sp. nov., the pore is lacking in K. draconis sp. nov.
Kinnecaris iulianae sp. nov. has the strongest affinities with species from the Oriental and Australian regions. In particular: (1) a pitted cuticle is present in all Australian species except K. solitaria, in the Papua New Guinean K. giselae Schminke (Citation2008), in the Indian K. godavari; (2) K. iulianae sp. nov. is well characterized by the ventral ornamentation of the genital double-somite and urosomites in the female: this species has two arched spinular rows on the ventral surface of the genital double somite, at half the length of the segment, and no spinules on the other urosomites. Spinular rows are missing in K. giselae, K. eberhardi (Karanovic Citation2005) K. solitaria and K. linesae Karanovic and Cooper (Citation2011). In K. godavari there is a “transverse row of about 8 dorsal spinules on either side at about the middle of distal half” (Reddy and Schminke Citation2009, p. 318) of the genital double-somite, and the ventral ornamentation is represented by a long row extending to almost the entire ventral surface, and a transverse row of five spinules midventrally on the third urosomite. Kinnecaris lakewayi Karanovic and Cooper (Citation2011) has “four groups of large spinules ventrally on third and fourth urosomites” (Karanovic and Cooper Citation2011, p. 17); K. barrambie Karanovic and Cooper (Citation2011) has “many short rows of minute spinules, and two parallel short rows of large spinules (five and each spinules respectively)” (Karanovic and Cooper Citation2011, p. 17) on the genital double-somite, and four rows of four large spinules ventrolaterally, and one row of three large spinules dorsolaterally on the third urosomite. Kinnecaris esbe Karanovic and Cooper (Citation2011) has numerous transverse rows of minute spinules, two parallel short rows of four large spinules laterally in the posterior half of the genital double-somite and two groups of four large spinules ventrally on the third urosomite. Kinnecaris linel Karanovic and Cooper (Citation2011) and K. uranusi Karanovic and Cooper (Citation2011) have several dorsolateral rows of minute spinules, one lateral short row of five large spinules in the posterior half of the genital double-somite and two groups of four large spinules ventrolaterally on the third urosomite. Kinnecaris lined Karanovic and Cooper (Citation2011) does not have spinules on the genital double somite but two ventrolateral rows of four large spinules on the third urosomite; (3) the ornamentation of the urosomites in males differs as well. K. iulianae sp. nov. has the most common ornamentation present in Kinnecaris, i.e., two (one of each side) ventrolateral short row of spinules on the third urosomite and no spinules on the second (genital) somite. The same ornamentation is present in the males of K. eberhardi, K. giselae, K. godavari and K. linesae. K lakewayi has ventral rows of large spinules on the fourth and fifth (preanal) urosomites, with no spinules on the second urosomite; K. barrambie has ventral or lateral rows of large spinules on the third and fourth urosomites and three short lateral rows of spinules (from five to seven) at midlength of the second urosomite. The third and fourth urosomites of K. esbe, K. uranusi, K. lined, and K. linel have some ventral and/or lateral additional larger spinules but no large spinules on the genital somite; (4) the caudal rami are as long as the last urosomite in K. iulianae sp. nov., K. barrambie and K. lined; the caudal rami are much shorter than the last urosomite in K. giselae, K. solitaria, K. godavari, K. lakewayi and K. linesae, and are much longer in K. esbe, K. linel and K. uranusi; (5) the A1 in females carries a seta on both segments 5 and 6 in K. iulianae sp. nov. and K. giselae, only on segment 5 in K. godavari, K. solitaria and K. eberhardi and only on segment 6 in K. lakewayi, K. barrambie, K. esbe, K. uranusi, K. linel, K. lined and K. linesae; (6) the P5 in both sexes has an intercoxal sclerite in K. iulianae sp. nov., K. giselae and K. eberhardi; the intercoxal sclerite is absent in all remaining Australian species and in K. godavari; (7) K. iulianae sp. nov. is characterized by the lack of ornamentation at the insertion of the P4 endopod in both sexes: an ornamentation represented by a row of spinules more or less transformed is present in all the Australian species, in K. giselae and K. godavari; (8) the thumb of male P3 is much longer than the apophysis, which is enlarged at the apex in K. iulianae sp. nov., K. eberhardi, K. giselae, K. lakewayi, K. barrambie and K. esbe, the P3 is longer but with different shape of apophysis and thumb in K. godavari; (9) P3 in male with endopod in K. iulianae sp. nov., K. giselae and all Australian species except K. eberhardi where the endopod is missing, as it is missing in K. godavari; (10) P3 in male with two rows of spinules along the outer margin in K. iulianae sp. nov., K. godavari, K. giselae and K. eberhardi, and only one row in K. lakewayi, K. barrambie, K. lined, K. linesae and K. forficulata, and no spinule in K. esbe, K. linel and K. uranusi.
Discussion
When Schminke (Citation2008) re-established the genus Kinnecaris, he gave an emended diagnosis, based partly on previously presented ideas (Schminke Citation1986). The diagnosis was based on 11 characters that, according to this author, were common to the 17 species that he included in the genus. In the same paper, Schminke discussed each character, listing the species where the character was not present (because it was not recorded in an incomplete description), and stated also that “Not all of these characters have been documented for all species, but it can be expected that re-examination would reveal them (as is already the case with the redescription of P. arenosus by L. Fischer and Th. Glatzel, in prep.)” (1986, p.1251). For K. aetiophica (Cottarelli & Bruno Citation1995), K. impervia (Cottarelli & Bruno Citation1995), K. quollensis (Cottarelli & Bruno Citation1995; for the last two species, only males were described) and K. lyncaea (Cottarelli & Bruno Citation1994; this latter species will be further discussed below), some of the 11 characters were not in the original description, but the re-examination of the specimens in our collection allowed us to assess their status. The analysis of these species from Sierra Leone and Ethiopia, of the Indian species described after Schminke’s paper by Ranga Reddy and Schminke (Citation2009), of the seven species described from Australia by Karanovic and Cooper (Citation2011) and of the three species reported in this paper, shows instead that some of the characters listed by Schminke (Citation2008) and used in his diagnosis of the genus are not shared by all the species, even in those recently and accurately described. In more detail, the discrepancies are the following:
Character 1: Pitted cuticle. The two new Turkish species have a smooth integument, as occurs in K. solitaria, K. lakewayi, K. linesae, K. arenicola (Chappuis, 1954), K. aetiophica and K. impervia. This character state is unknown for K. arenosus (Fryer, 1956), K. fluviatilis (Wells, 1964) and K. sinoiaca (Wells, 1964), although Schminke (Citation2008) reports unpublished data stating that the cuticle of K. arenosus is indeed pitted. In all remaining Kinnecaris and K. iulianae sp. nov., the pitted cuticle is present, with pits of varying depth and density. The lack of this character in several Kinnecaris is not surprising, since it is present in other taxa of Parastenocarididae, e.g., Monodicaris christiani (Dumont, 1981), M. larsi Schminke, 2009, Parastenocaris janae Karanovic, 2006, Cottarellicaris luciae (Cottarelli, Bruno and Berera, 2008) and Remaneicaris ignotus (Dussart, 1983) and, although Corgosinho et al. (Citation2007) consider this character synapomorphic for R. ignotus, its presence in the phylogenetically distant Parastenocarididae suggests that this feature might be the result of convergence.
Character 2: Latero-ventral integumental windows on urosomites 4 and 5 are missing only in K. variolata (Chappuis, 1952) and in the male of K. impervia; their presence in K. arenicola and K. muscicola (Chappuis, 1936) and in the females of K. quollensis and K. impervia, is unknown. This feature is widespread in Kinnecaris, but it is also diagnostic for other genera (e.g., Monodicaris Schminke, 2009).
Character 3: Two groups of spinules ventrally on the third urosomite in male. They are present in the three new species described here, in K. aethiopica, K. eberhardi, K. lyncaea, K. quollensis, K. giselae, K. godavari, K. arenosus, K. linel, K. barrambie, K. linesae, K. uranusi and K. esbe. Therefore, this character is missing only in K. impervia and in two of the recently described Australian species.
Character 4: Caudal rami with setae I–III located at 2/3 of the ramus length; dorsal seta (VII) inserted at 2/3 or more proximally. This character is present in all Kinnecaris except K. iulianae sp. nov., where the setae are inserted at about half of the ramus length. However, setae I–II and VII are distally inserted in several taxa of Parastenocarididae (e.g., in Stammericaris Jakobi Citation1972; Cottarellicaris, Schminke Citation2013).
Character 5: Antennule in male with typical “pocket-knife shape” sensu Schminke (Citation2010). This character is very conservative in Kinnecaris, being present for all the species where the male antennules were described, in the new three species as well and in K. arenosus (Schminke Citation2008). This character is probably not exclusive to the genus (see discussion on K. lyncaea).
Character 6: Endopod of P1 longer than exopod, with enp-1 mostly longer than exp-1 + exp-2. There is no information for K. arenicola, K. cornuta or K. variolata. In five species (K. aethiopica, K. lyncaea, K. sinoiaica, K. impervia and K. quollensis – for the latter two, only males have been described), they appear to be of the same length, whereas in all of the remaining species, not only is the endopod longer than the exopod, but also the first segment of the endopod is longer than the first two segments of the exopod together. But this character is present in other taxa of Parastenocarididae, e.g., Stammericaris diversitatis (Cottarelli and Bruno, Citation2012), Dussartstenocaris idioxenos Karanovic and Cooper (Citation2011), Asiacaris dispar Cottarelli et al. (Citation2010) and Remaneicaris ivoneae Corgosinho, Martínez Arbizu and dos Santos-Silva, 2010.
Character 7: Spinules arranged longitudinally along the outer border of exp-2 and exp-3 of P2 and P4 of both sexes, and of exp-2 of P3 in females. The spinular rows are present in all species except possibly in K. madagascarensis, for which there is no information. However, as reported by Schminke (Citation2008), this character was not taken into account in the earlier descriptions, and these rows are not documented for all three appendages in all cases. The species from Australia, Ethiopia (the status in the females of K. quollensis and K. impervia is unknown), India and Papua New Guinea, however, have spinular rows on all legs and segments. Only in K. iulianae sp. nov. is the row missing on the exp-2 of the female P2. Although this character seems to well characterize the genus Kinnecaris, it is present as well in M. larsi and M. cataractae (Cottarelli pers. obs.).
Character 8: Outer terminal seta of exp-3 of P4 in both sexes, and of P3 in female, remarkably shorter than inner terminal seta, in female P3 even shorter than seta at outer distal corner of exp-1. The outer terminal seta is very short in all species, including the three described here, with the possible exceptions of K. arenicola and K. madagascarensis, and of the females of K. quollensis and K. impervia, for which there is no information (see also discussion on K. lyncaea below). However, this character is present in M. larsi as well.
Character 9: P3 exopod in male with two longitudinal rows of spinules along outer border, one proximally, the other distally near the thumb insertion. For K. fluviatilis and K. solitaria, this character is unknown since males were not described. There are no spinules in K. madagascarensis (Chappuis, 1952), K. esbe, K. linel or K. uranusi, whereas in K. draconis sp. nov., K. arenicola, K. arenosus, K. cornuta, K. lakewayi, K. barrambie, K. lined, K. linesae and K. forficulata (the type species of the genus), there is only one row. Two rows are present in species belonging to other genera, e.g., Monodicaris larsi, Stammericaris diversitatis.
Character 10: Basis of male P4 with a row of spinules above insertion of endopod. For K. fluviatilis and K. solitaria, males were not described. The row of spinules is missing in K. iulianae sp. nov., K. arenosus, K. caffer (Chappuis, 1936), K. muscicola and K. variolata. In K. quollensis, near the origin of the P4 endopod, “two curved, finger like appendices, are inserted” (Cottarelli & Bruno Citation1995, p. 473). According to Schminke (Citation2008), the row has been lost secondarily in a few species.
Character 11: P5 similar in shape and size for both sexes, triangular, with long posteriorly extended, outwardly curved, spiniform process; P5 with three setae with the middle one longest. The middle seta is not the longest one in K. xanthi sp. nov. or K. draconis sp. nov. The shape, size and number of setae of P5 are peculiar and conservative within the genus, whereas the relative lengths of the three setae (excluded ancestral baseoendopodal one) are more variable. Sexual dimorphism in shape and size occurs in K. lyncaea and, according to Karanovic and Cooper (Citation2011), the P5 of males in K. madagascarensis is much smaller than the female’s, to the point that Karanovic and Cooper (Citation2011) suggest that the two sexes might belong to different species.
Schminke (Citation2008) stated that some of these characters cannot be assessed in the old and incomplete description, in K. solitaria and K. fluviatilis for the lack of description of the males, and these characters might be found if some of the older species were re-examined, as it occurred for K. arenosus which is presently being redescribed by Fisher and Glatzel (cited as in preparation by Schminke Citation2008), and for the A1, P5 and P6 of K. lyncaea which are redescribed in this paper (see below). The absence of some characters could be due to a secondary loss in a few species but, in our opinion, such characters should not be included in the diagnosis of the genus.
Notwithstanding the recorded variability in some diagnostic characters, Kinnecaris is a valid genus because it includes species which all share the following characterizing features: the peculiar shape of male A1 (character 5); the ornamentation of the second and last segment of P2 and P4 exopods in both sexes, and of the last segment of P3 in females (character 7), although the status of this character in the closely related genus Monodicaris must be further investigated; and the shape and size of P5 (character 11) and also its insertion and position on the genital somite. However, Schminke (Citation2008) used all 11 characters for the diagnosis of the genus, regardless of whether they were ubiquitously present in all the species of Kinnecaris. In our opinion, the diagnosis of the genus should be emended by omitting some of these characters, as follows:
Kinnecaris Jakobi Citation1972
Emended diagnosis: Body frequently with pitted cuticle. Lateroventral integumental windows often present on urosomites 4 and 5 of both sexes. Furcal rami with lateral group of three setae located from half to more distally of ramus length, dorsal seta also inserted there or even more proximally. Antennules of the male eight-segmented, with much-dilated fifth segment and forming with sickle-shaped seventh segment a powerful “pocket-knife”. Antennule of female seven-segmented. Exp-2 and exp-3 of leg 2 and of leg 4 of both sexes, and exp-2 of leg 3 in females, with longitudinal rows of spinules along outer border. Leg 5 in both sexes of similar size and shape, plate-like and triangular, with long posteriorly extended, outwardly curved, spiniform process; apart from basal outer seta with three setae, inner border without spinules. P5 reaching the insertion of P6 in males or to half of the double genital somite in females, in both sexes the P5 does not adhere to the urosome but instead projects outward to form a pronounced angle with the genital somite (see for instance in Karanovic & Cooper (Citation2011), in Ranga Reddy & Schminke (Citation2009) and , in this paper).
Type species: Kinnecaris forficulata (Chappuis, 1952).
Other species: K. aethiopica (Cottarelli & Bruno, Citation1995), K. arenicola (Chappuis, 1954), K. arenosus (Fryer, 1956), K. barrambie Karanovic and Cooper Citation2011, K. caffer (Chappuis, 1936), K. cornuta (Chappuis, 1955), K. draconis sp. nov., K. eberhardi (Karanovic, Citation2005), K. esbe Karanovic and Cooper Citation2011, K. fluviatilis (Wells, 1964), K. giselae (Schminke, Citation2008), K. godavari (Ranga Reddy & Schminke Citation2009), K. impervia (Cottarelli & Bruno Citation1995), K. iulianae sp. nov., K. lakewayi Karanovic and Cooper Citation2011, K. lined Karanovic and Cooper Citation2011, K. linel Karanovic and Cooper Citation2011; K. linesae Karanovic and Cooper Citation2011, K. madagascarensis (Chappuis, 1952), K. muscicola (Chappuis, 1936), K. quollensis (Cottarelli & Bruno, Citation1995), K. sinoiaica (Wells, 1964), K. solitaria (Karanovic, Citation2004), K. uranusi Karanovic and Cooper Citation2011, K. variolata (Chappuis, 1952), K. xanthi sp. nov.
Remarks on the taxonomic status of Kinnecaris lyncaea (Cottarelli & Bruno, Citation1995)
The re-examination of the material of the African Kinnecaris in our collection allowed us to assess that all but one species fit well in Schminke’s (Citation2008) diagnosis for the genus, and to the emended diagnosis we present here. The only exception is Kinnecaris lyncaea, which will be discussed hereafter.
We carefully analyzed the specimens of Kinnecaris lyncaea from the typical series in Cottarelli’s collection to verify some of Schminke’s (Citation2008) characters, which had not been originally described, with the aim of emending the original description. We observed that: (1) the double dorsal cuticular window is present on the cephalothorax and three lateral windows on the urosomites in both sexes; (2) the male A1 was portrayed and described in lateral view in the original paper (Cottarelli & Bruno Citation1994), which does not allow detection of the characteristic pocket-knife shape sensu Schminke (Citation2010). This shape can, in fact, be observed in , which shows the male A1 in dorsal view. The same figure shows the correct ornamentation of the antennule; (3) the exp-3 of P2 in both sexes does not carry a lateral spinular row and a row of few spinules (three or four) is laterally aligned on the exp-2 of P2. This latter feature was already shown in in Cottarelli and Bruno (Citation1994) for the male; (4) the P3 endopod of females (Figure 1g in Cottarelli & Bruno Citation1994, erroneously labelled as P2 endopod in captions) is represented by a small, flattened article with round tip, which does not resemble that of any other Kinnecaris; the exopod of females carries four small spinules aligned on the second segment; (5) the P4 endopod is missing in females. This latter character was recorded and discussed by Cottarelli and Bruno (Citation1994) but it was not evaluated by Schminke (Citation2008) nor, later on, by Karanovic and Cooper (Citation2011); (6) the last exopodal segment of P4 in both sexes does not have a lateral spinular row; (7) the apical outer seta of the last exopodal segment of P4 in both sexes, and of the last exopodal segment of P3 in females, is not remarkably shorter than the apical inner seta; (8) P5: from the original drawings, it is difficult to detect if the P5 are sexually dimorphic in size. The re-examination of the specimens and of the drawings in the original paper showed that the P5 of females are smaller than those of males (length P5 female/length P5 male = 1.15). Moreover, in the female P5, the cuticular pore is missing, the seta close to the basipodal one is very short and strong, almost spiniform and the inner distal corner is produced into a stronger and more sharpened tip than in the male (see Figure 1d in Cottarelli & Bruno Citation1994). A new drawing of the male holotype P5 is presented here (). Moreover, the recent re-examination of a male paratype in lateral view showed how the P5 do not project outwards as in all members of the genus Kinnecaris, but they adhere to the somites; (8) lateral elliptical windows are present on the third, fourth and fifth urosomites in both sexes.
Figure 13. Kinnecaris lyncaea (Cottarelli and Bruno, Citation1994). A, male, antennule, dorsal view. B, male, first and second urosomites, leg 5 and leg 6, ventral view. Scale bar: 50 µm.
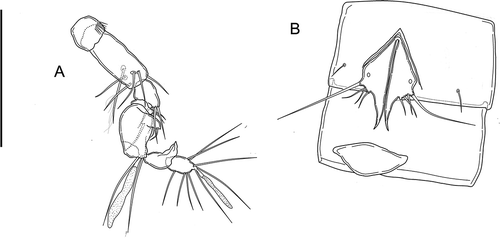
This new analysis suggests that K. lyncaea does not fit into to the generic diagnosis of Schminke (Citation2008), nor into the new diagnosis proposed in this paper. In our opinion, the differential characters described above are more taxonomically discriminant than the (few) remaining characters diagnostic for the genus. Moreover, we think that the inclusion of this species in Kinnecaris would start transforming this genus into a taxonomic repository, as has occurred for other genera of Parastenocarididae. Kinnecaris lyncaea should be considered species inquirenda until new research on the close genus Monodicaris, and further comparison with other African taxa such as, for instance, K. madagascarensis, and the desirable finding of new taxa from this continent, will help to define clear and solid phylogenetic relationships among these Parastenocarididae.
Distribution
Because parastenocaridids have no marine relatives or modern pathways between different continents (Boxshall & Jaume Citation2000), it has been postulated that they have a Pangean origin (Karanovic Citation2004). According to Karanovic (Citation2004), for instance, Parastenocarididae started colonizing subterranean waters of Australia just after the Permo–Carboniferous glaciation, which reached almost all the land that become the Gondwana supercontinent and covered the entire Australian plate (Playford Citation2003, cited in Karanovic & Cooper Citation2011). According to Corgosinho et al. (Citation2012), three genera of Parastenocarididae originated in Gondwana, i.e., Kinnecaris, Siolicaris Jakobi (Citation1972) and Remaneicaris Jakobi (Citation1972). In particular, the genus Kinnecaris had so far been found in Africa, the Oriental region sensu Morrone (Citation2002) and Australia (Schminke Citation2008; Ranga Reddy & Schminke Citation2009; Karanovic & Cooper Citation2011) and, due to the ancient origin of harpacticoids (e.g., 310 Ma according to Selden et al. Citation2010), it is plausible to assume that parastenocaridids occurred on the Gondwana landmass prior to its breakup, and justify the present distribution in all continents (excluded, presently, Antarctica) through vicariance events. However, it is not possible to disprove that short-term dispersal could have led to the present distribution of some taxa of this family. Antrophic activities involving passive dispersal and transport by wind, rain or human vectors cannot be ruled out either (Kulhavy & Noodt Citation1968; Schminke Citation1971; Schabetsberger et al. Citation2009; Karanovic & Lee Citation2012).
As regards the origin of the distribution of the new species described here, K. iulianae was collected on the small continental island of Pha-Ngan, which today is located 47 km offshore from the mainland in an area which was emergent terrestrial for much of the Mesozoic and Cenozoic (Hall, pers. comm.), i.e., before 45 Ma. The continental core of Southeast Asia was assembled in the Late Palaeozoic and Early Mesozoic by the addition of continental fragments carried from Gondwana to Southeast Asia (Metcalfe Citation2011). The origin of the stygofauna of Pha-Ngan Island might as a consequence be related to vicariance and dispersal events, and is complicated by the complex geological history of the island, and by the paucity of information on the harpacticoid biocoenosis from freshwater habitats of the adjacent mainland. In fact, most of the investigations on groundwater-dwelling fauna of Southeast Asia have been conducted in caves (see Brancelj et al. Citation2013 for a review); the only other Parastenocarididae recorded so far are Asiacaris dispar Cottarelli et al. (Citation2010) from the interstitial hyporheic habitat of Pha-Ngan Island (see below), Parastenocaris arganoi Cottarelli and Mura, 1982 from a cave in Malaysia, Parastenocaris distincta Cottarelli, Bruno and Berera, 2006 and Horstkurticaris mangyans (Bruno & Cottarelli, 1999) from the interstitial hyporheic habitat in the Philippines. All of these taxa are phylogenetically distant from Kinnecaris.
Turkey consists of several continental fragments which were joined together into a single landmass in the late Tertiary. Turkey is geologically part of the great Alpine belt that extends from the Atlantic Ocean to the Himalaya Mountains and was formed during the Cenozoic Era (about 66 to 1.6 Ma). Present-day Anatolia comprises several lithospheric fragments; while the fragments now in northern Anatolia are assumed to be part of Eurasia, the other continental pieces were detached from Gondwana and carried northward during the Late Mesozoic and Cenozoic (see Çinku et al. Citation2011 and citations therein). The two watersheds now hosting the two new species (K. xanthi sp. nov. in the Marmara and K. draconis sp. nov. in the Antalya and West Mediterranean watersheds) are in an area that was one such Gondwanian continental piece (Okay Citation2008). Since Kinnecaris had not been recorded from the Palearctic Region before, the discovery of these two new species in Anatolia supports the hypothesized ancient and, probably, Gondwanian origin of the genus Kinnecaris, and speciation by means of vicariance. The new Anatolian Kinnecaris species do not show strict affinities with the congeneric species; they could represent a distinct phyletic lineage within the genus as occurred, for instance, for the Australian Kinnecaris (Karanovic & Cooper Citation2011). However, inferences should be drawn cautiously, since large geographical areas represent terra incognita for the presence (or absence) of this genus and of other Parastenocarididae.
The genus Kinnecaris is, or was, rather well distributed in Anatolia; in fact, in June 1970, we collected few females and several copepodites of Kinnecaris, possibly belonging to one of the two new Anatolic species, from the Isparta Stream, near Antalya, the Biçkici River and other smaller streams flowing from the Taurus Mountains into the Mediterranean Sea. Hence, based on the few data from our collections, the genus Kinnecaris is the most widespread Parastenocarididae in Anatolia, having been collected from several water bodies. In fact, only one other Parastenocarididae was so far known for Turkey: Parastenocaris phyllopora, collected from the mesopsammal of Lake Iznik, but we also collected two new species of Proserpinicaris from three different rivers and one new Stammericaris from Lake Psammal (Cottarelli, unpub. data). One more species of Kinnecaris, probably different from those described here, was also collected by Dr. Ahmet Bozkurt of Mustafa Kemal University Fisheries Faculty, Turkey (pers. comm. to V. Cottarelli) and is possibly under study. All of these unpublished records underline how Turkish groundwater might be very rich in Parastenocarididae.
Acknowledgements
We would like to thank the personnel of Than Sadet Koh Pha-Ngan National Park for the useful information provided to V. Cottarelli, which allowed collection of the material. Three referees greatly improved the manuscript with their comments and editing. Research was partly supported by the Ministero dell’Istruzione, dell’Università e della Ricerca ex-60% funding to V. Cottarelli.
References
- Boxshall GA, Jaume D. 2000. Making waves: the repeated colonization of fresh water by copepod crustaceans. Advances in Ecological Research 31:61–79. doi:10.1016/S0065-2504(00)31007-8.
- Brancelj A, C Boonyanusith, Watiroyram S, Sanoamuang LO. 2013. The groundwater-dwelling fauna of South East Asia. Journal of Limnology 72:327–343. doi:10.4081/jlimnol.2013.s2.e16.
- Çinku MC, Hisarlı ZM, Heller F, Orbay N, Ustaömer T. 2011. Middle Eocene paleomagnetic data from the eastern Sakarya Zone and the central Pontides: Implications for the tectonic evolution of north central Anatolia. Tectonics 30:TC1008. doi:10.1029/2010TC002705.
- Corgosinho PHC, Martínez-Arbizu P. 2005. Two new interstitial species of Remaneicaris Jakobi (Copepoda, Harpacticoida, Parastenocarididae) from the Ribeirão do Ouro river, Brazil, with a redefinition of the genus. Senckenbergiana Biologica 85:147–162.
- Corgosinho PHC, Martínez-Arbizu P, dos Santos-Silva EN. 2007. Redescription of Remaneicaris ignotus (Dussart, 1983), a Parastenocarididae (Copepoda, Harpacticoida) with an unusual set of plesiomorphic characters. Invertebrate Zoology 4:31–44.
- Corgosinho PHC, Martínez-Arbizu P, dos Santos-Silva EN. 2010. Revision of Brasilibathynellocaris Jakobi, 1972 (Copepoda: Harpacticoida: Parastenocarididae) with redefinition of the genus. Zoological Journal of the Linnean Society 159:527–566. doi:10.1111/j.1096-3642.2009.00574.x.
- Corgosinho PHC, Ranga Reddy Y, Martínez-Arbizu P. 2012. Revision of the genus Siolicaris Jakobi, 1972, with redescriptions of S. sioli (Noodt, 1963) and S. jakobi (Noodt, 1963) from South America, and S. sandhya (Ranga Reddy, 2001) comb. nov. from India (Copepoda, Harpacticoida, Parastenocarididae). Zootaxa 3493:9–71.
- Cottarelli V, Bruno MC. 1994. Parastenocaris lyncaea n. sp. and Stygoelaphoidella africana n. sp. (Crustacea, Harpacticoida) from interstitial continental waters of Sierra Leone. Quaderni Accademia Nazionale dei Lincei 267:97–109.
- Cottarelli V, Bruno MC. 1995. First record of Parastenocarididae (Crust., Cop., Harpact.) from subterranean waters of Ethiopia and the description of three new species. Journal of African Zoology 109:467–479.
- Cottarelli V, Bruno MC, Berera R. 2010. First record of Parastenocarididae from Thailand and description of a new genus (Copepoda: Harpacticoida). Journal of Crustacean Biology 30:478–494. doi:10.1651/09-3201.1.
- Delamare Deboutteville C. 1960. Biologie des eaux souterraines littorales et continentales. Paris: Hermann.
- Huys R, Boxshall GA. 1991. Copepod Evolution. London: The Ray Society.
- Huys R, Gee JM, Moore CG, Hamond R. 1996. Marine and brackish water harpacticoid copepods part 1: keys and notes for identification of the species. London: The Linnean Society of London.
- Jakobi H. 1972. Trends (Enp. P4) innerhalb der Parastenocarididen (Copepoda Harpacticoidea). Crustaceana 22:127–146. doi:10.1163/156854072X00390.
- Karanovic T. 2004. Subterranean Copepoda from arid Western Australia. Crustaceana Monograph 3:1–366.
- Karanovic T. 2005. Two new subterranean Parastenocarididae (Crustacea, Copepoda, Harpacticoida) from Western Australia. Records of the Western Australian Museum 22:353–374.
- Karanovic T, Cooper SJB. 2011. Molecular and morphological evidence for short range endemism in the Kinnecaris solitaria complex (Copepoda: Parastenocarididae), with descriptions of seven new species. Zootaxa 3026:1–64.
- Karanovic T, Lee W. 2012. Invertebrate Fauna of the World. Volume 21, Number 2. Arthropoda: Crustacea: Harpacticoida: Parastenocarididae Parastenocaridid Copepods. Korea: National Institute of Biological Resources.
- Kulhavy V, Noodt W. 1968. Über Copepoden (Crustacea) aus dem limnischen Mesopsammal Islands. Gewässer und Abwässer 46:50–61.
- Metcalfe I. 2011. Palaeozoic-Mesozoic History of SE Asia. Geological Society London Special Publications(1):7–35. doi:10.1144/SP355.2.355.
- Morrone JJ. 2002. Biogeographical regions under track and cladistic Scrutiny. Journal of Biogeography 29:149–152. doi:10.1046/j.1365-2699.2002.00662.x.
- Okay AI. 2008. Geology of Turkey: A synopsis. Anschnitt 21:19–42.
- Playford P. 2003. The Permo-Carboniferous glaciations of Gondwana: its impact on Western Australia. Western Wildlife 7:1–5.
- Ranga Reddy Y, Schminke HK. 2009. Discovery of the genus Kinnecaris Jakobi, 1972 (Copepoda, Harpacticoida,Parastenocarididae) in southeastern India, with description of a new species. Crustaceana 82:311–326. doi:10.1163/156854009X409117.
- Reid JW. 1995. Redescription of Parastenocaris brevipes Kessler and description of a new species of Parastenocaris (Copepoda: Harpacticoida: Parastenocarididae) from the U.S.A. Canadian Journal of Zoology 73:173–187. doi:10.1139/z95-020.
- Schabetsberger R, Drozdowski G, Rott E, Lenzenweger R, Jersabek C, Fiers F, Traunspurger W, Reiff N, Stoch F, Kotov A, Martens K, Schatz H, Kaiser R. 2009. Losing the Bounty? Investigating species richness in isolated freshwater ecosystems of oceania. Pacific Science 63:153–179. doi:10.2984/049.063.0201.
- Schminke HK. 1971. Zwei neue Parastenocaris-Arten (Copepoda Harpacticoidea) von Tenerife. Gewässer und Abwässer 50–51:66–75.
- Schminke HK. 1986. The systematic confusion within the family Parastenocarididae (Copepoda, Harpacticoida). In: Schriever G, Schminke HK, Shih CT, editors. Proceedings of the Second International Conference on Copepoda, Ottawa, Canada, 13-17 August, 1984. Syllogeus 58:635. Ottawa: National Museum of Natural Sciences.
- Schminke HK. 2008. First report of groundwater fauna from Papua New Guinea: Kinnecaris Jakobi, 1972 redefined (Copepoda, Harpacticoida, Parastenocarididae), and description of a new species. Crustaceana 81:1241–1253.
- Schminke HK. 2010. High-level phylogenetic relationships within Parastenocarididae (Copepoda, Harpacticoida). Crustaceana 83:343–367. doi:10.1163/001121610X12627655658168.
- Schminke HK. 2013. Stammericaris Jakobi, 1972 redefined and a new genus of Parastenocarididae (Copepoda, Harpacticoida). Crustaceana 86:704–717. doi:10.1163/15685403-00003196.
- Selden PA, Huys R, Stephenson MH, Heward AP, Taylor PN. 2010. Crustaceans from bitumen clast in Carboniferous glacial diamictite extend fossil record of copepods. Nature Communications 1:1–6. doi:10.1038/ncomms1049.
