Abstract
The medfly (Ceratitis capitata Wiedemann, 1824) is a widespread pest for horticulture, mainly targeting fruits of pomaceous and citrus cultivars. Scanty data is available about its chemosensory responses to contact salty, sweet, sour and bitter stimuli. High Resolution Scanning Electron Microscopy (HRSEM) and Transmission Electron Microscopy (TEM) observations indicate the presence of six pairs of Long type and about 40 pairs of Intermediate type labellar chemosensilla, each containing four different neurons. Spike activity was electrophysiologically recorded from the labellar sensilla in response to sodium chloride (NaCl), fructose, acids (citric and malic) and bitter compounds (quinine, quercetin and 6-n-propyl-thiouracil (PROP)). By analysis of spike waveforms in the responses, four neurons were identified responding to different stimuli in both sensillum types of C. capitata. The response specificity of three of these cells (named M1, M2 and S) was determined on the basis of their dose-response profiles. “M1” was specific to NaCl, “M2” to fructose. Both acids appeared to excite the “S” cell and to inhibit the “M1” cell. Citric acid also partly inhibited the response of the “M2” cell. No cell clearly responded to any of the bitter stimuli.
Introduction
The Mediterranean fruit fly or medfly, Ceratitis capitata (Wiedemann 1824) (Diptera: Tephritidae), is a polyphagous pest that infests numerous plant species of agronomic interest, such as pomaceous (apples and pears) and citrus (oranges) cultures, and easily adapts to novel host plants according to their availability (Malacrida et al. Citation2007).
The reproductive behaviour of the medfly is relatively well studied (Eberhard Citation2000; Sivinski et al. Citation2000; Yuval & Hendrichs Citation2000; Marchini & Del Bene Citation2006; Gomulski et al. Citation2012; Harwood et al. Citation2013). Receptive females are attracted to gatherings (leks) of males emitting sex pheromone (Papadopoulos et al. Citation2004). The after-mating behaviour of females diverges from that of males, switching to host-fruit locating for oviposition (Jang et al. Citation1998). Medfly females use different sensory modalities such as sight, smell and taste to identify potential host-plant fruits on which to lay eggs (Fletcher & Prokopy Citation1991). Ovipositing females mark fruits with a deterrent pheromone to prevent further egg-laying by other females (Arredondo & Díaz-Fleischer Citation2006). The behavioural strategies underlying male aggregation, mating, selection and localization of host plants are largely conditioned by the neural input arising from olfactory and taste sensilla (Jang et al. Citation1998). Moreover, the gustatory system represents a model to study the mechanism of food intake, and its chemosensory pathways are considered ideal targets for the development of novel and useful control methods for this species.
Typically, taste neurons in Diptera are bipolar cells: somata are located at the base of the sensillum and dendrites extend into the sensillum shaft up to the tip, whereas axons project into the subesophageal ganglion (Ishimoto & Tanimura Citation2004; Wang et al. Citation2004; Amrein & Thorne Citation2005; Sellier et al. Citation2011).
On the basis of the morphology of their cuticular surface, supported by ultrastructure and electrophysiology, chemosensilla have been classified in a variety of morphological types (Schneider Citation1964; Zacharuk Citation1985) possessing a chemosensory function (Zacharuk & Shields Citation1991). Taste chemosensilla of flies are typically uniporous and house a variable number (i.e. two to six) of sensory neurons (Adams et al. Citation1965; Dethier Citation1976).
In tephritid flies, information about size and distribution of the labellar taste sensilla is limited to a few species (Gothilf et al. Citation1971; Crnjar & Prokopy Citation1982; Crnjar et al. Citation1989). In C. capitata, Gothilf and colleagues (Citation1971) showed that tarsal and labellar chemosensilla respond to salts and sugars.
Pest management of the medfly has mainly being done by the Sterile Insect Technique (SIT) that uses infertile matings of sterile males to wild females with the aim of decreasing its population distribution (Ogaugwu et al. Citation2013). Tephritid fruit flies have been recently used as laboratory models for aging and biodemography research (Papadopoulos et al. Citation2010; Carey Citation2011). Obtaining new information on the chemosensory profiles of the medflies could be helpful in designing novel pest control strategies.
The aim of the present study is to provide information about the taste chemosensory system in the labellum of the medfly, in both sexes, and to describe the response profiles of the labellar sensilla to salty, sweet, sour and bitter taste stimuli.
Materials and methods
Insects
Morphological and electrophysiological experiments were performed on adult male and female medflies 3–5 days old, obtained from a colony reared at the Department of Animal Biology, University of Pavia (Italy). Males and females were kept separate to avoid reciprocal exposure, under the following conditions: 22 ± 1°C and 70–80% relative humidity.
Electron microscopy
Sixteen females and males of C. capitata were treated for transmission electron microscopy (TEM) and high resolution scanning electron microscopy (HRSEM). For HRSEM analysis, C. capitata flies were fixed with a mixture of 0.5% glutaraldehyde and 0.5% paraformaldehyde in 0.1 M cacodylate buffer, pH 7.2, at room temperature for 2 hours. Some specimens were fixed up to 24 hours in order to prevent collapse of sensilla. The medflies were sonicated (3 × 20 s) in 2% Triton X-100 at room temperature, in order to remove any material that would prevent observation of details from the cuticular surface. Finally, insects were washed in a phosphate buffered saline (PBS) (3 × 30 min) at room temperature; specimens were dehydrated through a graded acetone series, and subjected to Critical Point Drying (CPD) with carbon dioxide (CO2). Samples were immobilized on aluminium electron microscopy stubs and coated with platinum (2 nm) by an Emitech 575 turbo sputtering apparatus. Observations were carried out with a Hitachi S-4000 field emission HRSEM, operated at 15–20 kV. Image acquisitions were obtained by the software Quartz PCI v. 5 (Quartz Imaging Corporation, Vancouver, BC, Canada). Over 500 images were collected by HRSEM at different magnifications. Measurements were obtained on HRSEM images and carried out with Image Tool v. 3.0 for Windows (University of Texas Health Science Centre in San Antonio). For TEM analysis, insects were fixed with a mixture of 1.25% glutaraldehyde and 1% paraformaldehyde in 0.1 M cacodylate buffer, pH 7.2, at room temperature for 2 hours. Specimens were included in epoxy resin and ultrathin sections, stained with uranyl acetate and bismuth subnitrate (Riva Citation1974), and observed by a JEOL 100S TEM operated to 80 kV.
Electrophysiology
Experiments were performed on medflies of both sexes deprived of food and water for 12 hours before taste stimulation. Room temperature and humidity conditions (22 ± 1°C and 70–80% relative humidity) were kept constant during electrophysiological experiments.
Spike activity from the chemosensory cells of the two types of sensilla, identified through the morphological observations, was recorded by means of the tip-recording technique (Hodgson et al. Citation1955). All recording operations were carried out by means of micromanipulators under the field of a stereomicroscope. A thin silver/silver chloride (Ag/AgCl) wire serving as a ground electrode was inserted into an isolated head through the “foramen magnum”. The recording electrode, a glass micropipette (tip diameter = 40 μm) filled with the stimulating solution, was placed over the sensillum tip.
A second Ag/AgCl wire connected the stimulating/recording electrode to the input of the electrometer (WPI Duo 773). Recordings were band-pass filtered (0.1–3 KHz), digitized by means of the Axon Digidata 1442A A/D converter (sampling rate: 10 KHz) and stored on a PC for further analyses. Stimulating solutions were applied in increasing concentrations to the sensilla for 2–3 s, and a 3-min interval was allowed between consecutive solutions to minimize adaptation phenomena. Spike firing frequency was analysed in the first second, starting 30 ms after stimulation onset, in order to skip any baseline shift induced by the contact artefact.
In order to avoid any drift in solution concentration due to evaporation, a piece of filter paper was used to draw a small amount of solution from the electrode tip just before each stimulation.
Stimuli
The following stimuli were chosen on the basis of preliminary experiments and/or previous studies (Gothilf et al. Citation1971): sodium chloride (NaCl) (10, 100, and 500 mM) and fructose (1, 10, and 50 mM), the latter being present in citrus fruits (Rodriguez-Saona et al. Citation2001). Two acids were selected: malic and citric (0.1, 1 and 20 mM); these are present in medfly host-plants fruits (Bernays et al. Citation1998).
The following bitter stimuli were also assayed: 6-n-propyl-thiouracil (PROP), a compound present in cruciferous plants, which contains a chemical thiocyanate moiety N − C = S responsible for their bitter taste for humans (Fox Citation1932; Harris & Kalmus Citation1949; Guo & Reed Citation2001); quercetin, a flavonol present in citrus fruits (Drewnowski & Gomez-Carneros Citation2000; Masek & Scott Citation2010), and quinine, a natural alkaloid, bitter for humans and a deterrent for insects (Liscia & Solari Citation2000). All bitter stimuli were tested at the following concentrations: 0.01, 0.1 and 1 mM (Sellier et al. Citation2011; Weiss et al. Citation2011).
All these substances were purchased by Sigma-Aldrich and their solutions were prepared using distilled water. NaCl 25 mM was used as a control stimulus and was added to all solutions in order to ensure adequate electrical conductance.
Data analysis and statistics
Active cells were sorted out by performing analysis of spike discharges obtained from the same sensilla with the SAPID Tools software (Smith et al. Citation1990). Responding cells were identified as higher (“H”), middle 1 (“M1”), middle 2 (“M2”) and small (“S”) units, on the basis of the shape and amplitude of their spike responding to salt, sugar and bitter solutions as described in previous papers (Dethier Citation1976; Liscia et al. Citation1998; Liscia & Solari Citation2000).
Statistical differences among data obtained on HRSEM images were determined by a non-parametric test (Mann-Whitney U-test). Differences in spike frequency were evaluated by means of “two-sided” Student “t” (STATISTICA software package) test with a 95% confidence level (p ≤ 0.05).
Results
Morphology
On the basis of HRSEM observations, mouthpart apparatus of C. capitata were formed by two hemilabella. The labellar external surface shows different types of sensilla trichodea (A, B).
Sensilla trichodea have a prominent socket at the base and present a few grooves along the sensillum shaft that converge near the tip. As reported in Drosophila (Shambhag et al. Citation2001), we have identified three sensillum types: Long (L-type), Intermediate (I-type a and b) and Short, according to their length.
Figure 1. A, scanning electron micrograph of the proboscis labella in Ceratitis capitata showing the chemosensilla on the aboral surfaces. B, schematic diagram of a labellum showing the localization of Long, Intermediate and Short type sensilla. The sensilla outlined in white (Long) and black (Intermediate a) are those used for electrophysiological experiments. Other types of sensilla (Intermediate b and Small) are indicated by solid circles and empty circles, respectively. Scale bar: 66 μm.
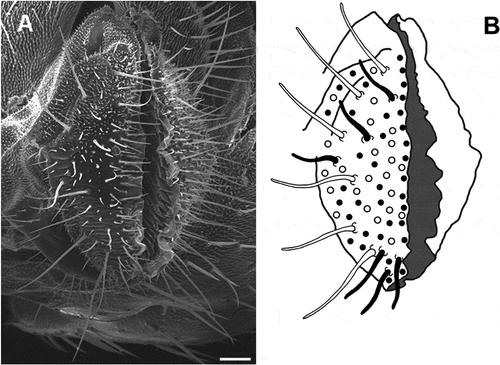
Long sensilla trichodea have a length of 174 ± 47 µm (mean length ± S.D.) and a base width of 5.3 ± 0.39 µm and they are located near the base of the labellum. We have consistently counted six Long sensilla in each hemilabellum (A, B).
Taking length and position into account, Intermediate sensilla trichodea can be classified in two subtypes: a and b. “a” sensilla show a mean length of 64 ± 17 µm and a base width of 4.3 ± 0.67 µm, and they are located in the region spanning from the Long type area to the labellar inner surface. “b” sensilla are shorter (mean length of 48 ± 10 µm) than the previous ones, and are located both immediately beyond the margin of the labellar lobe protruding in front of the oral cavity and among Long type sensilla. About 40 Intermediate type sensilla are observed on each hemilabellum. No variations in the total number of Intermediate and Long type are observed in either sex or between the two lobes.
Short type sensilla trichodea are scattered among Long and Intermediate type; they number about 20 and have a mean length of 17 ± 4 µm and a base width of 1.95 ± 0.22 µm.
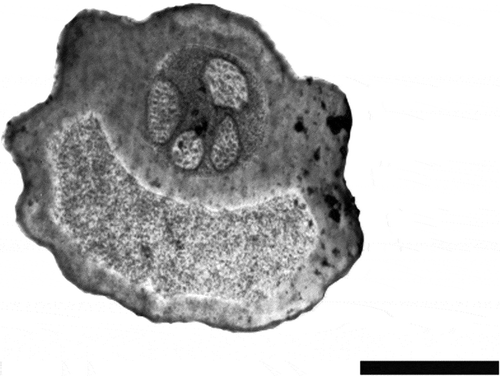
By TEM, cross sections of Long and Intermediate type sensilla trichodea show an external cuticle and a circular lumen, which contains four dendrites of different diameter (n = 26) (). On the basis of their morphology, Long and Intermediate type sensilla have been assigned to the uniporous type.
Electrophysiology
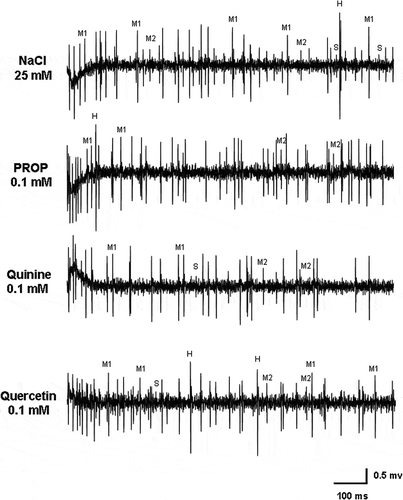
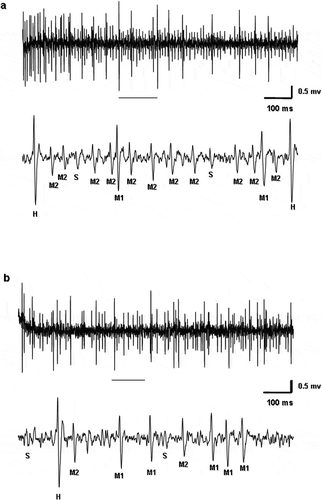
By the electrophysiological approach, salty, sweet, sour and bitter taste stimuli were tested in Long and Intermediate type sensilla, both in males and females. shows a sample discharge from the two types of sensilla in response to NaCl and fructose. In response to these stimuli, we noted that spike activity is produced by different neurons. By analysing spike waveforms, we were able to distinguish four types of neurons that were named by their apparent amplitude as high (H), middle 1 (M1), middle 2 (M2), and small (S) in response to salt and sweet ().
Figure 3. Samples of spike discharges from the (a) Intermediate and (b) Long type sensilla in Ceratitis capitata responding to 50 mM fructose and 500 mM sodium chloride (NaCl) respectively. Portion of the discharges enlarged in the trace underscored. High (H), middle 1 (M1), middle 2 (M2), and small (S) spike types are shown.
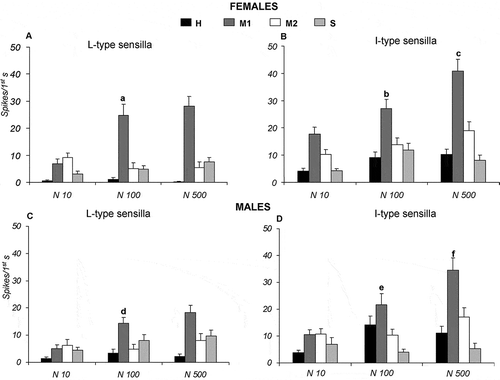
During stimulation with different concentrations (10, 100 and 500 mM) of NaCl, in both Long and Intermediate type sensilla of females and males, in response to increasing salt concentration, only “M1” cell enhances its activity. In particular, Long type sensilla elicit the highest spike activity of “M1” cells with a peak of response of 28.14 ± 3.54 and 18.30 ± 2.72 spikes/s at 500 mM NaCl, respectively, in females and males (A, C). Similarly, Intermediate type sensilla increased the activity of “M1” cells with a peak of response of 40.88 ± 4.24 and 34.59 ± 4.42 spikes/s at 500 mM NaCl, respectively, in females and males (B, D). For the “M1” cells of both sensillum types, all frequency mean values were statistically different from one another (p ≤ 0.05), with the exception of the responses in Long type sensilla of both sexes to NaCl between 100 and 500 mM (A, C).
Figure 4. Dose-response histograms following stimulation with 10, 100 and 500 mM sodium chloride (NaCl) in the Long and Intermediate type sensilla of Ceratitis capitata. A–B, spike firing frequencies (spikes/1st s) from high (H), middle 1 (M1), middle 2 (M2) and small (S) cells in Long and Intermediate type sensilla of females. Mean values (26–40 sensilla) ± SE (vertical bars); C–D, spike firing frequencies (spikes/1st s) from high (H), middle 1 (M1), middle 2 (M2) and small (S) cells in Long and Intermediate type sensilla of males. Mean values (31–59 sensilla) ± SE (vertical bars); “a” indicates significance difference (p < 0.05) in “M1” cell following stimulation with 10 and 100 mM NaCl of Long type sensilla in females; “b” and “c” indicate significant difference (p < 0.05) in “M1” cell of Intermediate type sensilla in females following stimulation with 10, 100 and 500 mM NaCl; “d” indicates significant difference (p < 0.05) in “M1” cell of Long type sensilla in males following stimulation with 10 and 100 mM NaCl; “e” and “f” indicate significant difference (p < 0.05) in “M1” cell of Intermediate type sensilla in males following stimulation with 10, 100 and 500 mM NaCl.
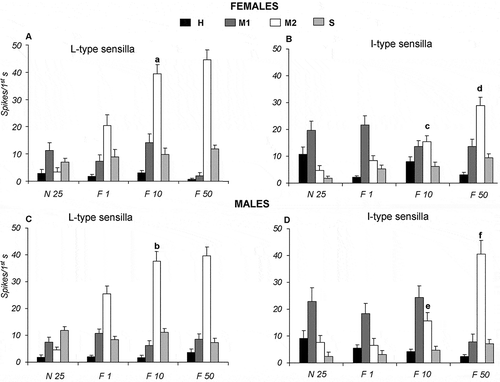
When the same sensilla are stimulated with 1, 10 and 50 mM fructose, a different cell, “M2”, shows an activity increase (A–D). In Long type sensilla of females and males, the results show that following stimulation with fructose, “M2” cells give the largest contribution with mean values (± SE) of 44.58 ± 3.64 and 39.62 ± 3.34 spikes/s, in females and males respectively (A, C). Instead, in Intermediate type in female and males, increasing the fructose concentration enhances significantly (p ≤ 0.05) the activity of “M2” cells in a dose-dependent manner (from 8.32 ± 1.84 to 29 ± 2.95 spikes/s for females and from 6.57 ± 2.59 to 40.57 ± 5.07 spikes/s for males) (B, D). In response to fructose, as observed for salt, all frequency values are statistically (p ≤ 0.05) different from one another for “M2” cells for both Long and Intermediate sensillum types, with the exception of the responses to 10 and 50 mM fructose of Long type of both sexes (A, C).
Figure 5. Dose-response histograms following stimulation with 1, 10 and 50 mM fructose in the Long and Intermediate type sensilla of Ceratitis capitata. A–B, spike firing frequencies (spikes/1st s) from high (H), middle 1 (M1), middle 2 (M2) and small (S) cells in Long and Intermediate type sensilla of females. Mean values (77–86 sensilla) ± SE (vertical bars); C–D, spike firing frequencies (spikes/1st s) from high (H), first intermediate (M1), second intermediate (M2), and small (S) cells in Long and Intermediate type sensilla of male. Mean values (23–70 sensilla) ± SE (vertical bars); “a” and “b” indicate significant difference (p < 0.05) in “M2” cell of Long type sensilla in both sexes following stimulation with 1 and 10 mM fructose; “c” and “d” indicate significant difference (p < 0.05) in “M2” cell of Intermediate type sensilla in female following stimulation with 1, 10, 50 mM fructose; “e” and “f” indicate significant difference (p < 0.05) in “M2” cell of Intermediate type sensilla in males following stimulation with 1, 10, 50 mM fructose.
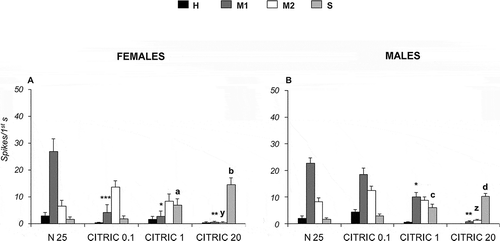
Following stimulation with malic acid at 0.1, 1 and 20 mM in Long type sensilla, the “S” cell increases its activity with respect to the control (25 mM NaCl), from 1.92 ± 0.72 to 4.87 ± 1.03 spikes/s in females and from 2.52 ± 0.90 to 10.53 ± 1.76 spikes/s in males (A, B). In addition, malic acid, at both 1 mM and 20 mM, produces an activity decrease in “M1” cells with respect to the control (p < 0.05). On the other hand, citric acid at 0.1, 1 and 20 mM enhances the spike firing frequency of “S” cells from 1.70 ± 0.92 to 14.60 ± 1.75 spikes/s in females and from 1.66 ± 0.45 to 10.17 ± 1.22 spikes/s in males (A, B). Besides, citric acid (1 mM and 20 mM) decreases the activity of “M1” cells with respect to the control (p < 0.05). Moreover, 20 mM citric acid decreases the activity of “M2” cells.
Figure 6. Dose-response histograms following stimulation with 0.1, 1 and 20 mM malic acid in the Long type sensilla of Ceratitis capitata. A–B, spike firing frequencies (spikes/1st s) from high (H), middle 1 (M1), middle 2 (M2) and small (S) cells in Long type sensilla of female and males respectively. Mean values (15–40 sensilla) ± SE (vertical bars); “a” and “d” indicate significant differences (p < 0.05) in “S” cell between 0.1 mM malic acid and the control 25 mM sodium chloride (NaCl) in females and males respectively; “b” and “e” indicate significant differences (p < 0.05) in “S” cell 1 mM malic acid and the control 25 mM NaCl in females and males respectively; “c” and “f” indicate significant differences (p < 0.05) in “S” cell 20 mM malic acid and the control 25 mM NaCl in females and males respectively; “*” indicates significant difference (p < 0.05) in “M1” cell between 1 mM malic acid and the control 25 mM NaCl in female and male; “**” indicates significant difference (p < 0.05) in “M1” cell between 20 mM malic acid and the control 25 mM NaCl in females and males.
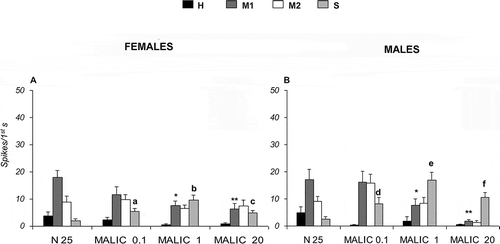
Figure 7. Dose-response histograms following stimulation with 0.1, 1 and 20 mM citric acid in the Long type sensilla of Ceratitis capitata. A–B, spike firing frequencies (spikes/1st s) from high (H), middle 1 (M1), middle 2 (M2) and small (S) cells in Long type sensilla of female and males respectively. Mean values (40–60 sensilla) ± SE (vertical bars); “a” and “c” indicate significant differences (p < 0.05) in “S” cell between 1 mM citric acid and the control 25 mM sodium chloride (NaCl) in females and males respectively; “b” and “d” indicate significant differences (p < 0.05) in “S” cell 20 mM citric acid and the control 25 mM NaCl in females and males respectively; “y” and “z” indicate significant differences (p < 0.05) in “M2” cell 20 mM citric acid and the control 25 mM NaCl in females and males respectively; “*” indicates significant difference (p < 0.05) in “M1” cell between 1 mM citric acid and the control 25 mM NaCl in females and males; “**” indicates significant difference (p < 0.05) in “M1” cell between 20 mM citric acid and the control 25 mM NaCl in females and males; “***” indicates significant difference (p < 0.05) in “M1” cell between 0.01mM citric acid and the control 25 mM NaCl in females.
Bitter stimulation was performed, in Long and Intermediate sensilla types of both sexes, using quinine, PROP and quercetin at the same concentrations (0.01, 0.1 and 1 mM). Our data show that no significant responses were obtained from any of the four neurons with respect to the control (25 mM NaCl) ().
Discussion
Our data provide a morpho-functional description of the taste chemosensory system located in the labellum of C. capitata, that plays a role in mediating food ingestion and the choice of oviposition sites (Gothilf et al. Citation1971; Arredondo & Díaz-Fleischer Citation2006; Coronado-Gonzales et al. Citation2008).
The labellum is characterized by a large population of sensilla trichodea housing taste receptor neurons and located in specific areas. In calliphorid flies, the labellar apparatus presents several subtypes of sensilla trichodea with a specific morphometry and distribution on the aboral surfaces: in Phormia regina, Wilczek (Citation1967) described six types. In Tephritid flies, available information on sensillum types and distribution is limited to a few species (Gothilf et al. Citation1971; Crnjar & Prokopy Citation1982; Crnjar et al. Citation1989).
Drosophila melanogaster is instead a valuable reference species, as a large number of studies exist describing its morphology and taste physiology (Hiroi et al. Citation2002; Montell Citation2009; Sellier et al. Citation2011; Weiss et al. Citation2011). In Drosophila, the labellar apparatus shows three types of chemosensory sensilla, classified into Long, Intermediate and Short (Shambhag et al. Citation2001; Hiroi et al. Citation2002; Montell Citation2009; Sellier et al. Citation2011). Long and Short types contain one mechanoreceptor and four gustatory neurons that respond to water, sugar, low salt or high salt concentration (Rodrigues & Siddiqi Citation1981; Hiroi et al. Citation2002; Sellier et al. Citation2011). On the other hand, Intermediate type sensilla contain only two neurons: one responds to sugar and low salt concentration, while the other responds to high salt concentration and bitter substances (Hiroi et al. Citation2004). Weiss et al. (Citation2011) described five classes of sensilla in Drosophila, on the basis of response to bitter stimuli; four were activated by bitter and one did not elicit any physiological response to bitter.
In the medfly, labellar sensilla trichodea are numerous and, as in Drosophila melanogaster, we have assigned them to four different groups: Long, Intermediate (a and b) and Short (Shambhag et al. Citation2001). According to HRSEM images, each sensillum type is characterized by its own length range and distribution on labellar surface. Moreover, TEM images show the presence of four neurons associated with each sensillum. The number of neurons in each sensillum may vary in different insects: in Drosophila, Long type sensilla house four gustatory receptor neurons and one mechanoreceptor, and Intermediate type sensilla only have two gustatory receptor neurons and one mechanoreceptor (Shambhag et al. Citation2001); labellar and tarsal hairs in Phormia regina contain four gustatory neurons and a mechanoreceptor (Dethier Citation1976).
In order to analyse the taste responses of the labellar chemosensilla in both sexes of the medfly, we described the spike activity of Long and Intermediate type sensilla evoked by salty, sweet, sour and bitter stimuli; we overlooked Short type sensilla as their short size and curved shape make electrophysiological access difficult. By cross-comparing spike amplitudes and shapes, our data confirm the presence of four neurons in C. capitata.
NaCl stimulation evoked, in Long and Intermediate type sensilla of both sexes, an increase of “M1” neuron activity. In particular, “M1” spike activity, in Intermediate type, increases significantly after stimulation with 100 and 500 mM NaCl; on the contrary, Long type spike frequency does not change between these concentrations. For fructose stimulation, a different cell (M2) increases its activity. In fact, in Long type sensilla, “M2” cell spike frequency does not change after stimulation with 10 and 50 mM fructose while, in Intermediate type, this value increases significantly.
Electrophysiological responses, after NaCl and fructose stimulation, indicate that Long type sensilla, in both sexes alike, probably reached response saturation at the highest value tested. In contrast, no saturation was observed in Intermediate type sensilla. This difference could provide space-related information on the localization of stimuli by using differential information from sensilla with different sensitivity profiles and distributions. In fact, fructose is important for the medfly not only as a high energy source, but also for recognizing suitable oviposition sites in host plants (Gothilf et al. Citation1971). Fructose is the most common monosaccharide present in medfly host plants (Rodriguez-Saona et al. Citation2001). On the other hand, salts are essential to regulate electrolyte homeostasis (Lindemann Citation1996; Hiroi et al. Citation2004) and have a phagostimulant effect (Smedley & Eisner Citation1995). Differences in the sensitivity threshold of the various chemosensillum types in response to the same stimuli have already been shown in other insects. In Protophormia terraenovae, Long and Intermediate sensilla have different response profiles to NaCl and sucrose. This suggests that different types of sensilla located on the rostral and caudal ends of the labellum provide a spatial representation of the taste stimuli (Liscia et al. Citation1995, Citation1998).
Citric and malic acid evoked both an increase in “S” cell response and a decrease in the response of “M1” cells in the two sexes. In this respect, acids have been shown to inhibit the activity of salt taste neurons in the blowfly Phormia regina (McCutchan Citation1969). The “S” cell was activated by both acids, but we do not exclude that it may respond to other stimuli as well, in agreement with Goldrich (Citation1973) who reported that blowflies can detect acids, but there is no evidence of the presence of a specific “acid” receptor: the responses to acids are mediated by the activity of the other cells (“salt”, “sugar” and “water”). The fact that the two acids inhibit the “M1” cell also supports what was suggested in Drosophila melanogaster, where acid stimuli both activate the bitter neurons and inhibit the sweet neurons (Charlu et al. Citation2013). In fact, citric acid also seems to inhibit to some extent the “M2” cell. Charlu et al. (Citation2013) suggest that a specific population of “sour” cells may not exist in flies, with acidity evoking excitatory responses in a defined subset of bitter cells.
The juice of apples and citrus fruits, common host plants in the medfly, contains fructose, sucrose, glucose, sorbitol, malic, citric, fumaric acids, sodium and calcium; malic acid is the major acid of apple juice at a concentration of 1738.2 mg/100 mL (Eisele & Drake Citation2005). Moreover, citric acid has been detected in different citrus fruits: at the highest concentration in grapefruit juice (64.7 mmol/L), and in lower concentrations in lemon juice (47.66 mmol/L), orange juice (47.36 mmol/L) and pineapple juice (41.57 mmol/L) (Haleblian et al. Citation2008). Acid stimuli are commonly found in overripe or rotting fruits that are common food sources for medflies.
To study the response to bitter stimuli in C. capitata, we used three substances with different molecular structures: PROP (a thiouracil-derived bitter compound), quinine (a natural alkaloid) and quercetin (a bitter flavonol present in citrus fruits). None of them elicited any response from each of the four neurons in both Long and Intermediate type sensilla. In the light of our data, we assume that labellar sensilla do not respond to deterrent stimuli. This fact is not so unusual as previous studies in Drosophila revealed that Long type sensilla respond to salt and sweet but not to bitter stimuli, while Intermediate and Short type sensilla are activated by only a few bitter substances (Weiss et al. Citation2011). However, one cannot exclude that bitter sensitivity may be confined to Short type sensilla, which we did not test, or to sensilla located in other body regions, such as the tarsi (Chapman Citation2003; Ishimoto & Tanimura Citation2004), the ovipositor (Crnjar et al. Citation1989) or the interpseudotracheal papillae (Coronado-Gonzales et al. Citation2008).
The input from the tarsal sensilla is known to regulate the first phase of feeding behaviour (Dethier Citation1976; Singh Citation1997). In Drosophila, the sensitivity to bitter stimuli is different in labellar and tarsal sensilla: tarsi are known to respond preferentially to quinine or berberine, while strychnine is detected by the labellar sensilla (Meunier et al. Citation2003). This effect suggests that the different input from the labellar and tarsal apparatus could control feeding behaviour. In our experiments, we only tested three bitter stimuli; given the great variety of bitter compounds, characterized by very different chemical structures and specific transduction mechanisms, we cannot rule out the possibility that other bitter substances may stimulate the labellar chemosensilla of C. capitata.
Besides, during specific steps of the medfly life cycle such as oviposition and mating, so-called bitter compounds may not have a toxic effect or even an attractive significance, as reported in caterpillars and in Drosophila (Yang et al. Citation2008; Singer et al. Citation2009; Weiss et al. Citation2011). Indeed, citrus fruits should be little infested by the medfly due to toxic effects of compounds in the flavedo and the chemical properties of essential oils (Salvatore et al. Citation2004). Furthermore, limonene, a common compound in all citrus fruits, has no deterrent effects but stimulates oviposition, while linalool, a chemical compound of immature citrus fruits, has a deterrent effect (Ioannou et al. Citation2012).
Finally, we identified the response profiles for three (“M1”, “M2” and “S”) of the four cells housed in both chemosensillum types of both sexes, on the basis of their dose-response curves: “M1” is specific to NaCl and “M2” to fructose. The “S” cell is somewhat activated by acids, but this does not necessarily imply a specificity of response to “sour”. Indeed, the sensitivity to acids seems to be somewhat “distributed” as excitation and inhibition of different cells, as suggested for other flies (McCutchan Citation1969; Goldrich Citation1973; Charlu et al. Citation2013). Instead, no response specificity was detected for the remaining unit, the “H cell”: it could be tuned on such compounds as the oviposition-deterring pheromone. Further electrophysiological and behavioural studies are necessary to elucidate the sensitivity to bitter stimuli in the medfly.
Acknowledgements
The authors wish to thank Dr. Marco Melis for his helpful cooperation and technical support in the experimental part of this study. We thank Mr. Alessandro Cadau for photography and image treatment assistance and the staff of Prof. Anna Malacrida, Department of Animal Biology, University of Pavia for the help in rearing the medflies. This work was supported by the Italian “Ministero dell’Istruzione, dell’Università e della Ricerca” (MIUR).
References
- Adams JR, Holbert PE, Forgash AJ. 1965. Electron microscopy of the contact chemoreceptor of the stable fly Stomoxys calcitrans. Annals of Entomological Society of America 58:909–917.
- Amrein H, Thorne N. 2005. Gustatory perception and behavior in Drosophila melanogaster. Current Biology 15:R673–R684. doi:10.1016/j.cub.2005.08.021.
- Arredondo J, Díaz-Fleischer F. 2006. Oviposition deterrents for the Mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae) from fly faeces extracts. Bulletin of Entomological Research 96:35–42. doi:10.1079/BER2005399.
- Bernays EA, Glendinning JI, Chapman RF. 1998. Plant acids modulate chemosensory responses in Manduca sexta larvae. Physiological Entomology 23:193–201. doi:10.1046/j.1365-3032.1998.233079.x.
- Carey JR. 2011. Biodemography of the Mediterranean fruit fly: Aging, longevity and adaptation in the wild. Experimental Gerontology 46:404–411. doi:10.1016/j.exger.2010.09.009.
- Chapman RF. 2003. Contact chemoreception in feeding by phytophagous insects. Annual Review of Entomology 48:455–484. doi:10.1146/annurev.ento.48.091801.112629.
- Charlu S, Wisotsky Z, Medina A, Dahanukar A. 2013. Acid sensing by sweet and bitter taste neurons in Drosophila melanogaster. Nature Communications 4:1–10. doi:10.1038/ncomms3042.
- Coronado-Gonzalez PA, Vijaysegaran S, Robinson AS. 2008. Functional morphology of the mouthparts of the adult Mediterranean fruit fly, Ceratitis capitata. Journal of Insect Sciences 73:2–11.
- Crnjar R, Angioy AM, Pietra P, Stoffolano JG, Liscia A, Tomassini Barbarossa I. 1989. Electrophysiological studies of gustatory and olfactory responses of the sensilla on the ovipositor of the apple maggot fly, Rhagoletis pomonella Walsh. Bolletino di zoologia 56:41–46. doi:10.1080/11250008909355620.
- Crnjar R, Prokopy RJ. 1982. Morphological and electrophysiological mapping of tarsal chemoreceptors of oviposition-deterring pheromone in Rhagoletis pomonella flies. Journal of Insect Physiology 28:393–400. doi:10.1016/0022-1910(82)90064-6.
- Dethier VG. 1976. The hungry fly. Cambrige: Harvard University Press. pp. 67–118.
- Drewnowski A, Gomez-Carneros C. 2000. Bitter taste, phytonutrients, and the consumer: A review. American Journal of Clinical Nutrition 72:1424–1435.
- Eberhard W. 2000. Sexual behaviour and sexual selection in the medfly Ceratitis capitata (Dacinae: Ceratitidini). In: Aluja M, Norrbom AL, editors. Fruit flies (Tephritidae): Phylogeny and evolution of behavior. Boca Raton, Florida, USA: CRC Press. pp. 459–489.
- Eisele TA, Drake SR. 2005. The partial compositional characteristics of apple juice from 175 apple varieties. Journal of Food Composition and Analysis 18:213–221. doi:10.1016/j.jfca.2004.01.002.
- Fletcher BS, Prokopy RJ. 1991. Host location and oviposition in tephritid fruit flies. In: Baily WJ, Ridsdill-Smith J, editors. Reproductive behaviour of insects. New York: Chapman & Hall. pp. 139–171.
- Fox AL. 1932. The relationship between chemical constitution and taste. Genetics 18:115–120.
- Goldrich NR. 1973. Behavioral responses of Phormia regina (Meigen) to labellar stimulation with Amino Acids. The Journal of General Physiology 61:74–88. doi:10.1085/jgp.61.1.74.
- Gomulski LM, Dimopoulos G, Xi Z, Scolari F, Gabrieli P, Siciliano P, Clarke AR, Malacrida AR, Gasperi G. 2012. Transcriptome profiling of sexual maturation and mating in the Mediterranean Fruit fly, Ceratitis capitata. PLoS One 7:e30857–11. doi:10.1371/Journal.pone.0030857.
- Gothilf S, Galun R, Bar-Zeev M. 1971. Taste reception in the Mediterranean fruit fly: Electrophysiological and behavioural studies. Journal of Insect Physiology 17:1371–1384. doi:10.1016/0022-1910(71)90201-0.
- Guo SW, Reed DR. 2001. The genetics of phenylthiocarbamide perception. Annals of Human Biology 28:111–142. doi:10.1080/03014460151056310.
- Haleblian GE, Leitao VA, Pierre SA, Robinson MR, Albala DM, Ribeiro AA, Preminger GM. 2008. Assessment of citrate concentrations in citrus fruit-based juices and beverages: Implications for management of hypocitraturic nephrolithiasis. Journal of Endourology 22:1359–1366. doi:10.1089/end.2008.0069.
- Harris H, Kalmus H. 1949. Chemical specificity in genetical differences of taste sensitivity. Annals of Eugenics 15:32–45. doi:10.1111/j.1469-1809.1949.tb02420.x.
- Harwood JF, Chen K, Müller HG, Wang JL, Vargas RI, Carey JR. 2013. Effects of diet and host access on fecundity and lifespan in two fruit fly species with different life-history patterns. Physiological Entomology 38:81–88. doi:10.1111/phen.12006.
- Hiroi M, Marion-Poll F, Tanimura T. 2002. Differentiated response to sugars among labellar chemosensilla in Drosophila. Zoological Science 19:1009–1018. doi:10.2108/zsj.19.1009.
- Hiroi M, Meunier N, Marion-Poll F, Tanimura T. 2004. Two antagonistic gustatory receptor neurons responding to Sweet-Salty and Bitter taste in Drosophila. Journal of Neurobiology 61:333–342. doi:10.1002/neu.20063.
- Hodgson ES, Lettvin JY, Roeder KD. 1955. Physiology of a primary chemoreceptor unit. Science 122:417–418. doi:10.1126/science.122.3166.417-a.
- Ioannou CS, Papadopoulos NT, Kouloussis NA, Tananaki CI, Katsoyannos B. 2012. Essential oils of citrus fruit stimulate oviposition in the Mediterranean fruit fly Ceratitis capitata (Diptera: Tephritidae). Physiological Entomology 37:330–339. doi:10.1111/j.1365-3032.2012.00847.x.
- Ishimoto H, Tanimura T. 2004. Molecular neurophysiology of taste in Drosophila. Cellular and Molecular Life Sciences (CMLS) 61:10–18. doi:10.1007/s00018-003-3182-9.
- Jang EB, McInnis DO, Lance DR, Carvalho LA. 1998. Mating-induced changes in olfactory-mediated behavior of laboratory-reared normal, sterile, and wild female Mediterranean fruit flies (Diptera: Tephritidae) mated to conspecific males. Annals Entomological Society of America 91:139–144.
- Lindemann B. 1996. Chemoreception: Tasting the sweet and the bitter. Current Biology 6:1234–1237. doi:10.1016/S0960-9822(96)00704-X.
- Liscia A, Majone R, Solari P, Tomassini Barbarossa I, Crnjar R. 1998. Sugar response differences related to sensillum type and location on the labella of Protophormia terraenovae: A contribution to spatial representation of the stimulus. Journal of Insect Physiology 44:471–481. doi:10.1016/S0022-1910(97)00114-5.
- Liscia A, Muroni P, Pietra P, Piroddi N, Tomassini Barbarossa I, Crnjar R. 1995. Variations in salt sensitivity related to type and position of labellar chemosensilla in Protophormia terraenovae. Comparative Biochemistry and Physiology Part A: Physiology 111:335–344. doi:10.1016/0300-9629(95)00045-9.
- Liscia A, Solari P. 2000. Bitter taste recognition in the blowfly: Electrophysiological and behavioral evidence. Physiology and Behavior 70:61–65. doi:10.1016/S0031-9384(00)00249-3.
- Malacrida AR, Gomulski LM, Bonizzoni M, Bertin S, Gasperi G, Guglielmino CR. 2007. Globalization and fruitfly invasion and expansion: The medfly paradigm. Genetica 131:1–9. doi:10.1007/s10709-006-9117-2.
- Marchini D, Del Bene G. 2006. Comparative fine structural analysis of the male reproductive accessory glands in Bactrocera oleae and Ceratitis capitata (Diptera, Tephritidae). Italian Journal of Zoology 73:15–25. doi:10.1080/11250000500502319.
- Masek P, Scott K. 2010. Limited taste discrimination in Drosophila. Proceedings of the National Academy of Sciences 107:14833–14838. doi:10.1073/pnas.1009318107.
- McCutchan MC. 1969. Behavioral and electrophysiological responses of the blowfly, Phormia regina Meigen, to acids. Zeitschrift für Vergleichende Physiologie 65:131–152. doi:10.1007/BF00297681.
- Meunier N, Marion-Poll F, Rospar JP, Tanimura T. 2003. Peripheral coding of bitter taste in Drosophila. Journal of Neurobiology 56:139–152. doi:10.1002/neu.10235.
- Montell C. 2009. A taste of the Drosophila gustatory receptors. Current Opinion in Neurobiology 19:345–353. doi:10.1016/j.conb.2009.07.001.
- Ogaugwu CE, Schetelig MF, Wimmer EA. 2013. Transgenic sexing system for Ceratitis capitata (Diptera: Tephritidae) based on female-specific embryonic lethality. Insect Biochemistry and Molecular Biology 43:1–8. doi:10.1016/j.ibmb.2012.10.010.
- Papadopoulos NT, Katsoyannos BI, Kouloussis NA, Carey JR, Müller H-G, Zhang Y. 2004. High sexual signalling rates of young individuals predict extended life span in male Mediterranean fruit flies. Oecologia 138:127–134. doi:10.1007/s00442-003-1392-3.
- Papadopoulos NT, Liedo P, Müller HG, Wang JL, Molleman F, Carey JR. 2010. Cost of reproduction in male medflies: The primacy of sexual courting in extreme longevity reduction. Journal of Insect Physiology 56:283–287. doi:10.1016/j.jinsphys.2009.10.014.
- Riva A. 1974. A simple and rapid staining method for enhancing the contrast of tissues previously treated with uranyl-acetate. Journal Microscopy 19:105–108.
- Rodrigues V, Siddiqi O. 1981. A gustatory mutant of Drosophila defective in pyranose receptors. MGG Molecular & General Genetics 181:406–408. doi:10.1007/BF00425621.
- Rodriguez-Saona LE, Fry FS, McLaughlin MA, Calvey EM. 2001. Rapid analysis of sugars in fruit juices by FT-NIR spectroscopy. Carbohydrate Research 336:63–74. doi:10.1016/S0008-6215(01)00244-0.
- Salvatore A, Borkosky S, Willink E, Bardón A. 2004. Toxic effects of lemon peel constituents on Ceratitis capitata. Journal of Chemical Ecology 30:323–333. doi:10.1023/B:JOEC.0000017980.66124.d1.
- Schneider D. 1964. Insect antennae. Annual Review of Entomology 9:103–122. doi:10.1146/annurev.en.09.010164.000535.
- Sellier MJ, Reeb P, Marion-Poll F. 2011. Consumption of bitter alkaloids in Drosophila melanogaster in multiple-choice test conditions. Chemical Senses 36:323–334. doi:10.1093/chemse/bjq133.
- Shambhag SR, Park S-K, Pikielny C, Steimbreck R. 2001. Gustatory organs of Drosophila melanogaster: Fine structure and expression of the putative odorant-binding protein PBPRP2. Cell and Tissue Research 304:423–437. doi:10.1007/s004410100388.
- Singer MS, Mace KC, Bernays EA. 2009. Self-medication as adaptive plasticity: Increased ingestion of plant toxins by parasitized caterpillars. PLoS One 4:e4796. doi:10.1371/Journal.pone.0004796.
- Singh RN. 1997. Neurobiology of the gustatory systems of Drosophila and some terrestrial insects. Microscopy Research Technique 39:547–563. doi:10.1002/(SICI)1097-0029(19971215)39:6<547::AID-JEMT7>3.0.CO;2-A.
- Sivinski J, Luja M, Dobson GN, Freidberg A, Headrick DH. 2000. Topics in the evolution of sexual behavior in the Tephritidae. In: Aluja M, Norrbom AL, editors. Fruit flies (Tephritidae): Phylogeny and evolution of behavior. Boca Raton, Florida, USA: CRC Press. pp. 751–792.
- Smedley SR, Eisner T. 1995. Sodium uptake by puddling in a moth. Science 270:1816–1818. doi:10.1126/science.270.5243.1816.
- Smith JJB, Mitchell BK, Rolseth BM, Whitehead AT, Albert PJ. 1990. SAPID tools: Microcomputer programs for analysis of multi-unit nerve recordings. Chemical Senses 15:253–270. doi:10.1093/chemse/15.3.253.
- Wang ZR, Singhvi A, Kong P, Scott K. 2004. Taste representations in the Drosophila brain. Cell 117:981–991. doi:10.1016/j.cell.2004.06.011.
- Weiss LA, Dahanukar A, Kwon JY, Banerjee D, Carlson JR. 2011. The molecular and cellular basis of bitter taste in Drosophila. Neuron 69:258–272. doi:10.1016/j.neuron.2011.01.001.
- Wilczek M. 1967. The distribution and neuroanatomy of the labellar sense organs of the blowfly Phormia regina Meigen. Journal of Morphology 122:175–201. doi:10.1002/jmor.1051220303.
- Yang CH, Belawat P, Hafen E, Jan LY, Jan YN. 2008. Drosophila egg-laying site selection as a system to study simple decision-making processes. Science 319:1679–1683. doi:10.1126/science.1151842.
- Yuval B, Hendrichs J. 2000. Behavior of the flies in the genus Ceratitis (Dacinae: Ceratidini). In: Aluja M, Norrbom AL, editors. Fruit flies (Tephritidae): Phylogeny and evolution of behavior. Boca Raton Florida, USA: CRC Press. pp. 429–458.
- Zacharuk RY. 1985. Antennae and sensilla. In: Kerkouk GA, Gilbert LI, editors. Comprehensive insect physiology, biochemistry and pharmacology, vol 6. Nervous system: Sensory. London: Pergamon Press. pp. 1–70.
- Zacharuk RY, Shields VD. 1991. Sensilla of immature insects. Annual Review of Entomology 36:331–354. doi:10.1146/annurev.en.36.010191.001555.