Abstract
The urohyal bone, located in the central part of the mandibular skeleton, plays an important role in the mouth opening-closing mechanism of fish, and is considered a synapomorphy in teleostean fish. Morphology of the urohyal bone in six species of Gerreidae (Diapterus brevirostris, D. auratus, Eugerres lineatus, E. plumieri, Eucinostomus entomelas and Gerres cinereus) from the Pacific and Atlantic coasts of México was compared, using size and shape measurements. The main goal of the study was to explore the effectiveness of urohyal measurements in discriminating Gerreidae species. Morphological variation of urohyal bones, in terms of size and shape parameters, allowed species differentiation. Discriminant function analysis (DFA) significantly separated the six species with high classification rates (overall mean 92%). The G-test and Cohen’s kappa confirmed the high rates of classification success obtained by DFA. Circularity, Feret minimum, roundness, rectangularity and area were the main urohyal measurements explaining inter-specific variability. These results suggest the usefulness of urohyal bone morphology in differentiating Gerreidae species analyzed, highlighting the taxonomic value of the urohyal bone, until now never quantitatively evaluated as a diagnostic character in the classification of teleostean fish.
Introduction
The urohyal bone in teleostean fish is a single solid bone with the anterior tip generally connected to ventral hypohyals, the anterodorsal part connected to the first basibranchial and the posterior part from which the large muscles are connected to the shoulder girdle (Kusaka Citation1974). Bone is formed as ossification of an unpaired tendon of the sternohyoideus muscle (Arratia & Schultze Citation1990). This structure, located in the central part of the mandibular skeleton, plays an important role in the mouth opening-closing mechanism of fish ().
Figure 1. (A). Left side view of an urohyal bone in Gerreidae fish. Terminology of urohyal as follows: Ha = hypohyal attachment; Ba = basibranchial attachment; Ve = ventral extension; De = dorsal extension; Dp = dorsal plate; Pde = postero-dorsal edge; Rb = radial band; Lp = lateral plate; Pe = posterior edge; Ve = ventral edge; Vp = ventral plate; Co = condyle (modified from Kusaka Citation1974). Circle and cross indicating the orientation of urohyal on the body plan: D = dorsal; V = ventral; A = anterior; P = posterior. (B). Urohyal bone position during closure (I) and opening (II) of the oral cavity, exemplified in Eugerres lineatus (Cuvier, 1830), the striped mojarra. Note that only for illustration purposes the bone is depicted over the opercle, disconnected from basibranchial (anterdorsal) and hypohyals (ventral).
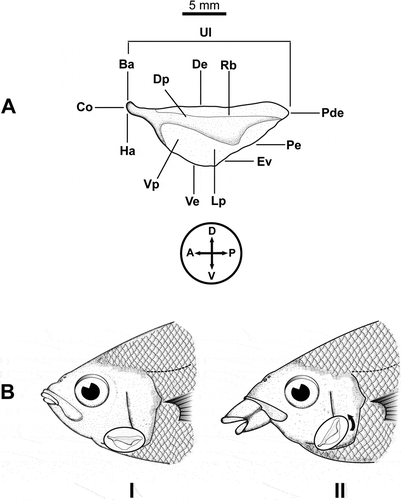
Morphology of the urohyals is related to species ecomorphlogy: slender-headed fish have an elongated urohyal; high-bodied fishes have vertically expanded urohyals; active swimmers have spatula-like shaped urohyals with an undeveloped ventral spread, which is present and developed in the omnivorous fishes (Kusaka Citation1974).
Urohyal bone morphology could be used to determine families, genera and even species (Arratia & Schultze Citation1990), and probably to infer phylogenetic relationships, because its presence is considered, among other characters, a synapomorphy for all living members of the division Teleostei (De Pinna Citation1996: 150).
Despite its diagnostic value in teleost fish, there are no quantitative studies about this structure, but some qualitative descriptions (e.g. Aprieto Citation1974; Sato et al. Citation1988), iconographic treatment in catalogues (e.g. Kusaka Citation1974; De La Cruz-Agüero & Chollet-Villalpando Citation2012), and age determination and body length-urohyal length relationships (e.g. Johdal et al. Citation2000, Citation2001). It has been emphasized that urohyal morphology is widely varying and that it could be used in fish taxonomy (Kusaka Citation1974; Esmaeili & Teymouri Citation2006).
The Gerreidae family (mojarras or silver biddies) is one of the most representative groups in aquatic systems in tropical and subtropical areas (Matheson & McEachrean Citation1984). Mojarras have an important role in their habitat as they play a role in the trophic web as transferor of energy between the primary consumers and top piscivores, since they prey on primary consumers of the benthic macrofauna (Kerschner et al. Citation1985; Chaves & Otto Citation1999) and, in turn, they are important preys ingested by avians, fish and mammalians (Fogarty et al. Citation1981; Cortés et al. Citation1990; Barros et al. Citation1998; De Oliveira et al. Citation2002; Moreira et al. Citation2003; Reeve et al. Citation2009). Besides, this fish family constitutes an abundant resource of commercial, artisanal and recreational fisheries around their areas of distribution, with average annual catches close to 9000 metric tons for the last 5 years (FAO Citation2011).
The six Gerreidae species considered in this study inhabit the Pacific and Atlantic coasts of México, including the Gulf of California, Gulf of México and Caribbean. These species are the most common among the 20 Mexican neotropical gerreid species, being important in the semi-subsistence fisheries of México (Aguirre & Yáñez-Arancibia Citation1986): Irish mojarra Diapterus auratus Ranzani, 1842, short-beaked mojarra D. brevirostris (Sauvage, 1879), dark-spot mojarra Eucinostomus entomelas Zahuranec in Yáñez-Arancibia, 1980, streaked mojarra Eugerres lineatus (Humboldt, 1821), striped mojarra E. plumieri (Cuvier, 1830) and yellow fin mojarra Gerres cinereus (Walbaum, 1792).
Members of the Gerreidae have a confusing taxonomic history, being an active issue of discussion among ichthyologists (e.g. Chen et al. Citation2007; De La Cruz-Agüero et al. Citation2012). Currently, eight genera are recognized, but the taxonomic status of more than 50 nominal species has not yet been revised, and some gerreid taxa in the Neotropics still have recognition problems, being treated by some authors as species inquirendae (e.g. validity and/or generic assignment uncertain or questionable), such as: Ulaema lefroyi, Eucinostomus havana, Eugerres periche, E. brevimanus and Gerres simillimus.
Hence, it seems necessary to perform comparative analyses and investigate in more detail the characteristics of urohyal bones in selected fish species of family Gerreidae, because even some possible difference could be indicative of taxonomic value and/or phylogenetic signal. The goals of the present study were, therefore, (1) to quantitatively investigate the morphometric variability of the urohyal bone in selected species of Gerreidae and (2) to test the hypothesis that this structure can be used to discriminate gerreid fish at the species level. Thus, the analysis of urohyal morphology is to contribute to enhancing taxonomy of mojarras inhabiting the coast of the Mexican Neotropics.
Materials and methods
Specimens, dissection and image acquisition
Fish specimens and the osteological material belong to the ichthyologic collection (CI) of Centro Interdisciplinario de Ciencias Marinas-Instituto Politécnico Nacional (CICIMAR-IPN) at La Paz, B.C.S., Mexico, and are available upon request (see details on the CI website: http://coleccion.cicimar.ipn.mx). The fish were captured between January 2009 and November 2011, in the Pacific, off three Mexican states: Baja California Sur (La Paz Bay, Magdalena Bay and Espiritu Santo Island), Sinaloa (Mazatlan harbour) and Guerrero (Acapulco harbour), and in the Atlantic, off two states: Veracruz (La Mancha estuary) and Quintana Roo (Chetumal harbour). The specimens came from artisanal catches of local fisheries and occasional samples by the authors, using the capture permit granted to the CI. Specimens were chosen without separating the sexes because there is no sexual dimorphism reported for gerreid fish (De La Cruz Agüero & Galvan Citation1993).
The urohyal bones were removed from the fresh samples as described in De La Cruz-Agüero and Chollet-Villalpando (Citation2012). After the extraction, cleaning and drying, the bones were stored in paper envelopes, catalogued and placed in the ichthyologic collection.
Number of specimens for each species, size range and mean are reported in . Digital images of each bone per fish and species were taken from the left side (as described by Kusaka Citation1974), using a stereo-microscope (model SZ61® Olympus, Tokyo, Japan) fitted with digital camera (model SP-320®, Olympus) with a 3× optical zoom lens and light source (SZ2-LGBST®, Olympus) to highlight structure edges.
Table I. Number of samples (n), standard length range (SL, mm) and mean (m SL) of Gerreidae fish collected and used in the urohyal morphology analysis.
Measurements
Urohyal size parameters were obtained from each structure using image software (Image Pro Plus® 6.0, Media Cybernetics, Bethesda, MD). Size parameters were used to calculate shape indices, which are independent of differences in size (Tuset et al. Citation2003b, Citation2006). Size parameters were: area (Au), perimeter (Pu), aspect ratio, Feret length (Fl), Feret width (Fw), Feret maximum (Fmx), Feret minimum (Fmn), Feret mean (Fm), diameter maximum (Dmx), diameter minimum (Dmn) and diameter mean (Dm). In microscopy and image analysis, Fl is the longest distance between any two points along the selection boundary or object profile under analysis and perpendicular to the ocular scale at given arbitrary angles. This measurement is also known as maximum calliper. The other measures of the Feret are self-explanatory (see also Pertusa Citation2010). Shape indices used in this study were: rectangularity, roundness, circularity, form factor and ellipticity, and were calculated using the following formulas:
Rectangularity describes variations in length and width with respect to the area (1.0 being a perfect square). Roundness and circularity are the ratios between the actual area and the area of a circle of the same diameter (higher ratios approximates a perfect circle, taking a minimum value of 1 and 4
, respectively). Form factor is an estimate of surface area irregularity and is defined as the inverse ratio of the squared perimeter of an object to the squared perimeter of a circle of the same surface (the smaller the form-factor, the lacier the outline of the urohyal, taking values of 1.0 as a perfect circle and < 1.0 when irregular). Ellipticity indicates if the changes in axis length are proportional. Aspect ratio is the ratio between the length and the width (the larger the value, the more elongated is the urohyal bone; see also Tuset et al. Citation2003b; Ponton Citation2006).
Analysis of data
Raw untransformed data for all urohyal bones of the six species were examined for outliers (that might indicate errors or damaged bones) and normality. The distributions of the variables were subjected to a Kolmogorov-Smirnov test, and to the inspection of the dispersion of the average values of Fmx (used as a proxy of urohyal size) by species. Because they were significantly different (, and ), the size-dependent variation was corrected by means of an allometric method, as suggested by Elliott et al. (Citation1995):
Table II. Kolmogorov-Smirnov (K-S) test of normality at the 95% confidence level for the urohyal raw untransformed data (RUD), and standardized measurements (SM). Perimeter was transformed by the Box-Cox function. *Not normally distributed.
This standardization reduces the resulting variability from allometric growth (Beacham Citation1985; Reist Citation1985), removing the influence of size in species differentiation (Elliott et al. Citation1995). Standardized measurements for each parameter were tested for normality and, when necessary, different models of transformation (e.g. logarithms, arc-sin, Box-Cox model, and others) were applied, seeking a better approximation to normal data distribution. Finally, each measurement was regressed against Fmx (i.e. a standard estimate of urohyal size). A significant interaction term between the regressor (i.e. size) and species measurements indicates that the slopes of the species lines are different (lack of homogeneity of regression), suggesting that the effect of allometric growth has not been removed (Tabachnick & Fidell Citation1983).
In the present study, a linear discriminant function analysis (DFA) was used to discriminate the six species, based on their urohyal bone measurements. This multivariate technique evaluates differences between groups, using several discriminant variables and predicts ownership to a specific group (Legendre & Legendre Citation1998). The aim of discriminant analysis (DA) is to combine (weight) the variable scores in some way so that a single new composite variable, the discriminant score, is produced. DA involves the determination of a linear equation that will predict which group the case belongs to:
The quadratic Mahalanobis distances (MD) and their approximation to the F-statistic were used to assess significant differences between centroid species in the multivariate space. Also, a phenogram was constructed by hierarchical cluster analysis (UPGMA), based on the Euclidian distance of MD values to assess the degree of phenetic similarity between Gerreidae species by urohyal bone measurements.
Statistical significance of DFA was determined based on Wilks’s lambda (λ) statistic. The λ provides an objective means of calculating chance-corrected percentage of agreement between a real and a predicted group’s membership, where with values close to zero the group centroids are greatly separated, which indicates a good model fit; values ≈1 indicate a lower power of discrimination, where the group centroids are identical (Klecka Citation1980; Lord et al. Citation2012).
Another way to judge the relevance of a discriminant function is by examining the canonical correlation coefficient (r*). This coefficient is a measure of association that summarizes the degree of relatedness between groups, such as species, and the discriminant functions (Klecka Citation1980). If the groups are not very different in the variables that are analyzed, then all of the correlations will be low (a value of zero denotes no relationship at all and one indicating the maximum correlation).
Classification efficiency, such as the estimated error rates in DFA, was cross-validated according to the procedure described by Lachenbruch and Mickey (Citation1968). The bias of the classification was determined with the Cohen-Kappa (κ) coefficient, which estimates the improvement over chance of the percent corrected classification rates (Tuset et al. Citation2003a). Values of κ range from zero to one, with zero indicating that discriminant analysis yielded no improvement over chance, while one indicates perfect agreement (Titus et al. Citation1984). The κ values were scaled up to a percentage, and the prior probability of classification was equal for all groups. Finally, a G-test of independence was used to test agreement between observed and expected classification rates (Tuset et al. Citation2003a).
All statistical analyses were performed in XLStat 2009 (Addinsoft, Paris, France), a statistical plug-in for Microsoft Excel 2007. Significance level was set at 0.05 for all statistical tests.
Results
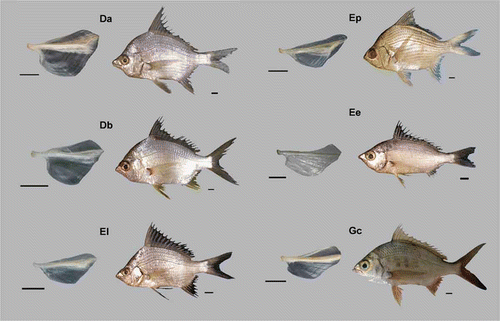
In our research, 222 urohyal bones were digitized, extracted from fish with a range of size from 46 mm standard length (SL) in D. brevirostris to 225 mm SL in G. cinereus. The urohyal bones per species are listed in (see also ). The gross morphology of the urohyal per species is described according to Kusaka (Citation1974); see also A and .
Figure 2. Morphology of the urohyal bone in Gerreidae species. (Da) Diapterus auratus, (Db) D. brevirostris, (El) Eugerres lineatus, (Ep) E. plumieri, (Ee) Eucinostomus entomelas and (Gc) Gerres cinereus. Scale bars: for urohyals, 0.5 mm, and 1 cm for fish.
Description of urohyal bone by species
Diapterus auratus
Basibranchial and hipohyal attachments reduced in their anterior margins, and moderately thickened. Condyle short and thick, compressed in the hipohyal union. Dorsal edge smooth, elevated after the first third of its length, rear end with a median decline towards the dorsum, with a slight prolongation. Radial band short and wide, dorsal plate median, ventral plate wide, and extending to the posterior border, fairly pronounced. Ventral border rounded, with a short extension, lateral plate very wide, ventral plate reduced.
D. brevirostris
Basibranchial and hipohyal attachment moderately elongated and thickened at its anterior margin. Condyle long and thickened. Dorsal extension smooth and elevated after the first third of its length, with a pronounced decline towards dorsum. Radial band short and wide; dorsal plate median. Ventral plate wide and extending to its postero-dorsal edge; dorsal plate with a slight prolongation sharpened and moderately pronounced. Ventral edge and ventral extension rounded. Lateral plate very broad in dorsal view.
Eucinostomus entomelas
Basibranchial and hipohyal attachments elongated in their anterior margins, slightly thickened. Condyle thickened at the front, moderate. Dorsal extension with a slight anterior projection, and subtly separated from the radial band, presents a slight elevation in the last third of its length, and with a sharp decline towards the dorsum. Radial band long and wide, its front reduced toward the posterior edge. Dorsal plate reduced moderately; ventral plate extending toward the posterior edge. Posterior edge concave, dorsal extension obtuse, ventral edge moderately pronounced, rounded. Ventral edge reduced, lateral plate extending ventrally.
Eugerres lineatus
Basibranchial attachment elevated; hipohyal attachment depressed at its anterior margin. Condyle thickened. Dorsal edge smooth, elevated to the dorsal extension. Radial band long and wide. Dorsal plate reduced and extended to the postero-dorsal edge. Ventral plate broad and extended towards the posterior edge, which is nearly straight. Dorsal extension dorsally beak-shaped. Ventral edge slightly pronounced. Ventral edge reduced; lateral plate reduced in dorsal view, extending toward the posterior edge.
E. plumieri
Basibranchial attachment elevated; hipohyal attachment slightly reduced. Condyle thick elevated anteriorly. Dorsal edge smooth and strongly elevated to the dorsal extension. Radial band long and very wide, without extending to the posterior edge. Dorsal plate small and uniform. Ventral plate moderated, extending toward the posterior edge, which is slightly convex in its dorsal portion. Dorsal extension beak-shaped; ventral edge very pronounced and with a slightly anterior projection. Ventral extension reduced. Lateral plate reduced in its dorsal portion.
Gerres cinereus
Basibranchial and hipohyal attachments wide at their anterior margins. Condyle short and uniform. Dorsal extension smooth with a continuous elevation toward the dorsal extension. Radial band long and wide, extended to the posterior edge. Dorsal plate median; ventral plate wide and extending toward the posterior edge. Posterior edge concave in the middle region. Dorsal and ventral extensions fully rounded. Ventral edge pronounced with a short projection. Lateral plate extended dorsally.
Adjustment of data
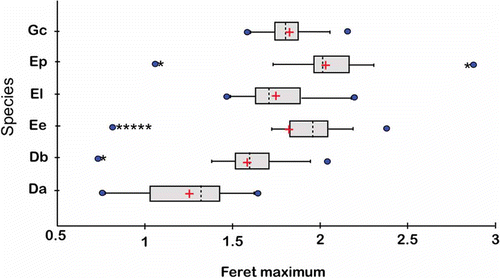
Kolmogorov-Smirnov test for normality (), and size distribution () showed the statistical inconsistency (e.g. non-normally distributed) of most of the raw untransformed data. Only the following measurements – maximum diameter, roundness and rectangularity – were normally distributed (). One urohyal bone from D. auratus and E. entomelas as well as two urohyals bones from D. brevirostris were identified as outliers and excluded from analyses.
Figure 3. Box plots comparing Feret maximum (Fmx, i.e. the size estimator) for the urohyal bone of six Gerreidae species (raw untransformed data): (Da) Diapterus auratus, (Db) D. brevirostris, (El) Eugerres lineatus, (Ep) E. plumieri, (Ee) Eucinostomus entomelas and (Gc) Gerres cinereus, showing the mean (+), median (dashed lines), quartiles, standard deviations (SDs; grey boxes) and range values (minimum and maximum). Sample sizes are given in (● and * indicate, respectively, individual values greater than 1.5 and 3 times the interquartile range from the nearest quartile).
All standardized measurements, except for aspect ratio, minimum diameter and perimeter, were not correlated with the size estimator (i.e. Fmx), indicating that the effects of size had been successfully removed. Perimeter was normalized by the Box-Cox transformation, and the aspect ratio and minimum diameter were transformed by natural logarithms (). Finally, a second analysis of regression of size estimator (Fmx, the dependent variable), explained by the measurements and species (the explanatory variables), suggested that none of the measurements were positively and significantly related to Fmx (e.g. slopes did not deviate from zero, p > 0.0001), and all measurements were not different among species (e.g. regressions coefficients were homogeneous, p > 0.0001). All measurements also showed non-significant interaction effects, ensuring the use of standardized data (r = 0.977; F = 485,967; p < 0.0001).
Size and shape discrimination among species by DFA
All five size-free discriminant functions produced with the 13 urohyal measurements were significant in discriminating urohyal bones of the six gerreid species. The first two functions explained variability 87% [e.g. F1 (71.73%): λ = 0.003, p < 0.001, r* = 0.96; F2 (15.02%): λ = 0.05, p < 0.001, r* = 0.86], while discriminant functions F3, F4 and F5 made a negligible contribution and therefore were not considered (r* = 0.74, r* = 0.69, and r* = 0.48, respectively). Interspecific discriminant analysis of urohyal descriptors demonstrated that 91.9% of all cases (204) were correctly classified, ranging from 96.6% in D. brevirostris to 86.4% in E. lineatus (). Incorrect classification (18 cases) varied between 3.5% (D. brevirostris) and 13.6% (E. lineatus), with an average of 8.89%. Cohen-Kappa (κ) indicates that a classification efficiency of 90% or better would have occurred by chance alone (κ = 0.90; standard error = 0.022; 95% confidence interval = 0.856 to 0.944; z = 40.9, p < 0.001). The G-test indicated that urohyal bones were assigned to the correct species at rates significantly greater than would be expected by chance (G = 638.95; d.f. = 25, p < 0.001).
Table III. Classification matrix as a result of discriminant function analysis testing for differences between analyzed Gerreidae species based on 13 urohyal shape and size descriptors. (Da) Diapterus auratus, (Db) D. brevirostris, (El) Eugerres lineatus, (Ep) E. plumieri, (Ee) Eucinostomus entomelas and (Gc) Gerres cinereus.
Scatter plots for the two discriminant functions show separation between the six species and, although some overlapping may be noticeable, all but the quadratic Mahalanobis distances were significant (p < 0.0001; , ). Variables with the highest contribution to the Eigen-values were (standardized coefficients in parentheses): circularity (5.07), roundness (–2.81) and Feret minimum (2.63) for Fl and Feret minimum (10.33), area (–7.70) and rectangularity (5.59) for F2.
Figure 4. Scatter plots of first (F1) and second (F2) discriminant functions. Discriminant function analysis (DFA) using 13 urohyal shape and size descriptors of the six species of Gerreidae. The first discriminant axis (F1) explains 71.73% and the second axis (F2) 15.02%. (Da) Diapterus auratus, (Db) D. brevirostris, (El) Eugerres lineatus, (Ep) E. plumieri, (Ee) Eucinostomus entomelas and (Gc) Gerres cinereus.
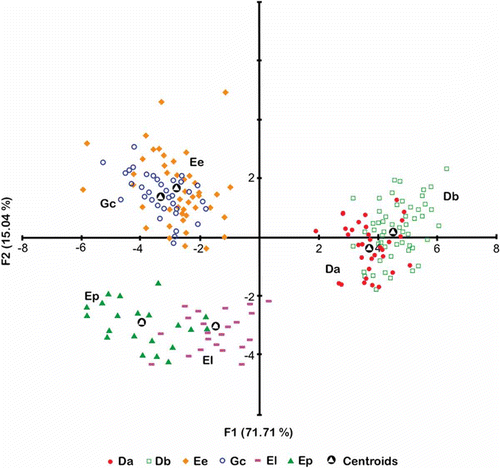
Table IV. Mahalanobis distances between groups (above the diagonal) and p value of the F test (below the diagonal). Da: Diapterus auratus, Db: D. brevirostris, Ee: Eucinostomus entomelas, El: Eugerres lineatus, Ep: E. plumieri and Gc: Gerres cinereus.
Discussion
The present study enhances knowledge about Gerreidae urohyal bone morphology, highlighting its diagnostic value for teleostean taxonomy. As far as we are aware, the present research is the first comparative and quantitative assessment of the urohyal bone in any teleost fish, following the establishment of its diagnostic value by Arratia and Schultze (Citation1990) .
Because intra-specific and inter-specific variations in the morphology of urohyal could be attributable to growth, to process the samples or other statistical artefacts, we applied a protocol for data exploration. This involves the detection of outliers, assessing homogeneity, normality, and other potential problems and undesirable artefacts. After data adjustment and using size-free measurements, we can say that our results reflect natural variation, unrelated to statistical artefacts.
Kusaka (Citation1974) and Esmaeili and Teymouri (Citation2006) state that morphological differences in the urohyal bone can be identified even at the species level. In this sense, osteological studies in the family Carangidae have shown differences in the morphology of the skull and urohyal bones between individuals from wild and cultivated populations (Aprieto Citation1974). Similarly, the morphology of urohyal bones and other bony structures were used in the description of a subspecies of the genus Merluccius (Lloris et al. Citation2003).
Other studies of age and growth of southern red snapper Lutjanus purpureus (Gonzalez & Nora Citation1999) and silver carp Hypophthalmichthys molitrix (Johdal et al. Citation2000, Citation2001) used annual rings of urohyal bones or length to body ratio and urohyal bone length.
However, within the family Gerreidae in studies of the osteology of some species, urohyal bones have been described only qualitatively and with no comparative purposes (Andreata & Barbieri Citation1981; Andreata Citation1989; Gonzalez-Acosta et al. Citation2005, Citation2007; De La Cruz-Agüero & Chollet-Villalpando Citation2012).
The family Gerreidae is characterized by a complex taxonomy, at both the generic and specific levels (Matheson & McEachrean Citation1984), primarily related to morphological plasticity in some taxa and uncertain definitions of valid genera. Hence, current taxonomy within family Gerreidae is under considerable debate.
The results of this study demonstrated the potential taxonomic value of the urohyal morphology for fish classification, judging by the rate of separation in size-free DFA (92% on average), and significant differences between species. Current classification rate is very similar to reports for gerreid species by other authors, by analyzing the morphology of sagittal otoliths (10 shape and size descriptors) among six species of mojarras (De La Cruz-Agüero et al. Citation2012), and body morphology (19 linear measurements) for Eucinostomus spp. of the Pacific coast (De La Cruz-Agüero & Galvan Citation1993), and Eugerres spp. (21 box truss distances) for the Pacific and Atlantic basins (Gonzalez-Acosta et al. Citation2005, Citation2007, respectively).
The present DFA shows that this set of morphological measurements of urohyal bones produced good discrimination among all the species. Thus, urohyal bone analysis could contribute to highlighting taxonomy of gerreid species because of its distinctive interspecific morphology. The urohyal measurements circularity, Feret minimum, roundness, rectangularity and area explain the highest percentage of the total variability in DFA and could be used as additional features to those already employed for taxonomic identification.
Besides, morphological affinity between species according to urohyal analysis is close to the current taxonomic arrangement, as shown in the phenogram based on the quadratic Mahalanobis distances (except for the E. entomelas and G. cinereus grouping; , ). A quasi-similar morphological subdivision has been proposed in most taxonomic keys, individualizing two subgroups: Diapterus and Eugerres, which exhibit serrated inferior margins of the preopercle, and Eucinostomus and Gerres, which have smooth inferior margins (e.g. Bussing Citation1995).
Figure 5. Phenogram indicating the phenotypic relationship of the urohyal morphology (shape and size measurements) for Gerreidae species: (Da) Diapterus auratus, (Db) D. brevirostris, (El) Eugerres lineatus, (Ep) E. plumieri, (Ee) Eucinostomus entomelas and (Gc) Gerres cinereus. Dissimilarity based on the Euclidian distance of Mahalanobis distance values, grouping by hierarchical cluster analysis (UPGMA).
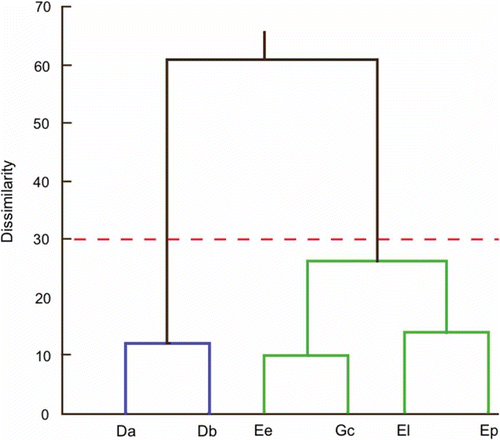
This morphological similarity is almost consistent, to the genus level, with the results from molecular systematic studies of selected species of Gerreidae, based on serum proteins (Espinosa et al. Citation1993), allozymes and restriction profiles of mtDNA (Ruiz-Carus & Uribe-Alcocer Citation2003a), karyotype analysis (Ruiz-Carus & Uribe-Alcocer Citation2003b), and mitochondrial 12Sr RNA and 16Sr RNA sequences and Rhodospin and RAG1 nuclear genes (Chen et al. Citation2007). In all these molecular proposals, Eugerres and Diapterus form one clade, and Eucinostomus and Gerres form another.
Moreover, given that it has been shown that urohyal bones can be used to identify gerreid fish, and that different mojarra species are important preys in their ecosystem, urohyal bone remains found in piscivorous gastric contents could be used in order to improve the assessment of the contribution of gerreids to the diet of marine piscivores.
Finally, since taxonomy of the mojarras is under considerable debate, we strongly encourage future studies that include new traits in the family, such as the urohyal bone, as well as other analytic strategies not yet applied in Gerreidae (e.g. DNA markers and geometric morphometrics). For the urohyal bone in particular, future research is needed to evaluate its phylogenetic signal, to help elucidate the taxonomy and systematics of a group as troublesome as Gerreidae.
Acknowledgements
We thank V.M. Cota, F.J. Vergara, A. Martínez and J.A. Payán for their help in the fieldwork, and I. Fogel (Centro de Investigaciones Biológicas del Noroeste, CIBNOR) for improving the English. Funding was provided by Secretaría de Investigación y Posgrado, Instituto Politécnico Nacional (SIP-IPN). JDA and FJGR are fellows of the Comisión para el Fomento de las Actividades Académicas (COFAA-IPN) and Estímulo al Desempeño de los Investigadores (EDI-IPN). JDA and FJGR appreciate the support of the Sistema Nacional de Investigadores (SNI-CONACyT). JGCV received grants from the Programa Institucional de Formación de Investigadores (PIFI-IPN) and Consejo Nacional de Ciencia y Tecnología (CONACyT). We would also like to thank the anonymous reviewers for valuable comments on the manuscript.
References
- Aguirre AL, Yañez-Arancibia A. 1986. Las mojarras de la Laguna de Términos: Taxonomía, biología, ecología y dinámica trófica (Pisces: Gerreidae). Anales del Instituto de Ciencias del Mar y Limnología 13:369–444.
- Andreata JV. 1989. Sobre a osteología cefálica das especies de Gerres Quoy y Gaimard, 1824 (Pisces, Perciformes, Gerreidae) que ocorren em águas Brasileiras. Acta Biologica Leopoldina 11:165–202.
- Andreata JV, Barbieri LR. 1981. Osteología de cráneo de Diapterus brasilianus (Cuvier, 1830) (Perciformes, Percoidei, Gerreidae). Revista Brazileira de Biologia 41:565–574.
- Aprieto VL. 1974. Early development of five carangid fishes of the Gulf of México and the south Atlantic coast of the United States. Fishery Bulletin 72:415–443.
- Arratia G, Schultze HP. 1990. The urohyal: Development and homology within osteichthyans. Journal of Morphology 203:247–282. doi:10.1002/jmor.1052030302.
- Barros NB, Wells RS, Barros, N.B. 1998. Prey and feeding patterns of resident bottlenose dolphins (Tursiops truncatus) in Sarasota Bay, Florida. Journal of Mammalogy 79:1045–1059. doi:10.2307/1383114.
- Beacham TD. 1985. Meristic and morphometric variation in pink salmon (Oncorhynchus gorbuscha) in southern British Columbia and Puget Sound. Canadian Journal of Zoology 63:366–372. doi:10.1139/z85-056.
- Bussing WA. 1995. Gerreidae. In: Fisher W, Krupp F, Schneider W, Sommer C, Carpenter KE, Niem VH, editors. Guía para la identificación de especies para los fines de la pesca. Pacífico Centro-Oriental. Roma: FAO. pp. 1114–1128.
- Chaves PTC, Otto G. 1999. The mangrove as a temporary habitat for fish: the Eucinostomus Species at Guaratuba Bay, Brazil (25º 52'S; 48º 39'W). Brazilian Archives of Biology and Technology 42:1–8.
- Chen WJ, Ruiz-Carus R, Ortíz G. 2007. Relationships among four genera of mojarras (Teleostei: Perciformes: Gerreidae) from the western Atlantic and their tentative placement among percomorph fishes. Journal of Fish Biology 70(Supp. B):202–218. doi:10.1111/j.1095-8649.2007.01395.x.
- Cortés E, Gruber SH, Cortes, E. 1990. Diet, feeding habits and estimates of daily ration of young lemon sharks, Negaprion brevirostris (Poey). Copeia 1990:204–218. doi:10.2307/1445836.
- De La Cruz-Agüero J, Chollet-Villalpando JG. 2012. Catálogo sinóptico del hueso urohial de las especies de la familia Gerreidae de México. In: del Moral LF, Martínez JA, Franco J, Ramírez AJ, Tello JL, editors. Investigación Ictiológica en México, tópicos selectos en honor al Dr. José Luis Castro Aguirre. México, D.F: UNAM-FES Iztacala, SIMAC. pp. 57–73.
- De La Cruz-Agüero J, Galvan FM. 1993. Morphological discrimination of Eucinostomus spp. from the pacific coast of México. Bulletin of Marine Science 52:819–824.
- De La Cruz-Agüero J, García-Rodríguez FJ, De La Cruz-Agüero G, Díaz Murillo BP. 2012. Identification of gerreid species (Actinopterygii: Perciformes: Gerreidae) from the Pacific coast of Mexico based on sagittal otolith morphology analysis. Acta Ichthyologica et Piscatoria 42:297–306. doi:10.3750/AIP2012.42.4.03.
- De Oliveira SMC, Rosso S, Dos Santos RA, Lucato SHB. 2002. Insights on small cetacean feeding habits in southeastern Brazil. Aquatic Mammals 28:38–45.
- De Pinna MCC. 1996. Teleostean monophyly. In: Stiassny MLJ, Parenti LR, Johnson GD, editors. Interrelationships of fishes. New York: Academic Press. pp. 147–162.
- Elliott NG, Haskard K, Koslow JA. 1995. Morphometric analysis of orange roughy (Hoplostethus atlanticus) off the continental slope of southern Australia. Journal of Fish Biology 46:202–220. doi:10.1111/j.1095-8649.1995.tb05962.x.
- Esmaeili HR, Teymouri A. 2006. Morphology of urohyal bone and its importance in taxonomy of some freshwater fishes of Iran. Iranian Scientific Fisheries Journal 15:1–8.
- Espinosa G, Gutierrez E, Baez-Hidalgo M. 1993. Relaciones entre cuatro especies de peces de la familia Gerreidae sobre la base de 9 loci electroforéticos. Revista de Investigaciones Marinas 14:132–137.
- FAO. 2011. Yearbook 2009: Fishery and aquaculture statistics. Roma: FAO.
- Fogarty MJ, Nesbitt SA, Gilbert CR. 1981. Diet of nestling brown pelicans in Florida. Florida Field Naturalist 9:38–40.
- Gonzalez LW, Nora E. 1999. Edad y crecimiento del pargo colorado, Lutjanus purpureus, Poey, 1867 (Teleostei: Lutjanidae) de la región oriental de Venezuela. Revista de Biología Marina y Oceanografía 34:99–107.
- Gonzalez-Acosta AF, De La Cruz-Agüero J, Castro-Aguirre JL. 2005. A review of eastern pacific species of the genus Eugerres (Perciformes: Gerreidae). Bulletin of Marine Science 76:661–673.
- Gonzalez-Acosta AF, De La Cruz-Agüero J, Castro-Aguirre JL. 2007. A review of the marine western Atlantic species of the genus Eugerres (Perciformes: Gerreidae). Bulletin of Marine Science 80:109–124.
- Johdal MS, Esmaeili HR, Tandon KK. 2000. Reliability of urohyal bone of silver carp, Hypophthalmichthys molitrix (Val. 1844) for age determination. Current Science 79:27–28.
- Johdal MS, Esmaeili HR, Tandon KK. 2001. A comparison of back-calculated lengths of silver carp derived from bony structures. Journal of Fish Biology 59:1483–1493. doi:10.1111/j.1095-8649.2001.tb00213.x.
- Kerschner BA, Peterson MS, Gilmore RG. 1985. Ecotopic and ontogenetic trophic variation in mojarras (Pisces: Gerreidae). Estuaries 8:311–322. doi:10.2307/1351492.
- Klecka WR. 1980. Discriminant analysis. Beverly Hills: Sage University Papers.
- Kusaka T. 1974. The urohyal of fishes. Tokyo: University of Tokyo Press.
- Lachenbruch PA, Mickey MR. 1968. Estimation of error rates in discriminant analysis. Technometrics 10:1–11. doi:10.1080/00401706.1968.10490530.
- Legendre P, Legendre LFJ. 1998. Numerical ecology. Amsterdam: Elsevier.
- Lloris D, Matallanas J, Oliver P. 2003. Merluzas del mundo (Familia Merlucciidae). Catálogo comentado e ilustrado de las merluzas conocidas. Roma: FAO.
- Lord C, Morat F, Lecomte-Finiger R, Keith P. 2012. Otolith shape analysis for three Sicyopterus (Teleostei: Gobioidei: Sicydiinae) species from New Caledonia and Vanuatu. Environmental Biology of Fishes 93:209–222. doi:10.1007/s10641-011-9907-y.
- Matheson RE, McEachran JD. 1984. Taxonomic studies of the Eucinostomus argenteus complex (Pisces: Gerreidae): Preliminary studies of external morphology. Copeia 1984:893–902. doi:10.2307/1445334.
- Moreira LG, de Andrade MA, Aguiar RS, Cascon P. 2003. Feeding habits of marine tucuxi, Sotalia fluviatilis, at Ceará State, northeastern Brazil. Latino American Journal of Aquatic Mammals 2:117–122.
- Pertusa GF. 2010. Técnicas de análisis de imagen: Aplicaciones en biología. Aldaia: Universitat de Valencia.
- Ponton D. 2006. Is geometric morphometrics efficient for comparing otolith shape of different fish species?. Journal of Morphology 267:750–757. doi:10.1002/jmor.10439.
- Reeve A, Handy RD, Gruber SH. 2009. Prey selection and functional response of juvenile lemon sharks Negaprion brevirostris. Journal of Fish Biology 75:276–281. doi:10.1111/j.1095-8649.2009.02265.x.
- Reist J. 1985. An empirical evaluation of several univariate methods that adjust for size variation in morphometric data. Canadian Journal of Zoology 63:1429–1439. doi:10.1139/z85-213.
- Ruiz-Carus R, Uribe-Alcocer M. 2003a. Phylogenetic assessment of Eucinostomus gula, Eugerres plumieri, and Diapterus auratus (Pisces; Gerreidae) based on allozyme and mtDNA analyses. Caribbean Journal of Science 39:109–115.
- Ruiz-Carus R, Uribe-Alcocer M. 2003b. Karyotype analysis of Eucinostomus argenteus, E. gula, E. harengulus, and Eugerres plumieri (Teleostei, Gerreidae) from Florida and Puerto Rico. Environmental Biology of Fishes 67:269–276. doi:10.1023/A:1025899418162.
- Sato Y, Hasegawa Y, Yonezawa A. 1988. The urohyal of Japanese Miocene clupeid fish Eosardinella hishinaiensis. Scientific Report of Yokohama National University 35:57–59.
- Tabachnick G, Fidell LS. 1983. Using multivariate statistics. New York: Harper and Row Publishers.
- Titus K, Mosher J, Williams BK. 1984. Chance-corrected classification for use in discriminant analysis: Ecological applications. American Midland Naturalist 111:1–7. doi:10.2307/2425535.
- Tuset VM, Lombarte A, González JA, Pertusa JF, Lorente MJ. 2003a. Comparative morphology of the sagittal otolith in Serranus spp. Journal of Fish Biology 63:1491–1504. doi:10.1111/j.1095-8649.2003.00262.x.
- Tuset VM, Lozano IJ, Gonzalez JA, Pertusa JF, Garcia-Diaz MM. 2003b. Shape indices to identify regional differences in otolith morphology of comber, Serranus cabrilla (L., 1758). Journal of Applied Ichthyology 19:88–93. doi:10.1046/j.1439-0426.2003.00344.x.
- Tuset VM, Rosin PL, Lombarte A. 2006. Sagittal otolith shape used in the identification of fishes of the genus Serranus. Fisheries Research 81:316–325. doi:10.1016/j.fishres.2006.06.020.
