Abstract
Evidence of necrophagous behaviour has been reported for 8.43% of nocturnal raptors. To determine whether preys were captured alive or consumed as carrion is challenging, as the diet of Strigiformes is mainly studied through pellet analysis, rather than direct observation. The diet of the long-eared owl Asio otus has been widely studied all over the distribution range of this species, but proven evidence of this feeding habit is still lacking. We collected 106 pellets under a suburban dormitory in Southern Tuscany (Central Italy) between December 2012 and April 2013. Prey remains (skulls, mandibles) were compared with a specific atlas. Four fragments of crested porcupine (Hystrix cristata) quills and a jawbone of Martes sp. were found in pellets collected after a snowfall (20–40 cm in depth). Although voles and mice constituted the staple of the diet of this species, accordingly with previous studies, these findings represent the first proof of carrion consumption by the long-eared owl. Body sizes of crested porcupine and Martes sp,, as well as the defence behaviour of the rodent, rule out a direct predation by the owl. Our study enlarges the trophic spectrum of the long-eared owl, thus adding a further dimension to the behavioural plasticity of this species.
Introduction
Although improved by large eyes with respect to the skull, sight does not represent the principal sense in owls (Aves: Strigiformes) because of their nocturnal habits. The specialized auditory systems of nocturnal raptors represent the main aid for food searching (Konishi Citation1973; Knudsen & Konishi Citation1978). Evolution has acted on owls by adapting hearing functions and by making them highly sensitive (Van Dijk Citation1972; Bye et al. Citation1992). Moreover, the fringed outer parts of the flight feathers allow owls to emit very little noise during flight (Lilley Citation1998; Bachmann et al. Citation2007). Such considerations may mitigate in favour of hunting behaviour, which includes the capture of live preys. Owls, flying silently, hear potential preys moving on the ground (e.g. small mammals), or flying (e.g. birds, bats). The diet of Strigiformes is often assessed by analysing content of pellets (i.e. bones, feathers and other food remains: e.g. Errington Citation1930; Alivizatos et al. Citation2005). Thus, to determine whether preys were captured alive or consumed as carrion is challenging, and not always verifiable.
Consumption of carcasses has been documented for 8.43% (21/249) of Strigiformes species (e.g. Kapfer et al. Citation2011; Allen & Taylor Citation2013). Among European nocturnal raptors, necrophagy have been described only for eagle owls Bubo bubo (Linnaeus, 1758) (Hiraldo et al. Citation1975; Serrano Citation2000; Dìaz-Ruiz et al. Citation2010) and barn owls Tyto alba Scopoli, 1769 (Welch Citation2012).
The long-eared owl Asio otus (Linnaeus, 1758) is a medium-sized nocturnal raptor widely distributed all over the Holarctic (BirdLife International Citation2012). The main preys of this species are small mammals, with seasonal variations recorded (e.g. Cramp Citation1985; Tome Citation2009): voles and mice represent the staple of the diet (see Birrer Citation2009, for a review). When access to these ground-dwelling rodents is denied (e.g. by snow cover), long-eared owls may supplement their diet with birds (Canova Citation1989; Birrer Citation2009).
The winter diet of this species has been analysed throughout its distribution range (see Birrer Citation2009 for a review). A group of individuals may share the same roost outside the breeding season (Cramp Citation1985); thus, collecting a high number of pellets in the surroundings of communal roosting areas may be easy, especially in urban environments, and it requires little time (Korpimäki Citation1992; Maccarone & Janzen Citation2005). Scavenging behaviour has been suggested also for this species: a small bone fragment, maybe belonging to a roe deer (Capreolus capreolus), was detected within a pellet (Erfurt & Stubbe Citation1987), but other irrefutable, well-ascribed evidence is still lacking.
On the other hand, pellet analysis is a valid method used to obtain new data on small mammal (Rodentia and Soricomorpha) communities and dynamics of a certain area, which is essential for research and conservation (e.g. Cecere & Vicini Citation2000). Since the long-eared owl is commonly considered a feeding specialist (Cramp Citation1985; Tome Citation2009), pellets of generalist species (e.g. barn owl: Michel et al. Citation2006; Obuch & Benda Citation2009) are preferred for this purpose; this may partly feed a limit in the knowledge of the diet of Asio otus. We performed an analysis of long-eared owl pellets in the framework of a checklist project about mammal communities of a Natura 2000 site in southern Tuscany. In this paper, we report the first evidence of carrion consumption in the winter diet of this nocturnal raptor.
Materials and methods
Pellets (N = 106) were gathered from one site inside the village of Prata, in the municipality of Massa Marittima (Province of Grosseto, Southern Tuscany: 43°05’N 10°59’E), from 20 December 2012 to 30 April 2013. The study area is located in a rural hilly landscape (475–903 m above sea level); a Natura 2000 network site (“Poggi di Prata”: Tuscan Regional Law 56/2000) and an International Waterbird Census area (IWC “Gabellino”) are part of it. Habitat types were determined through an ortophoto, provided by a local hunting district (ATC Gr6), and by on-the-spot investigations. Habitat composition resulted as follows: mixed deciduous woods (52.14%), fallows (19.49%), chestnut woods (14.89%), cultivated areas (7.78%), pinewoods (2.02%), built-up areas (1.97%) and scrubwoods (1.71%) (Cantini et al. Citation2013). Four other species of Strigiformes are recorded within the study area (Vannini et al. Citation2013): the barn owl, the tawny owl Strix aluco Linnaeus, 1758, the little owl Athene noctua Scopoli, 1769 and the scops owl Otus scops Linnaeus, 1758. According to a 2-year survey, the long-eared owl is the least common owl species within the study area, and the tawny owl the most detected (Vannini et al. Citation2013). Data on local small mammal densities and composition are scanty and incomplete (Sforzi & Ragni Citation1997). Pellets were collected under a winter roost, the only one identified during the survey, used by six individuals of Asio otus located on a stone pine (Pinus pinea L., 1753) at the side of the main street of the village. All the pellets detected on the ground were collected once every 3 days, then dried, softened in hot water and in pure ethanol, to better separate parts corresponding to different prey species (skulls, mandibles: Cecere & Vicini Citation2000). We considered a single individual for each species detected per pellet, as no more than one hemimandible per side was observed within the same pellet.
Identification of preys, from both broken and whole pellets, was carried out to the genus or species level, by comparison with Nappi (Citation2001), and with the aid of a binocular microscope (20 × and 40 ×). Skulls and mandibles belonging to the genus Apodemus presented similar morphology and measurements (Nappi Citation2001; Chassovnikarova & Markov Citation2007), so it has been not possible to assess whether remains belonged to A. sylvaticus (Linnaeus, 1758) or to A. flavicollis (Melchior, 1834). Both species are present within the study area (Mori, unpublished). Diet composition was calculated as the relative percentage of prey items belonging to each identified species.
Results
We determined 112 prey items belonging to 9–10 species (N = 5–6 Rodentia, 3 Soricomorpha, 1 Carnivora) from a total of 106 pellets. Remains from the other 34 pellets were not identified. The relative percentage of prey items belonging to each identified species, and the number of pellets, are reported in .
Table I. Winter diet composition (number of pellets containing each prey and percent by number of prey items) of long-eared owl in the study area. Apodemus spp. includes both A. sylvaticus and A. flavicollis.
Wood mice and bank voles represent the bulk of the diet (53.62% and 30.14% respectively, Table I). Other species are less represented. No insects, bats or birds were found in our sample.
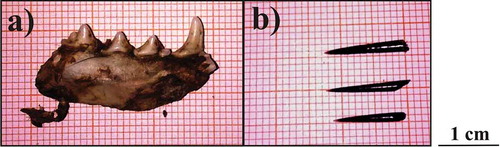
We reported the presence of crested porcupine (fragments of quills) and Martes sp. (fragment of a jawbone) in the diet of the long-eared owl (). The type of teeth (permanent teeth) and the level of dental wear found in the fragment indicate that the Martes sp. is an adult individual (Marshall Citation1951).
Discussion
In our study, the diet of the long-eared owl was strongly dominated by Apodemus spp. and bank voles (53.62% and 30.14% of prey items, respectively), consistent with other studies (see Birrer Citation2009, for a review). Accordingly, both black rats and Soricomorpha are poorly represented.
Fragments of crested porcupine quills were detected in four pellets, the day after a snowfall (20–40 cm in depth); their sturdiness suggests that they do not belong to a newborn individual (Mohr Citation1965). All four pellets were collected in the same location and on the same date, suggesting that they may belong to the same porcupine. The weight of crested porcupines (median weight of adult porcupines: 11.2 kg, Q1–Q3 = 9.9–13.3: Mori & Lovari Citation2014) is higher than the heaviest verified species preyed by long-eared owls (Bonasa umbellus, 400–500 g: Cramp Citation1985; Birrer Citation2009). Given also the anti-predatory behaviour displayed by porcupines (Mori et al. Citation2014), the hypothesis of a direct predation by the long-eared owl can be ruled out, thus suggesting a necrophagous behaviour. This feeding habit have never been described with certainty for A. otus, but only suggested (Erfurt & Stubbe Citation1987). The crested porcupine is inhomogeneously distributed throughout Italy, and higher population densities seem to occur in Central Italy, on the Tyrrhenian side (http://www.naturaesocialmapping.it/istrice, accessed 6 December 2013). At least 13 den setts inhabited by porcupines have been identified through camera trapping within the study area (Mori, unpublished). The peak of road-killed porcupines seems to occur during the winter (in February: Mori et al. Citation2013). Local hunters report that a similar rate is observed when the ground is covered by snow in vegetation-covered areas (i.e. scrubwoods, woodlands), thus possibly increasing the availability of this trophic source for the long-eared owl.
Three days later, when the ground was still covered by snow, a pellet containing a fragment of a jawbone of Martes sp. was collected. As for Mustelid remains, Cramp (Citation1985) reports only stoats Mustela erminea and weasels Mustela nivalis in the diet of A. otus, while Martes spp. are never mentioned (see Birrer Citation2009, for a review). The presence of Martes spp. has been sporadically observed in pellets of other European strigiforms: Bubo bubo (one M. foina in 2533 vertebrate preys: Wassink & Hingmann Citation2010; one M. martes in 164 vertebrate preys: Obuch & Karaska Citation2010), Strix aluco (one M. foina and one M. martes in 2651 vertebrate preys: Obuch Citation2011) and Tyto alba (one M. foina in 135 vertebrate preys: Obuch & Benda Citation2009). We assume that a medium-sized owl, which Asio otus is, cannot kill an adult Martes spp., because of its body size (Reig Citation1992, for marten size; Cramp Citation1985; Birrer Citation2009 for long-eared owl prey sizes) and aggressiveness (cf. Fryxell et al. Citation1999). Moreover, the level of tissue wear suggests that the fragment here described may belong to an individual that had been dead for a few days, and not to a directly preyed animal (D. Scaravelli, personal communication, 2013; cf. Behrensmeyer Citation1978).
Bertolino et al. (Citation2001) showed that diet of A. otus is more variable in Italy than in other countries. According to the optimal foraging theory (Pyke et al. Citation1977), variations in the abundance of small rodents in the field may trigger changes in the diet of this species, as a functional response to food scarcity (Holling Citation1959).
Surprisingly, the possible limited availability of small rodents has not been bypassed through predation on birds, whose activity is not obstructed by the snow cover (cf. Canova Citation1989; Sergio et al. Citation2008). High mortality peaks of mammals after snowfalls in the study area (Mori et al. Citation2013) may also provide an easily accessible trophic source to species adapted to hunt live prey, when these last are not available. Our findings provide new evidence that the long-eared owl is a specialized predator, which preys mainly on voles and wood mice. However, it may adapt its trophic spectrum when environmental conditions (e.g. snowfall, winter temperatures) reduce access to its preferred preys (e.g. ground-dwelling rodents: Canova Citation1989; Bertolino et al. Citation2001; Birrer Citation2009), including also carrion in its diet.
Acknowledgements
We thank Simon Birrer, Dino Scaravelli and Leonardo Ancillotto, who provided us with literature and helped us to improve the draft of our paper. We acknowledge Samuele Lozzi (ATC Gr6) for the ortophoto of the study area. Two anonymous reviewers and Paolo Trotti kindly took the time to make comments on the manuscript. Diego Consiglio allowed us to collect pellets in his private garden.
References
- Alivizatos H, Gourtner V, Zogaris S. 2005. Contribution to the study of the diet of four owl species (Aves, Strigiformes) from mainland and island areas of Greece. Belgian Journal of Zoology 135:109–118.
- Allen ML, Taylor AP. 2013. First record of scavenging by a western screech-owl (Megascops kennicottii). The Wilson Journal of Ornithology 125:417–419. doi:10.1676/12-176.1.
- Bachmann T, Klän S, Baumgartner W, Klaas M, Schröder W, Wagner H. 2007. Morphometric characterisation of wing feathers of the barn owl Tyto alba pratincola and the pigeon Columba livia. Frontiers in Zoology 4:23. doi:10.1186/1742-9994-4-23.
- Behrensmeyer AK. 1978. Taphonomic and ecologic information from bone weathering. Paleobiology 4:150–162.
- Bertolino S, Ghiberti E, Perrone A. 2001. Feeding ecology of the Long-eared owl (Asio otus) in northern Italy: Is it a dietary specialist? Canadian Journal of Zoology 79:2192–2198. doi:10.1139/cjz-79-12-2192.
- BirdLife International. 2012. Asio otus. In: IUCN, editors. IUCN Red List of Threatened Species. Version 2012.2. Available: http://www.iucnredlist.org. Accessed Jun 2013 04.
- Birrer S. 2009. Synthesis of 312 studies on the diet of the Long-eared owl Asio otus. Ardea 97:615–624. doi:10.5253/078.097.0430.
- Bye FN, Jacobsen BV, Sonerud GA. 1992. Auditory prey location in a pause-travel predator: Search height, search time, and attack range of Tengmalm’s Owls (Aegolius funereus). Behavioral Ecology 3:266–276. doi:10.1093/beheco/3.3.266.
- Canova L. 1989. Influence of snow cover on prey selection by Long-eared Owls Asio otus. Ethology, Ecology and Evolution 1:367–372. doi:10.1080/08927014.1989.9525506.
- Cantini M, Menchetti M, Vannini A, Bruni G, Borri B, Mori E. 2013. Checklist of Amphibians and Reptiles in a hilly area of Southern Tuscany (Central Italy): An update. Herpetology Notes 6:223–228.
- Cecere F, Vicini G. 2000. Micromammals in the diet of the long eared owl (Asio otus) at the W.W.F.’s Oasi San Giuliano (Matera, Southern Italy). Hystrix, the Italian Journal of Mammalogy 11:47–53.
- Chassovnikarova T, Markov G. 2007. Wood mice (Apodemus sylvaticus Linnaeus, 1758 and Apodemus flavicollis Melchior, 1834) from Bulgaria: Craniometric characteristics and species discrimination. Forest Science 3:39–52.
- Cramp S. 1985. The birds of the Western Palearctic. Vol. 4. New York, USA: Oxford University Press.
- Díaz-Ruiz F, Buenestado F, Fernàndez-de-Simòn J, Ferreras P. 2010. First record of rabbit carrion consumption by Eurasian eagle-owl (Bubo bubo) on the Iberian Peninsula. Journal of Raptor Research 44:78–79. doi:10.3356/JRR-09-21.1.
- Erfurt J, Stubbe M. 1987. Gewöllanalysen zur Untersuchung der Ernärungsbiologie von Eulen. In: Stubbe M, editor. Populationsökologie von Greifvogel – und Eulenarten. 1. 1987/14 (P27). Halle: Publisher. pp. 429–452.
- Errington PE. 1930. The pellet analysis method of raptor food habits study. The Condor 32:292–296. doi:10.2307/1363377.
- Fryxell JM, Falls JB, Falls EA, Brooks RJ, Dix L, Strickland MA. 1999. Density dependence, prey dependence, and population dynamics of martens in Ontario. Ecology 80:1311–1321. doi:10.1890/0012-9658(1999)080[1311:DDPDAP]2.0.CO;2.
- Hiraldo F, Andrada J, Parreño FF. 1975. Diet of the Eagle owl (Bubo bubo) in Mediterranean Spain. Doñana Acta Vertebrata 2:161–177.
- Holling CS. 1959. Some characteristics of simple types of predation and parasitism. The Canadian Entomologist 91:385–398. doi:10.4039/Ent91385-7.
- Kapfer JM, Gammon DE, Groves JD. 2011. Carrion-feeding by Barred Owls (Strix varia). The Wilson Journal of Ornithology 123:646–649. doi:10.1676/11-015.1.
- Knudsen EI, Konishi M. 1978. A neural map of auditory space in the owl. Science 200:795–797. doi:10.1126/science.644324.
- Konishi M. 1973. Experiments with trained barn owls reveal how their acute sense of hearing enables them to catch prey in the dark. American Scientist 61:414.
- Korpimäki E. 1992. Diet composition, prey choice, and breeding success of Long-eared Owls: Effects of multiannual fluctuations in food abundance. Canadian Journal of Zoology 70:2373–2381. doi:10.1139/z92-319.
- Lilley G. 1998. A study on the silent flight of the owl. AIAA Paper: 2004–2186.
- Maccarone AD, Janzen P. 2005. Winter diet of long-eared owls (Asio otus) at an urban roost in Wichita, Kansas. Transactions of the Kansas Academy of Science 108:116–120. doi:10.1660/0022-8443(2005)108[0116:WDOLOA]2.0.CO;2.
- Marshall WM. 1951. An age determination method for the pine marten. The Journal of Wildlife Management 15:276–283. doi:10.2307/3797220.
- Michel N, Burel F, Butet A. 2006. How does landscape use influence small mammal diversity, abundance and biomass in hedgerow networks of farming landscapes? Acta Oecologica 30:11–20.
- Mohr E. 1965. Altweltliche Stachelschweine. Wittenburg Lutherstadt, Germany: A. Ziemsen Verlag.
- Mori E, Corsini B, Mazza G, Menchetti M. 2013. From the shores to the cities: Road mortality of the crested porcupine in Southern Tuscany. In: Bertolino S, Capizzi D, Mori E, Colangelo P, Scaravelli D, editors. Pavia, Italy: Associazione Teriologica Italiana. Secondo Convegno Italiano sui Piccoli Mammiferi, Ercolano (Naples). pp. 24–25. October 2013. Abstract Book:41.
- Mori E, Lovari S. 2014. Sexual size monomorphism in the crested porcupine (Hystrix cristata). Mammalian Biology - Zeitschrift für Säugetierkunde 79:157–160. doi:10.1016/j.mambio.2013.07.077.
- Mori E, Maggini I, Menchetti M. 2014. When quills kill. The defence strategy of the crested porcupine Hystrix cristata L., 1758. Mammalia 78:229–234.
- Nappi A. 2001. I Micromammiferi d’Italia. Naples, Italy: Publisher Simoni. editors.
- Obuch J. 2011. Spatial and temporal diversity of the diet of the tawny owl (Strix aluco). Slovak Raptor Journal 4:83–98.
- Obuch J, Benda P. 2009. Food of the Barn Owl (Tyto alba) in the Eastern Mediterranean. Slovak Raptor Journal 3:41–50. doi:10.2478/v10262-012-0032-4.
- Obuch J, Karaska D. 2010. The Eurasian eagle-owl (Bubo bubo) diet in the Orava Region (N Slovakia). Slovak Raptor Journal 4:83–98. doi:10.2478/v10262-012-0048-9.
- Pyke GH, Pulliam HR, Charnov EL. 1977. Optimal foraging: A selective review of theory and tests. The Quarterly Review of Biology 52:137–154. doi:10.1086/409852.
- Reig S. 1992. Geographic variation in pine marten (Martes martes) and beech marten (M. foina) in Europe. Journal of Mammalogy 73:744–769. doi:10.2307/1382193.
- Sergio F, Marchesi L, Pedrini P. 2008. Density, diet and productivity of Long-eared Owls Asio otus in the Italian Alps: The importance of Microtus voles: Capsule Relatively large populations, feeding predominantly upon voles, were present at higher elevations. Bird Study 55:321–328. doi:10.1080/00063650809461538.
- Serrano D. 2000. Use of farm chicken carcasses by the Eagle Owl. Ardeola 47:101–103.
- Sforzi A, Ragni B. 1997. Atlante dei Mammiferi della Provincia di Grosseto. Supplemento al n. 16 degli Atti del Museo di Storia Naturale della Maremma, Grosseto, Italy.
- Tome D. 2009. Changes in the diet of long-eared owl Asio otus: Seasonal patterns of dependence on vole abundance. Ardeola 56:49–56.
- Van Dijk T. 1972. A comparative study of hearing in owls of the family Strigidae. Netherlands Journal of Ornithology 23:131–167.
- Vannini A, Menchetti M, Mori E. 2013. L’avifauna del pSIC “Poggi di Prata” (Grosseto, Italia centrale): analisi faunistica, quantitativa e considerazioni sulla gestione ambientale del sito. Alula 20:101–112.
- Wassink G, Hingmann W. 2010. Year-round diet of Eagle Owl Bubo bubo in The Netherlands and adjacent Germany. Limosa 83:97–108.
- Welch S. 2012. A possible instance of a Barn Owl scavenging in severe weather. Scottish Birds 32:300–301.