Abstract
Environmental change is commonly considered as a driver for the extinction of rare species. This belief, long established on land, may not apply to marine species. Dramatic environmental change in the shallow marine ecosystems of Kos, an island in the Aegean (east Mediterranean Sea) caused algal reefs to shift to sponge reefs. Among the sponge species that gained supremacy on Kos reefs, the Mediterranean endemic Ircinia retidermata was previously a rare species. Comparing surveys carried out in 1981 and 2013 by the same method (time-based visual census along random paths by scuba diving), in the same sites, by the same people, showed that I. retidermata increased its overall abundance by one order of magnitude, and expanded its occurrence to all the habitats examined. This outcome contradicts the current common belief that rare species in semi-enclosed seas are prone to extinction. Besides being the state preceding final extinction, rarity could represent the source of variation that marine ecosystems need in order to face environmental change. However, for many marine invertebrates, and especially sponges, inferred rarity may simply be the result of insufficient investigation. This study represents an attempt to assess change with time in a rare sponge species’ abundance using visual census by scuba diving.
Introduction
Global change is affecting the planet’s biodiversity and ecosystem functioning (Reid et al. Citation2005), driving unprecedented rates of species extinction that are altering key processes of Earth’s ecosystems (Hooper et al. Citation2012). Extinctions can be caused by the combined action of a complex mixture of driver variables, but natural rarity is normally considered the main ecological trait that predisposes species to extinction (Brook et al. Citation2008): it has long been believed that rare species are the most endangered, rarity being usually used as a surrogate for extinction risk (Gaston Citation2013).
Marine ecosystems are centrally important to the ecology of the planet (Hoegh-Guldberg & Bruno Citation2010), and global environmental change (including climate change, biodiversity loss, modified hydrological and biogeochemical cycles, and intensive exploitation of natural resources together with the consequences of other anthropogenic pressures) is having significant impacts on the world’s oceans (Barangé et al. Citation2010). This notwithstanding, extinction risk in the sea is still little understood (Roberts & Hawkins Citation1999; del Monte-Luna et al. Citation2007).
The Mediterranean Sea is a biodiversity hotspot with many endemic and rare species (Bianchi & Morri Citation2000). It is a semi-enclosed basin experiencing heavy demographic, urban and industrial pressures (Airoldi & Beck Citation2007), and where climate change is showing large impacts (Bianchi & Morri Citation2003). Complete disappearance of a species from a basin as wide as the Mediterranean in a few decades is perhaps unrealistic, and represents a highly debated issue (Bianchi et al. Citation2012). Examples do exist, however, of local extinctions or severe decrease of certain species (del Monte-Luna et al. Citation2007). The commercial sponge Spongia officinalis Linnaeus, 1759, for instance, has been dramatically reduced in the last decades due to overexploitation and disease (Pronzato & Manconi Citation2008; Voultsiadou et al. Citation2011). The opposite, i.e., that environmental change causes a rare species to become more abundant and/or to colonise habitats from which it was formerly excluded, has been envisaged by Boero (Citation1994), who hypothesised that rare species may represent those components that ecosystems employ to take over functional or structural aspects of community makeup when change occurs (see also Boero & Bonsdorff Citation2007). Little evidence is presently available in support of Boero’s hypothesis. Yet a recent survey of the shallow-water marine benthos of the Island of Kos (southeast Aegean) found abundant and widespread Ircinia retidermata Pulitzer-Finali & Pronzato, Citation1980 (Demospongiae: Dictyoceratida), a species of sponge that proved extremely rare in a previous survey in 1981 (Bianchi & Morri Citation1983: Ircinia sp.).
First described in 1980 on the basis of two specimens collected by trawls 70–80 m deep (Pulitzer-Finali & Pronzato Citation1980), I. retidermata has been recorded only four times thereafter. Ilan et al. (Citation1994) reported it dubiously from a depth of 830 m off the Israeli Mediterranean coast. On the contrary, Bianchi and Morri (Citation1983) and Schiaparelli et al. (Citation2007) reported it as shallow as 3 m, in the Aegean, and 12 m, in the Ligurian Sea, respectively. In all these records, the species came from rocks dispersed amidst sand or mud, or from rocky reefs. Only recently, Manconi et al. (Citation2013) found it inside a submarine cave at the base of a rocky cliff in Sardinia. Thus, I. retidermata, which can be considered endemic to the Mediterranean Sea at the present status of knowledge, exhibits the main forms of rarity recognised in conservation biology (Manne & Pimm Citation2001, and references therein): low abundance, habitat specialisation and narrow geographic range. I. retidermata is a conspicuous species living in the scuba zone (), and the fact that it has never been illustrated in any of the numerous guidebooks to the marine fauna of the Mediterranean Sea (for a recent example see Weinberg Citation2013) may be taken as a further indication of its actual rarity.
Figure 1. A massive specimen of Ircinia retidermata on shallow rocks at Kos in 2013 (underwater photograph by G. Chrisopoulous).

The present paper is aimed at documenting the recent habitat expansion and increased abundance of I. retidermata at Kos, where a recent survey evidenced dramatic environmental change with respect to 30 years before (Bianchi & Morri Citation1983; Bianchi et al. Citation2014).
Materials and methods
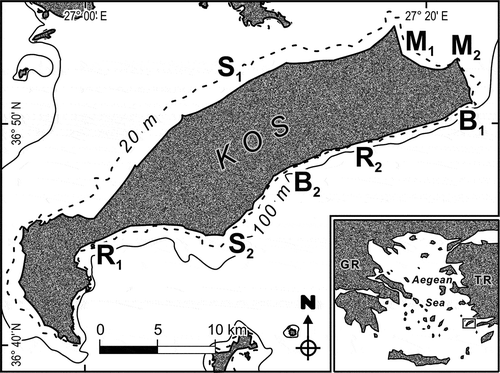
Kos is one of the major islands of the Dodecanese Archipelago, located in the southeastern corner of the Aegean Sea near the coasts of Turkey (). In 1981 and 2013, four habitats were studied in both years, each represented by two sites (): (i) habitat S, submerged beachrocks in sandy seafloor (sites S1 and S2); (ii) habitat M, rocks within seagrass meadows (sites M1 and M2); (iii) habitat B, boulder fields (sites B1 and B2); (iv) habitat R, rocky reefs (sites R1 and R2). Ircinia retidermata was visually inventoried by scuba diving to about 10 m depth; the number of specimens was counted on a time basis (40-minute dives) along random paths. Also, the total number of sponges and macroalgal species was counted contextually. For each of the two reef sites, the total macroalgal and sponge cover was estimated in three randomly placed point intercept transects (PITs) (Bianchi et al. Citation2004).
Results and discussion
In 2013, a total of 32 specimens was recorded in the eight sites, compared to just two specimens recorded in 1981 – an increase of one order of magnitude. While in 1981 Ircinia retidermata was found in one of the sandy sites only (S1), in 2013 it thrived in all four habitats surveyed. In the sandy sites (S1 and S2), its abundance doubled (). It was especially common in boulder fields (B1 and B2) and, secondarily, rocky reefs (R1 and R2), but scarcer within seagrass meadows (M1 and M2). Its occurrence in such different habitat types was unprecedented in the few other Mediterranean areas for which the species was known.
Figure 3. Mean (± standard error, SE) abundance of Ircinia retidermata in four coastal marine habitats of Kos in 1981 and 2013. S = beachrocks in sandy seafloor; M = rocks within seagrass beds; B = boulders fields; R = rocky reefs.
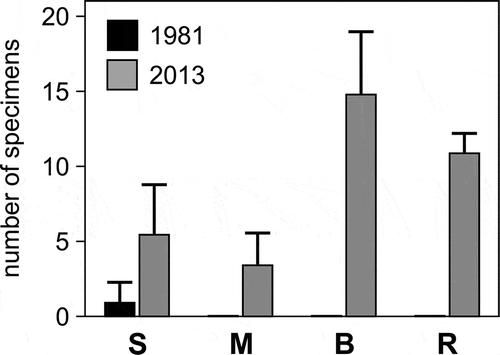
As the surveys of 1981 and 2013 have been conducted using the same method (time-based visual census along random paths), in the same sites, by the same people, the differences observed in both abundance and number of habitats colonised by the species may be taken as significant. We used a revisitation approach and two points in time are obviously too few to track long-term change, but the fact that I. retidermata has not been included in the sponge fauna checklists previously published for the region (Voultsiadou Citation2005; Evcen & Çinar Citation2012) suggests that its increased abundance and habitat expansion are recent phenomena.
Together with Ircinia retidermata, several other sponge species – such as Agelas oroides (Schmidt, 1864), Aplysina aerophoba Nardo, 1833, Chondrosia reniformis Nardo, 1847, Crambe crambe (Schmidt, 1862), Petrosia ficiformis (Poiret, 1789) and Sarcotragus foetidus Schmidt, 1862 – were more abundant in 2013 than in 1981, especially on rocks. None of those species, however, can be qualified as rare in the whole Mediterranean basin, making the case of I. retidermata unusual.
During the last few decades, Kos has undergone an impressive change in environmental conditions, with a sea surface temperature rise of more than 1°C, an exponential increase of human pressure (the resident population has nearly doubled and tourism has grown about eight times), and the invasion by tens of alien marine species (Pancucci-Papadopoulou et al. Citation2012). The combined action of these changes produced an evident alteration of coastal marine ecosystem composition, structure and functioning, with reduction in submerged macrophyte cover and habitat homogenisation (Bianchi et al. Citation2014).
The increased sponge abundance at Kos was paralleled by the near extirpation of macroalgae (), caused by the combined action of temperature rise, growing human pressure and, especially, excess herbivory by the invasive fish Siganus (Bianchi et al. Citation2014). Kos represents a rather unique example for the Mediterranean Sea of habitat switching due to the introduction of an alien species (Sala et al. Citation2011; Giakoumi Citation2014). The relative-dominance paradigm of Littler and Littler (Citation1985) for tropical reefs suggests corals gain primacy upon macroalgae under intense herbivory. Whether this paradigm is applicable to the rocky reefs of the Mediterranean Sea, which are presently undergoing a process of tropicalisation (Bianchi & Morri Citation2003), is debatable (Peirano et al. Citation1998; Serrano et al. Citation2012). No coral species were abundant at Kos, where algal depletion implied the first known example in a warm-temperate sea of algal reefs shifting to sponge reefs (); on the other hand, there are examples in the tropics of coral reefs becoming sponge reefs under the influence of climate change (Bell et al. Citation2013). Are sponges becoming the dominant organisms in both warm-temperate and tropical reefs of the future?
Figure 4. Shift from algal dominance to sponge dominance, both in terms of substrate cover (left panel) and species richness (right panel), on Kos reefs between 1981 and 2013.
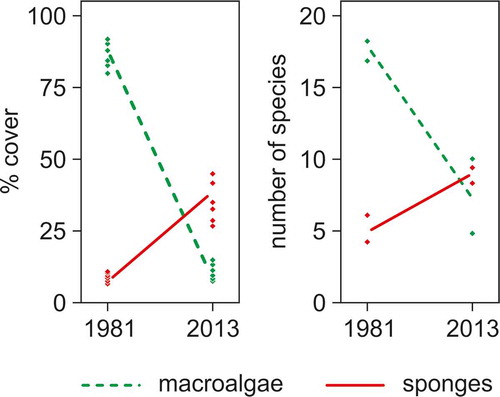
Figure 5. A shallow rocky reef of Kos in 2013 deprived of macroalgal cover and with large massive sponges (underwater photograph by G. Chrisopoulous).

Among the sponge species that gained supremacy in Kos reefs, I. reticulata was previously a rare species. Now, not only is it common but it has also expanded its occurrence to various habitats, thus participating, together with many newly arrived aliens, in the process of biotic homogenisation. This outcome contradicts the current common belief that rare species in semi-enclosed seas are prone to extinction under the impact of climate change and species invasions (Cheung et al. Citation2009). According to Boero (Citation1994), rarity, besides being the state preceding final extinction, could represent the source of potential variation and alternation that marine ecosystems need in order to face environmental change: the case of I. reticulata at Kos might represent an example in support of Boero’s hypothesis.
The idea that rarity anticipates extinction was erected for terrestrial species (Chapman Citation1999), and might not apply to the sea. For many marine invertebrates, alleged rarity may simply be the result of insufficient investigation, and the real pattern of species distributions is often obscured by extrinsic factors linked to the availability of the relevant taxonomic specialists (Giangrande & Licciano Citation2004). This situation is particularly obvious for sponges. The case of the genus Darwinella (Demospongiae: Dendroceratida) may serve as an example. After a detailed note on this genus by Pronzato (Citation1975), no other records of Darwinella species have been reported from the Mediterranean Sea. The world distribution pattern of this genus, the very low number of findings and their chronology may help in illustrating the problem of defining rarity for sponges. Among the ca. 30 specimens globally recorded of this taxon from 1865 to the present day, in temperate and tropical marine coastal waters, six were collected by a single scientist in only two Mediterranean localities (Portofino and Ischia), in less than two years (October 1974–June 1976); these six specimens represent half of the Mediterranean records. When Pronzato stopped looking for Darwinella, this taxon “disappeared” from the Mediterranean Sea, as no other sponge specialist found it again.
Unfortunately, studies aiming at detecting change with time in the populations of conspicuous, easily recognisable sponge species are few and partial in the Mediterranean Sea. Similarly to what done by Voultsiadou et al. (Citation2011), our example at Kos represents an attempt using visual census by scuba diving and comparison with historical information through revisitation after many years: regular monitoring would be needed in order to understand the link between environmental change and the increased abundance of Ircinia retidermata.
Acknowledgements
Kos Divers (Psalidi, www.kosdivers.com) and Spirit of the Sea (Kardamena, www.kosdiving.gr) provided logistic support to field activity in 2013. George Chrisopoulous ([email protected]) made freely available to us his underwater photographs.
References
- Airoldi L, Beck MW. 2007. Loss, status and trends for coastal marine habitats of Europe. Oceanography and Marine Biology Annual Revue 45:345–405.
- Barangé M, Field JG, Harris RP, Hofmann EE, Perry RI, Werner F, editors. 2010. Marine ecosystems and global change. Oxford, USA: University Press.
- Bell JJ, Davy SK, Jones T, Taylor MW, Webster NS. 2013. Could some coral reefs become sponge reefs as our climate changes? Global Change Biology 19:2613–2624. doi:10.1111/gcb.12212.
- Bianchi CN, Corsini-Foka M, Morri C, Zenetos A. 2014. Thirty years after: Dramatic change in the coastal marine habitats of Kos Island (Greece), 1981–2013. Mediterranean Marine Science 15. doi:http://dx.doi.org/10.12681/mms.678.
- Bianchi CN, Morri C. 1983. Note sul benthos marino costiero dell’Isola di Kos (Egeo sud—orientale). Natura 74:96–114.
- Bianchi CN, Morri C. 2000. Marine biodiversity of the Mediterranean Sea: Situation, problems and prospects for future research. Marine Pollution Bulletin 40:367–376. doi:10.1016/S0025-326X(00)00027-8.
- Bianchi CN, Morri C. 2003. Global sea warming and “tropicalization” of the Mediterranean Sea: Biogeographic and ecological aspects. Biogeographia 24:319–327.
- Bianchi CN, Morri C, Chiantore M, Montefalcone M, Parravicini V, Rovere A. 2012. Mediterranean Sea biodiversity between the legacy from the past and a future of change. In: Stambler N, editor. Life in the Mediterranean Sea: A look at habitat changes. New York, USA: Nova Science. pp. 1–55.
- Bianchi CN, Pronzato R, Cattaneo-Vietti R, Benedetti-Cecchi L, Morri C, Pansini M, Chemello R, Milazzo M, Fraschetti S, Terlizzi A, Peirano A, Salvati E, Benzoni F, Calcinai B, Cerrano C, Bavestrello G. 2004. Mediterranean marine benthos: A manual of methods for its sampling and study. 6: Hard bottoms. Biologia Marina Mediterranea 11(suppl. 1):185–215.
- Boero F. 1994. Fluctuations and variations in coastal marine environments. PSZN I: Marine Ecology 15:3–25.
- Boero F, Bonsdorff E. 2007. A conceptual framework for marine biodiversity and ecosystem functioning. Marine Ecology 28(suppl. 1):134–145. doi:10.1111/j.1439-0485.2007.00171.x.
- Brook BW, Sodhi NS, Bradshaw CJA. 2008. Synergies among extinction drivers under global change. Trends in Ecology and Evolution 23:453–460. doi:10.1016/j.tree.2008.03.011.
- Chapman MG. 1999. Are there adequate data to assess how well theories of rarity apply to marine invertebrates? Biodiversity and Conservation 8:1295–1318. doi:10.1023/A:1008909323840.
- Cheung WWL, Lam VWY, Sarmiento JL, Kearney K, Watson R, Pauly D. 2009. Projecting global marine biodiversity impacts under climate change scenarios. Fish and Fisheries 10:235–251. doi:10.1111/j.1467-2979.2008.00315.x.
- del Monte-Luna P, Lluch-Belda D, Serviere-Zaragoza E, Carmona R, Reyes-Bonilla H, Aurioles-Gamboa D, Luis Castro-Aguirre J, Guzmán del Próo SA, Trujillo-Millán O, Brook BW. 2007. Marine extinctions revisited. Fish and Fisheries 8:107–122. doi:10.1111/j.1467-2679.2007.00240.x.
- Evcen A, Çinar ME. 2012. Sponge (Porifera) species from the Mediterranean coast of Turkey (Levantine Sea, eastern Mediterranean), with a checklist of sponges from the coasts of Turkey. Turkish Journal of Zoology 36:460–464.
- Gaston KJ. 2013. Rarity. Reprint of the original 1st edition (1994). London, UK: Chapman and Hall.
- Giakoumi S. 2014. Distribution patterns of the invasive herbivore Siganus luridus (Rüppell, 1829) and its relation to native benthic communities in the central Aegean Sea, Northeastern Mediterranean. Marine Ecology 35:96–105. doi:10.1111/maec.12059.
- Giangrande A, Licciano M. 2004. Factors influencing latitudinal pattern of biodiversity: An example using Sabellidae (Annelida, Polychaeta). Biodiversity and Conservation 13:1633–1646. doi:10.1023/B:BIOC.0000029327.63397.6b.
- Hoegh-Guldberg O, Bruno JF. 2010. The impact of climate change on the world’s marine ecosystems. Science 328:1523–1528. doi:10.1126/science.1189930.
- Hooper DU, Adair EC, Cardinale BJ, Byrnes JEK, Hungate BA, Matulich KL, Gonzalez A, Duffy JE, Gamfeldt L, O’Connor MI. 2012. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486:105–108.
- Ilan M, Ben-Eliahu MN, Galil B. 1994. Three deep water sponges from the Eastern Mediterranean and their associated fauna. Ophelia 39:45–54. doi:10.1080/00785326.1994.10429901.
- Littler MM, Littler DS. 1985. Factors controlling relative dominance of primary producers on biotic reefs. In: Gabrie C, Salvat B, editors. Proceedings of the 5th International Coral Reef Congress. Volume 4: Symposia and Seminars (B). Moorea, French Polynesia: Antenne Museum-Ephe. pp. 35–39.
- Manconi R, Cadeddu B, Ledda F, Pronzato R. 2013. An overview of the Mediterranean cave-dwelling horny sponges (Porifera, Demospongiae). ZooKeys 281:1–68. doi:10.3897/zookeys.281.4171.
- Manne LL, Pimm SL. 2001. Beyond eight forms of rarity: Which species are threatened and which will be next? Animal Conservation 4:221–229. doi:10.1017/S1367943001001263.
- Pancucci-Papadopoulou MA, Raitsos DE, Corsini-Foka M. 2012. Biological invasions and climatic warming: Implications for south-eastern Aegean ecosystem functioning. Journal of the Marine Biological Association of the United Kingdom 92:777–789. doi:10.1017/S0025315411000981.
- Peirano A, Morri C, Mastronuzzi G, Bianchi CN. 1998. The coral Cladocora caespitosa (Anthozoa, Scleractinia) as a bioherm builder in the Mediterranean Sea. Memorie Descrittive della Carta Geologica d’Italia 52(1994):59–74.
- Pronzato R. 1975. Note tassonomiche sul genere Darwinella (Porifera). Bollettino dei Musei e degli Istituti Biologici dell’Università di Genova 43:5–20.
- Pronzato R, Manconi R. 2008. Mediterranean commercial sponges: Over 5000 years of natural history and cultural heritage. Marine Ecology 29:146–166. doi:10.1111/j.1439-0485.2008.00235.x.
- Pulitzer-Finali G, Pronzato R. 1980. The Keratosa in a collection of Mediterranean sponges, mainly from the Italian coasts. Annali del Museo Civico di Storia Naturale ‘Giacomo Doria’ di Genova 83:123–158.
- Reid WV, Mooney HA, Cropper A, Capistrano D, Carpenter SR, Chopra K, Dasgupta P, Dietz T, Duraiappah AK, Hassan R, Kasperson R, Leemans R, May RM, McMichael TAJ, Pingali P, Samper C, Scholes R, Watson RT, Zakri AH, Shidong Z, Ash NJ, Bennett E, Kumar P, Lee MJ, Raudsepp-Hearne C, Simons H, Thonell J, Zurek MB. 2005. Millennium ecosystem assessment synthesis report. Washington, USA: Island Press.
- Roberts CM, Hawkins JP. 1999. Extinction risk in the sea. Trends in Ecology and Evolution 14:241–246. doi:10.1016/S0169-5347(98)01584-5.
- Sala E, Kizilkaya Z, Yildirim D, Ballesteros E. 2011. Alien marine fishes deplete algal biomass in the Eastern Mediterranean. Public Library of Science One 6:e17356.
- Schiaparelli S, Castellano M, Povero P, Sartoni G, Cattaneo-Vietti R. 2007. A benthic mucilage event in North-Western Mediterranean Sea and its possible relationships with the summer 2003 European heatwave: Short term effects on littoral rocky assemblages. Marine Ecology 28:341–353. doi:10.1111/j.1439-0485.2007.00155.x.
- Serrano E, Coma R, Ribes M. 2012. A phase shift from macroalgal to coral dominance in the Mediterranean. Coral Reefs 31:1199. doi:10.1007/s00338-012-0939-3.
- Voultsiadou E. 2005. Sponge diversity in the Aegean Sea: Check list and new information. Italian Journal of Zoology 72:53–64. doi:10.1080/11250000509356653.
- Voultsiadou E, Dailianis T, Antoniadou C, Vafidis D, Dounas C, Chintiroglou C. 2011. Aegean bath sponges: Historical data and current status. Reviews in Fisheries Science 19:34–51. doi:10.1080/10641262.2010.531794.
- Weinberg S. 2013. Découvrir la vie sous-marine: Méditerranée. Challes-les-eaux, France: Gap.