Abstract
Increased attention has been focused on marine invertebrates as a source of bioactive molecules for biomedical applications. Many bioactive molecules are part of the innate immune system. Some more recently isolated compounds, mainly from the sea urchin and the sea cucumber, are antimicrobial peptides (AMPs) active against Gram-positive and Gram-negative bacteria, and fungi. In this review we described the most recent studies on AMPs isolated from echinoderms. AMPs are small peptides (< 10 kDa) with cationic charge and amphipathic structure. Recently, it was demonstrated that in the coelomocyte lysates of Paracentrotus lividus and Holothuria tubulosa, AMPs possess activity against staphylococcal and Pseudomonas aeruginosa biofilms. The data shows a great potential for application of AMPs in biotechnology for developing novel therapeutic agents that are either alternative or complementary to conventional antibiotic therapy to combat multi-resistant pathogens.
Biofilms and antimicrobial peptides
Biofilms are a complex community of microbial cells, enclosed in three-dimensional networks of microbial cells embedded in a self-produced polymeric matrix (slime) (). The capacity to organize a biofilm community is present in almost all Gram-negative and Gram-positive bacteria. The process of building a biofilm structure consists, generally, of four stages: (1) adherence of planktonic cells to the abiotic surface or tissue through weak van der Waals forces where the colonists are anchored tightly or irreversibly by pili (Pratt & Kolter Citation1998) (); (2) the microcolonies’ recruitment of other planktonic cells and growth. Normally, they are surrounded by a large amount of extracellular polymeric protective matrix (extracellular polymeric substance: EPS) (Lawrence et al. Citation1991). The matrix includes a wide variety of proteins, glycoproteins and glycolipids, and, in some cases, surprising amounts of extracellular DNA (e-DNA) (Hall-Stoodley & Stoodley Citation2009), and can interact with the environment, e.g., by attaching biofilms to surfaces, and through its sorption properties, which allow for sequestering of dissolved and particulate substances from the environment, providing nutrients for biofilm organisms and influencing predator-prey interactions (Joubert et al. Citation2006). These components are very important targets for overcoming both biofilms and drug-resistant bacteria (Hancock & Diamond Citation2000), (). (3) Biofilm maturation involving the development of water channels and specialized zones (), and (4) dispersion of cells and/or parts of the biofilm with subsequent colonization of other locations (Costerton et al. Citation1981; Donlan & Costerton Citation2002; Fey Citation2010; Høiby et al. Citation2010) (). The architecture of a mature biofilm is variable, ranging from flat, homogeneous layers of cells to highly organized cell clusters with a mushroom-shaped structure containing water-filled channels (Wimpenny et al. Citation2000).
Figure 1. The cycle of biofilm. (a) Attachment of planktonic cells to a surface; (b) recruitment of other planktonic cells and production of extracellular polymeric substance (EPS); (c) growth and development of biofilm through cell division and recruitment; (d) dispersion of bacteria from biofilm.
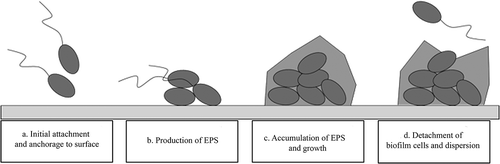
Although there is great scientific interest and there have been important research advances in the biofilm area, we are still far from being able to control and suppress biofilms. Approximately 80% of human bacterial infections are caused by biofilms (Harro et al. Citation2010), mainly due to healthcare-associated infections related to the implantation of medical devices, e.g. urinary catheters, intravascular catheters and prosthetic heart valves. As such, microbial adhesion onto surfaces and the subsequent formation of biofilms are critical concerns for many biomedical applications (Donlan & Costerton Citation2002; Fey Citation2010). Indeed, the increasing resistance of biofilms to traditional antimicrobial treatments is considered the major cause of dissemination of antibiotic resistance in nosocomial infections (Fey Citation2010; Spizek et al. Citation2010).
Antibiotics can be effective against planktonic (free-living) pathogens but are quite often barely effective against the bacteria organized in a community, which can increase antibiotic resistance by 10–1000 fold (Nickel et al. Citation1985; Evans & Holmes Citation1987; Gristina et al. Citation1987; Prosser et al. Citation1987). It has been estimated that biofilms are associated with nosocomial infections and represent the fourth leading cause of death in the US with ~10% of American hospital patients leading to more than $5 billion in added medical costs per annum (Wenzel Citation2007). The bacteria structured in biofilms develop a multifactorial mechanism of resistance to antibiotics (Obst et al. Citation2006) and there are several factors that contribute to biofilm resistance. The biofilm environment allows a higher frequency of mutation and horizontal gene transmission when compared to planktonic bacteria, which explains the rapid development of antibiotic resistance in biofilms (Ghigo Citation2001). Within the structure of the biofilm are formed oxygen and nutrient gradients, which cause some form of nutrient limitation. This induces the bacteria to enter into a stationary phase, like dormancy, and under this form bacteria are tolerant to antimicrobials (Brown et al. Citation1988; Wentland et al. Citation1996). The resistance can also be due to a general stress response initiated by growth within a biofilm (Brown & Barker Citation1999). So, bacteria can resist, protecting themselves from the detrimental effects of heat shock, cold shock, changes in pH and many chemical agents (Hengge-Aronis Citation1996). The matrix is able to delay antibiotic penetration into the biofilm structure, which contains polymers that bind to antibiotics and hinder their action, and antibiotic-degrading enzymes that deactivate them (Hoyle et al. Citation1992; Tseng et al. Citation2013).
The difficulty of successfully treating biofilm-associated infections, and the increasing resistance of microbes to traditional treatments, call for the discovery of compounds with novel mechanisms of action. Most of the antimicrobial products that have been developed are derivatives of compounds that are already known, and hit the same targets, so their action can only be somewhat better. Now, research is drifting towards the discovery of non-traditional sources of antimicrobials, and a series of natural compounds that exhibit antimicrobial activity has been isolated in the past 20 years from many plant, insect and animal species (Roch et al. Citation1996; Andreu & Rivas Citation1998; Bulet et al. Citation1999; Zasloff Citation2002; Rosetto et al. Citation2003; Dalla Valle et al. Citation2013) as defense molecules as humoral parts of the innate immune response (). Animals, in particular, are the most important source of antimicrobial compounds, and several hundreds of antimicrobial peptides have been found in a wide range of invertebrate and vertebrate species. These natural antimicrobial substances, named antimicrobial peptides (AMPs), stand out because they have a much higher hit rate in high-throughput screens than the combinational libraries of traditional antimicrobials. Moreover, natural products are usually much more complex than synthetic products and present scaffolds with viable and biologically validated starting points for the design of chemical libraries (Spizek et al. Citation2010). According to antimicrobial peptide databases, more than 10,000 AMPs have been discovered to date ().
Table I. List of antimicrobial web databases.
The largest group of AMPs is that of cationic molecules, which are widely distributed in animals and plants. They have a small molecular size with no more than 100 amino acids and a molecular weight < 10 kDa. They differ considerably in amino acid sequence and structural conformation, and most of them are positively charged, showing a net positive charge of +2 to +9 due to an excess number of basic amino acids like arginine or lysin, with 50% or more of the amino acids hydrophobic and forming a hydrophobic face opposite to a hydrophilic one (Brogden Citation2005; Hancock & Sahl Citation2006). On the basis of their structural features, cationic AMPs can be divided into linear peptides forming α-helical structures, cysteine-rich open-ended peptides containing single or several disulfide bridges and molecules rich in specific amino acids such as proline, glycine or histidine. These chemo-physical characteristics allow these peptides to be soluble in water but react simultaneously with the hydrophobic layer of microbial membranes. Such peptides are found in all species of life including bacteria, fungi, plants, insects, birds, crustaceans, amphibians and mammals. A single animal can contain different classes of peptides and a number of variants in a given class.
The mode of action of AMPs has rarely been addressed and is therefore not yet understood (Brogden Citation2005). It is generally agreed that the prevalent mechanism of action of the AMPs is due to their ability to permeabilize and/or to form pores within the cytoplasmic membranes. AMPs are initially recruited on the microbial surface through electrostatic interaction between the cationic portion of the peptides and the negatively charged microbial cell walls and/or membranes. Bacterial pathogens’ membranes are composed predominantly of phosphatidylglycerol, cardiolipin or phosphatidylserine and tend to be highly electronegative. Studies support a non-receptor type interaction for antimicrobial peptides with most pathogen membranes (Bessalle et al. Citation1990; Wade et al. Citation1990). When the peptide/lipid ratio increases, the peptides start forming multimers or self-associating on top of the membrane. When the peptides reach a high concentration, they orientate perpendicularly and insert into the bilayer, thereby interfering with membrane integrity. The microorganisms are then destroyed via either membrane destabilization and/or pore formation (Brogden Citation2005; Yount et al. Citation2006), or through interference with several essential metabolic functions, such as protein, nucleic acid and cell wall syntheses acting on nucleic acids and/or enzymatic proteins, leading to bacterial cell death (Kamysz et al. Citation2003; Brogden Citation2005; Yount et al. Citation2006; Hale & Hancock Citation2007; Nicolas Citation2009). Moreover, it appears that many AMPs may be multifunctional microbicides, acting simultaneously at the cell membrane and internal sites (Yount et al. Citation2006).
Recent research has shown that AMPs also have a high potential for inhibiting the formation of or destroying biofilm. In fact, they can act at several stages of biofilm formation and with different mechanisms of action: they may minimize the initial adhesion of microbial cells to abiotic surfaces by altering the adhesive features of abiotic surfaces, reducing flagellum-dependent swimming motility, stimulating twitching motility, a type of surface motility that promotes the disassembly of biofilm structures or by binding to microbial surfaces via electrostatic interactions; they may prevent biofilm maturation by killing the early surface colonizers, or by inhibiting quorum sensing (QS) – that is, the communication system used by many bacteria to build a biofilm. QS is a system composed of small molecules that control collective behaviors, such as bioluminescence, virulence factor production and biofilm formation (Spoering & Gilmore Citation2006; Horswill et al. Citation2007; Picioreanu et al. Citation2007; Huang et al. Citation2010; Brogden & Brogden Citation2011; de la Fuente-Nunez et al. Citation2012).
AMPs are produced by living organisms throughout the bacteria and animal kingdoms, including also fungi and plants (O’Keefe Citation2001; Rodriguez et al. Citation2002; Zasloff Citation2002; Castro & Fontes Citation2005; Cotter et al. Citation2005; Riley & Chavan Citation2007; Strominger Citation2009; da Rocha Pitta et al. Citation2010). Currently, more than 4000 peptides have been isolated and characterized from tissues and organisms, and have been listed in the main databases or in journal publications (Thomas et al. Citation2010).
In invertebrates, AMPs are ubiquitously distributed, found especially in hemolymph and in tissues such as epidermis, gut and respiratory organs where exposure to pathogenic microorganisms is most likely to occur, expressed constitutively or in response to a pathogen stimulus. The AMPs defend the organism not only through their ability to kill bacteria, but it has been shown that they have antitumor effects and mitogenic activity and, most importantly, participate in immunoregulatory mechanisms by modulating signal transduction and cytokine production and/or release (Hancock & Diamond Citation2000; Zasloff Citation2002; Bals & Wilson Citation2003; Kamysz et al. Citation2003; Bowdish et al. Citation2005; Brown & Hancock Citation2006; Hancock et al. Citation2006; Yount et al. Citation2006; Easton et al. Citation2009; Lai & Gallo Citation2009; Guani-Guerra et al. Citation2010).
The purpose of this review will be to present the most recent data on microbial antibiofilm peptides isolated and characterized in the phylum Echinodermata. In particular we will focus on their structure and biological functions, and on their potential application as antimicrobial and antibiofilm agents to combat human pathogens.
Antimicrobial and antibiofilm peptides in echinoderms
Echinoderms are deuterostome invertebrates, an ancient group of marine invertebrates that live in both the intertidal and deep-sea benthos, composed of approximately 7000 extant species including sea stars (asteroids), sea urchins (echinoids), sea cucumbers (holothurians), brittle stars (ophiuroids) and sea lilies (crinoids). Because many species live in or near the coastal or estuary waters overloaded with infectious organisms – such as viruses, bacteria, fungi and other parasites – they are exposed to a broad variety of challenges to their self-integrity. They have evolved efficient defense strategies in order to survive in these high-impact environments.
Echinoderms, similarly to other invertebrates, do not possess a specific immune response. Therefore, they ensure the protection of their organism and its homeostasis using natural immunity responses.
The echinoderm humoral immune response consists of the production of proteins that are sequestered within the cells, having hemolytic and hemagglutinating properties (Canicattì Citation1987, Citation1988, Citation1992; Arizza et al. Citation2007), and also includes the synthesis of AMPs that are secreted from the coelomocyte.
Beauregard et al. (Citation2001) discovered, for the first time in echinoderms, the presence of AMPs, a peptide of ~ 6 kDa active against Gram-positive and Gram-negative bacteria, in the coleomic fluid of the sea cucumber Cucumaria frondosa (Gunnerus, 1767). Since 2001, the number of such discovered molecules has increased steadily. Stabili et al. (Citation1996) found in coelomic fluid and in coelomocytes of Paracentrotus lividus (Lamarck, 1816) the effector cells of immunity, a peptide of 60 kDa that was able to inhibit the growth of Vibrio alginolyticus. Other antimicrobial activity was found in the jelly coat, in seminal plasma (Stabili & Canicattì Citation1994) and in the larval lysate of the same species (Stabili et al. Citation1994). In some cases, antimicrobial proteins were also found in the gastrointestinal organs and in the eggs of Strongylocentrotus droebachiensis (Müller, 1776) and Asterias rubens (Linnaeus, 1758) (Haug et al. Citation2002; Li et al. Citation2008). Many AMPs are derived from larger proteins that could be enzymatically digested and produce active fragments. This has been demonstrated by (Ghanbari et al. Citation2012). In fact, digesting the tissues of sea cucumber Actinopyga lecanora (Jaeger, 1833) with bromelain, peptides with inhibitory activities against Pseudomonas sp., P. aeruginosa, and Escherichia coli, respectively, were obtained and one papain-digested fraction showed antibacterial activity against Staphylococcus aureus. Maltseva et al. (Citation2007) found that among the AMPs isolated in the starfish Asterias rubens, two peptides were part of the histone molecule H2A. Maltseva and co-workers also found that two other peptides were fragments of actin, while one peptide was a fragment of filamin A. Gowda et al. (Citation2008) showed that an agglutinin that can agglutinate Gram-positive and Gram-negative bacteria exhibited strong antibacterial activity both under in vivo and in vitro conditions. Defensin-like peptides were isolated by Ng et al. (Citation2013) in Strongylocentrotus droebachiensis. In a recent study, Li et al. (Citation2010) showed, in S. droebachiensis, an AMP heterodimer structure named centrocin. Centrocins possess a strong activity, not only against Gram-positive and Gram-negative bacteria, but also against fungi and yeasts.
The simultaneous presence of diverse AMPs found in the same echinoderms as S. droebachiensis, probably acting in synergy or complementary to each other, may provide the organisms with an extended defense against a wide range of pathogenic microorganisms. Such interactions have been reported, at least in vitro, between different AMPs isolated from the horseshoe crab (Iwanaga et al. Citation1998) and the oyster, Crassostrea gigas (Thunberg, 1793) (Gueguen et al. Citation2009).
Recently, our research group has found novel cationic peptides in the echinoderm species Paracentrotus lividus and in Holothuria tubulosa (Gmelin, 1788). They were isolated from coelomocyte lysate supernatant and showed good activity in preventing the biofilm formation of important pathogens involved in human and animal diseases, like staphylococcal or P. aeruginosa strains (Schillaci et al. Citation2010, Citation2013).
The antimicrobial peptides were discovered in a protein fraction at low molecular weight (< 5 kDa) from acid extract of coelomocytes. P. lividus showed a peptide, Paracentrin 1, of 1251.7, the peptide belonging to a segment (9–19) of a β-thymosin, a ubiquitous peptide that exerts several biological effects such as the induction of metalloproteinases, chemotaxis, angiogenesis and inhibition of inflammation (Huff et al. Citation2001). The β thymosins are a family of highly conserved polar 5-kDa peptides originally thought to be thymic hormones. They are present at high concentrations in almost every cell from vertebrate phyla and have several biological functions due to direct and indirect effects on the actin cytoskeleton. β-Thymosin is also described as one of the AMPs of platelets from animals, including human beings (Tang et al. Citation2002). There is little information about the function of thymosins in invertebrates, but their presence has been reported in marine invertebrates (Safer & Chowrashi Citation1997; Saelee et al. Citation2013) and in insects where they are up-regulated by microbial infections (Zhang et al. Citation2011). H. tubulosa possessed two peptides, Holothuroidin 1 and Holothuroidin 2, whose molecular weights are respectively 1389.5 and 1547.6 Da (Schillaci et al. Citation2013).
The peptides of both species showed a similarity with other antimicrobial peptides produced by different organisms. Indeed, Paracentrin 1 had a amino acid sequence similarity of 38.46% with Jelleine-III, a short peptide presenting a broad spectrum of activity against Gram-positive and Gram-negative bacteria, and also against yeasts present in the royal jelly produced by Apis mellifera (Linnaeus, 1758) worker bees (Fontana et al. Citation2004); both Holothuroidin 1 and Holothuroidin 2 showed an amino acid sequence similarity ≥ 35% respectively with protonectins, peptides present in the venom of the neotropical social wasp Agelaia pallipes (Lepeletier, 1836), with a potent antimicrobial action against both Gram-positive and Gram-negative bacteria (Mendes et al. Citation2004) and signiferins, a naturally occurring cationic peptide produced by an Australian frog, Crinia signifera (Girard, 1853), that showed a wide spectrum of activity against Gram-positive and Gram-negative bacteria including Bacillus cereus, Enterococcus faecalis, Lactococcus lactis, Listeria innocua, Micrococcus luteus, Staphylococcus aureus, Staphylococcus epidermidis and Streptococcus uberis (Maselli et al. Citation2004).
The peptides of both species had α-helix structures with a considerable amphipathic character, with the polar and mainly cationic residues segregated on one polar face and the hydrophobic or nonpolar residues on the opposite, apolar face. (). They were cationic peptides with a net charge of +0.9, with a total hydrophobic ratio and deduced pI ranging respectively from 36.36 and 8.72 for Paracentrin 1 to 42.86 and 7.56 for both Holothuroidins ().
Table II. Chemical-physical characteristics and amino acid sequences of echinoderm antibiofilm peptides.
The activity of synthetic peptides constructed from the sequences indicated by the tandem mass spectrometry (MS/MS) data were active against free-living (planktonic) Gram-positive and Gram-negative pathogens as Staphylococcus aureus, Staphylococcus epidermidis and Pseudomonas aeruginosa () (Schillaci et al. Citation2010).
Figure 2. A ribbon representation of (A) Paracentrin 1, (B) Holoturoidin 1 and (C) Holoturoidin 2. The amphipathic nature of the peptide is shown in this representation with the hydrophilic side above and the hydrophobic side below the polypeptide backbone. The potential surface is superimposed. Color code: acidic residues in red, basic residues in blue and hydrophobic residues in white.
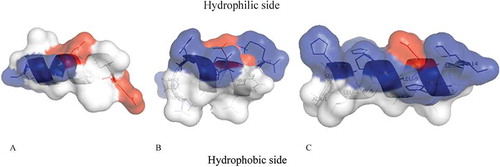
Table III. Antimicrobial activity (MIC) of < 5-kDa peptide fraction from celomocytes lysate supernatant (Paracentrin 1) or synthetic peptides (Holothuroidin 1 and 2). Values expressed in mg/mL.
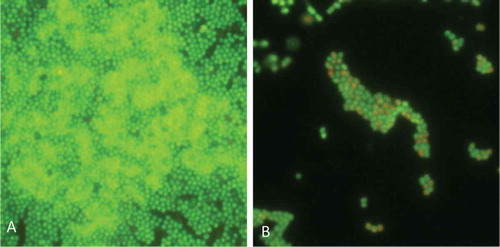
The three echinoderm antimicrobial peptides were also active to combat the biofilm formation at sub-minimum inhibitory concentration (MIC) concentrations. Indeed, Paracentrin 1 was able to inhibit either the formation of a young biofilm (6-h old) of S. epidermidis 1457 or the formation of a mature biofilm (24 h old) of the same clinical strain (Schillaci et al. Citation2010). The antimicrobial peptides from H. tubulosa were able to inhibit the biofilm formation of two staphylococcal reference strains, S. aureus ATCC 25923 and S. epidermidis ATCC 35984 (Schillaci et al. Citation2013).
Figure 3. Anti-biofilm activity of Paracentrin 1 against Staphylococcus epidermidis. (A) Control; (B) biofilm treated with sub-minimum inhibitory concentration concentration of Paracentrin 1.
We observed that synthetic Paracentrin 1, Holothuroidin 1 and Holothuroidin 2 did not show, in phosphate buffered saline (PBS), hemolytic activity, probably because the high ionic strength of PBS allows negatively charged sialic acid present on the erythrocyte membrane to neutralize the peptides. On the basis of this evidence, they could be classified as peptide antibiotics (Saberwal & Nagaraj Citation1994).
Conclusions
AMPs are evolutionarily ancient defensive molecules. Their extraordinary distribution in all kingdoms, within both unicellular and multicellular organisms, suggests that they have a key and fundamental role in the biology of organisms that probably has evolved through positive selection (Tennessen Citation2005; Viljakainen & Pamilo Citation2008; Fernandes et al. Citation2010).
AMPs from marine invertebrates could play a complementary but critical role in helping acquired immunity in vertebrates to combat bacterial infections that normally confound and even suppress the immune system with their sophisticated and multiantigenic cycle of life (Chiu et al. Citation2013).
AMPs from marine invertebrates can be applied in biotechnology and in medicine. These natural compounds constitute potential candidates for the development of alternative strategies to prevent and treat bacterial infections, including biofilm-associated infections that are particularly resistant to conventional antibiotics.
In conclusion, the discovery of novel peptides in echinoderms represents a good starting point to design new synthetic derivatives with modified chemical-physical properties, with the aim being to improve their antimicrobial activity against pathogens, and their pharmaceutical potential (Huang et al. Citation2010; Brogden & Brogden Citation2011).
References
- Andreu D, Rivas L. 1998. Animal antimicrobial peptides: An overview. Biopolymers 47:415–433. doi:10.1002/(SICI)1097-0282(1998)47:6<415::AID-BIP2>3.0.CO;2-D.
- Arizza V, Giaramita FT, Parrinello D, Cammarata M, Parrinello N. 2007. Cell cooperation in coelomocyte cytotoxic activity of Paracentrotus lividus coelomocytes. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 147:389–394. doi:10.1016/j.cbpa.2007.01.022.
- Bals R, Wilson JM. 2003. Cathelicidins a family of multifunctional antimicrobial peptides. Cellular and Molecular Life Sciences (CMLS) 60:711–720. doi:10.1007/s00018-003-2186-9.
- Beauregard KA, Truong NT, Zhang H, Lin W, Beck G, Sugumaran, M, Cooper, EL. 2001. The detection and isolation of a novel antimicrobial peptide from the echinoderm, Cucumaria frondosa. Phylogenetic Perspectives on the Vertebrate Immune System 484:55–62. doi:10.1007/978-1-4615-1291-2_5.
- Bessalle R, Kapitkovsky A, Gorea A, Shalit I, Fridkin M. 1990. All-D-magainin: Chirality, antimicrobial activity and proteolytic resistance. FEBS Letters 274:151–155. doi:10.1016/0014-5793(90)81351-N.
- Bowdish DM, Davidson DJ, Lau YE, Lee K, Scott MG, Hancock RE. 2005. Impact of LL-37 on anti-infective immunity. Journal of Leukocyte Biology 77:451–459. doi:10.1189/jlb.0704380.
- Brogden KA. 2005. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nature Reviews Microbiology 3:238–250. doi:10.1038/nrmicro1098.
- Brogden NK, Brogden KA. 2011. Will new generations of modified antimicrobial peptides improve their potential as pharmaceuticals? International Journal of Antimicrobial Agents 38:217–225.
- Brown MRW, Allison DG, Gilbert P. 1988. Resistance of bacterial biofilms to antibiotics a growth-rate related effect? Journal of Antimicrobial Chemotherapy 22:777–780. doi:10.1093/jac/22.6.777.
- Brown MRW, Barker J. 1999. Unexplored reservoirs of pathogenic bacteria: Protozoa and biofilms. Trends in Microbiology 7:46–50. doi:10.1016/S0966-842X(98)01425-5.
- Brown KL, Hancock RE. 2006. Cationic host defense (antimicrobial) peptides. Current Opinion in Immunology 18:24–30. doi:10.1016/j.coi.2005.11.004.
- Bulet P, Hetru C, Dimarcq JL, Hoffmann D. 1999. Antimicrobial peptides in insects; structure and function. Developmental & Comparative Immunology 23:329–344. doi:10.1016/S0145-305X(99)00015-4.
- Canicattì C. 1987. Evolution of the lytic system in echinoderms. I. Naturally occurring hemolytic activity in Paracentrotus lividus(Echinoidea) coelomic fluid. Bolletino di Zoologia 54:325–329. doi:10.1080/11250008709355604.
- Canicattì C. 1988. The lytic system of Holothuria polii (Echinodermata): A review. Bolletino di Zoologia 55:139–144. doi:10.1080/11250008809386610.
- Canicattì C. 1992. The echinoderm lytic system. Bolletino di Zoologia 59:159–166. doi:10.1080/11250009209386664.
- Castro MS, Fontes W. 2005. Plant defense and antimicrobial peptides. Protein Peptide Letter 12:13–18.
- Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, Wainger B, Strominger A, Muralidharan S, Horswill AR, Wardenburg JB, Hwang SW, Carroll MC, Woolf CJ. 2013. Bacteria activate sensory neurons that modulate pain and inflammation. Nature 501:52–57. doi:10.1038/nature12479.
- Costerton JW, Irvin RT, Cheng KJ. 1981. The bacterial glycocalyx in nature and disease. Annual Review of Microbiology 35:299–324. doi:10.1146/annurev.mi.35.100181.001503.
- Cotter PD, Hill C, Ross RP. 2005. Bacterial lantibiotics: Strategies to improve therapeutic potential. Current Protein Peptide Science 6:61–75. doi:10.2174/1389203053027584.
- da Rocha Pitta MG, Galdino M, Lins Galdino S. 2010. Development of novel therapeutic drugs in humans from plant antimicrobial peptides. Current Protein Peptide Science 11:236–247. doi:10.2174/138920310791112066.
- Dalla Valle L, Benato F, Paccanaro MC, Alibardi L. 2013. Bioinformatic and molecular characterization of cathelicidin-like peptides isolated from the green lizard Anolis carolinensis (Reptilia: Lepidosauria: Iguanidae). Italian Journal of Zoology 80:177–186. doi:10.1080/11250003.2013.783632.
- de la Fuente-Nunez C, Korolik V, Bains M, Nguyen U, Breidenstein EBM, Horsman S, Lewenza S, Burrows L, Hancock REW. 2012. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrobial Agents and Chemotherapy 56:2696–2704. doi:10.1128/AAC.00064-12.
- Donlan RM, Costerton JW. 2002. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clinical Microbiology Reviews 15:167–193. doi:10.1128/CMR.15.2.167-193.2002.
- Easton DM, Nijnik A, Mayer ML, Hancock REW. 2009. Potential of immunomodulatory host defense peptides as novel anti-infectives. Trends in Biotechnology 27:582–590. doi:10.1016/j.tibtech.2009.07.004.
- Evans RC, Holmes CJ. 1987. Effect of vancomycin hydrochloride on Staphylococcus epidermidis biofilm associated with silicone elastomer. Antimicrobial Agents and Chemotherapy 31:889–894. doi:10.1128/AAC.31.6.889.
- Fernandes JC, Tavaria FK, Fonseca SC, Ramos OS, Pintado ME, Malcata FX. 2010. In vitro screening for antimicrobial activity of chitosans and chitooligosaccharides, aiming at potential uses in functional textiles. Journal of Microbiology and Biotechnology 20:311–318. doi:10.4014/jmb.0904.04038.
- Fey PD. 2010. Modality of bacterial growth presents unique targets: How do we treat biofilm-mediated infections? Current Opinion in Microbiology 13:610–615. doi:10.1016/j.mib.2010.09.007.
- Fontana R, Mendes MA, de Souza BM, Konno K, César LM, Malaspina O, Palma MS. 2004. Jelleines: A family of antimicrobial peptides from the Royal Jelly of honeybees (Apis mellifera). Peptides 25:919–928. doi:10.1016/j.peptides.2004.03.016.
- Ghanbari R, Ebrahimpour A, Abdul-Hamid A, Ismail A, Saari N. 2012. Actinopyga lecanora hydrolysates as natural antibacterial agents. International Journal of Molecular Sciences 13:16796–16811. doi:10.3390/ijms131216796.
- Ghigo J-M. 2001. Natural conjugative plasmids induce bacterial biofilm development. Nature 412:442–445. doi:10.1038/35086581.
- Gowda NM, Goswami U, Islam Khan M. 2008. T-antigen binding lectin with antibacterial activity from marine invertebrate, sea cucumber (Holothuria scabra): Possible involvement in differential recognition of bacteria. Journal of Invertebrate Pathology 99:141–145. doi:10.1016/j.jip.2008.04.003.
- Gristina AG, Hobgood CD, Webb LX, Myrvik QN. 1987. Adhesive colonization of biomaterials and antibiotic resistance. Biomaterials 8:423–426. doi:10.1016/0142-9612(87)90077-9.
- Guaní-Guerra E, Santos-Mendoza T, Lugo-Reyes SO, Terán LM. 2010. Antimicrobial peptides: General overview and clinical implications in human health and disease. Clinical Immunology and Immunopathology 135:1–11. doi:10.1016/j.clim.2009.12.004.
- Gueguen Y, Bernard R, Julie F, Paulina S, Delphine DG, Franck V, Philippe B, Evelyne B. 2009. Oyster hemocytes express a proline-rich peptide displaying synergistic antimicrobial activity with a defensin. Molecular immunology 46:516–522. doi:10.1016/j.molimm.2008.07.021.
- Hale JD, Hancock RE. 2007. Alternative mechanisms of action of cationic antimicrobial peptides on bacteria. Expert Review of Anti-infective Therapy 5:951–959. doi:10.1586/14787210.5.6.951.
- Hall-Stoodley L, Stoodley P. 2009. Evolving concepts in biofilm infections. Cellular Microbiology 11:1034–1043. doi:10.1111/j.1462-5822.2009.01323.x.
- Hancock RE, Brown KL, Mookherjee N. 2006. Host defence peptides from invertebrates emerging antimicrobial strategies. Immunobiology 211:315–322. doi:10.1016/j.imbio.2005.10.017.
- Hancock RE, Diamond G. 2000. The role of cationic antimicrobial peptides in innate host defences. Trends in Microbiology 8:402–410. doi:10.1016/S0966-842X(00)01823-0.
- Hancock REW, Sahl HG. 2006. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nature Biotechnology 24:1551–1557. doi:10.1038/nbt1267.
- Harro JM, Peters BM, O’May GA, Archer N, Kerns P, Prabhakara R, Shirtliff ME. 2010. Vaccine development in Staphylococcus aureus: Taking the biofilm phenotype into consideration. FEMS Immunology Medical Microbiology 59:306–323.
- Haug T, Kjuul AK, Styrvold OB, Sandsdalen E, Olsen ØM, Stensvåg K. 2002. Antibacterial activity in Strongylocentrotus droebachiensis (Echinoidea), Cucumaria frondosa (Holothuroidea), and Asterias rubens (Asteroidea). Journal of Invertebrate Pathology 81:94–102. doi:10.1016/S0022-2011(02)00153-2.
- Hengge-Aronis R. 1996. Regulation of gene expression during entry into stationary phase. Cell and Molecular Biology 5:1497–1512.
- Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. 2010. Antibiotic resistance of bacterial biofilms. International Journal of Antimicrobial Agents 35:322–332. doi:10.1016/j.ijantimicag.2009.12.011.
- Horswill AR, Stoodley P, Stewart PS, Parsek MR. 2007. The effect of the chemical, biological, and physical environment on quorum sensing in structured microbial communities. Analytical Bioanalytical Chemistry 387:371–380. doi:10.1007/s00216-006-0720-y.
- Hoyle BD, Alcantara J, Costerton JW. 1992. Pseudomonas aeruginosa biofilm as a diffusion barrier to piperacillin. Antimicrobial Agents and Chemotherapy 36:2054–2056. doi:10.1128/AAC.36.9.2054.
- Huang YB, Huang JF, Chen YX. 2010. Alpha-helical cationic antimicrobial peptides: Relationships of structure and function. Protein & Cell 1:143–152. doi:10.1007/s13238-010-0004-3.
- Huff T, Müller CS, Otto AM, Netzker R, Hannappel E. 2001. β-Thymosins, small acidic peptides with multiple functions. The International Journal of Biochemistry & Cell Biology 33:205–220. doi:10.1016/S1357-2725(00)00087-X.
- Iwanaga S, Kawabata SI, Muta T. 1998. New types of clotting factors and defense molecules found in horseshoe crab hemolymph: Their structures and functions. Journal of Biochemistry 123:1–15. doi:10.1093/oxfordjournals.jbchem.a021894.
- Joubert LM, Wolfaardt GM, Botha A. 2006. Microbial exopolymers link predator and prey in a model yeast biofilm system. Microbial Ecology 52:187–197. doi:10.1007/s00248-006-9063-7.
- Kamysz W, Okroj M, Lukasiak J. 2003. Novel properties of antimicrobial peptides. Acta Biochimica Polonica 50:461–469.
- Lai Y, Gallo RL. 2009. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends in Immunology 30:131–141. doi:10.1016/j.it.2008.12.003.
- Lawrence JR, Korber DR, Hoyle BD, Costerton JW, Caldwell DE. 1991. Optical sectioning of microbial biofilms. Journal of Bacteriology 173:6558–6567.
- Li C, Haug T, Moe MK, Styrvold OB, Stensvåg K. 2010. Centrocins: Isolation and characterization of novel dimeric antimicrobial peptides from the green sea urchin, Strongylocentrotus droebachiensis. Developmental Comparative Immunology 34:959–968. doi:10.1016/j.dci.2010.04.004.
- Li C, Haug T, Styrvold OB, Jørgensen TØ, Stensvåg K. 2008. Strongylocins, novel antimicrobial peptides from the green sea urchin, Strongylocentrotus droebachiensis. Developmental Comparative Immunology 32:1430–1440. doi:10.1016/j.dci.2008.06.013.
- Maltseva AL, Aleshina GM, Kokryakov VN, Krasnodembsky EG. 2007. Diversity of antimicrobial peptides in acidic extracts from coelomocytes of starfish Asterias rubens L. Biologiya 1:10.
- Maselli VM, Brinkworth CS, Bowie JH, Tyler MJ. 2004. Host-defence skin peptides of the Australian Common Froglet Crinia signifera: Sequence determination using positive and negative ion electrospray mass spectra. Rapid Communications in Mass Spectrometry 18:2155–2161. doi:10.1002/rcm.1602.
- Mendes MA, de Souza BM, Marques MR, Palma MS. 2004. Structural and biological characterization of two novel peptides from the venom of the neotropical social wasp Agelaia pallipes pallipes. Toxicon 44:67–74. doi:10.1016/j.toxicon.2004.04.009.
- Ng TB, Cheung RCF, Wong JH, Ye XJ. 2013. Antimicrobial activity of defensins and defensin-like peptides with special emphasis on those from fungi and invertebrate animals. Current Protein & Peptide Science 14:515–531. doi:10.2174/13892037113149990068.
- Nickel JC, Ruseska I, Wright JB, Costerton JW. 1985. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrobial Agents and Chemotherapy 27:619–624. doi:10.1128/AAC.27.4.619.
- Nicolas P. 2009. Multifunctional host defense peptides: Intracellular targeting antimicrobial peptides. FEBS Journal 276:6483–6496. doi:10.1111/j.1742-4658.2009.07359.x.
- O’Keefe BR. 2001. Biologically active proteins from natural product extracts. Journal of Natural Products 64:1373–1381. doi:10.1021/np0103362.
- Obst U, Schwartz T, Volkmann H. 2006. Antibiotic resistant pathogenic bacteria and their resistance genes in bacterial biofilms. International Journal of Artificial Organs 29:387–394.
- Picioreanu C, Kreft JU, Klausen M, Haagensen JA, Tolker-Nielsen T, Molin S. 2007. Microbial motility involvement in biofilm structure formation a 3D modelling study. Water Science and Technology 55:337–343. doi:10.2166/wst.2007.275.
- Pratt LA, Kolter R. 1998. Genetic analysis of Escherichia coli biofilm formation: Roles of flagella, motility, chemotaxis and type I pili. Molecular Microbiology 30:285–293. doi:10.1046/j.1365-2958.1998.01061.x.
- Prosser BL, Taylor D, Dix BA, Cleeland R. 1987. Method of evaluating effects of antibiotics on bacterial biofilm. Antimicrobial Agents and Chemotherapy 31:1502–1506. doi:10.1128/AAC.31.10.1502.
- Riley MA, Chavan MA. 2007. Bacteriocins ecology and evolution. Berlin: Springer-Verlag.
- Roch P, Hubert F, van Der Knaap W, Noël T. 1996. Present knowledge on the molecular basis of cytotoxicity, antibacterial activity and stress response in marine bivalves. Italian Journal of Zoology 63:311–316. doi:10.1080/11250009609356151.
- Rodríguez JM, Martínez MI, Kok J. 2002. Pediocin PA-1, a wide-spectrum bacteriocin from lactic acid bacteria. Critical Reviews in Food Science and Nutrition 42:91–121. doi:10.1080/10408690290825475.
- Rosetto M, Belardinelli M, Fausto AM, Bongiorno G, Maroli M, Mazzini M. 2003. Antimicrobial activities in the haemolymph of Phlebotomus papatasi (Diptera, Psychodidae). Italian Journal of Zoology 70:221–224. doi:10.1080/11250000309356520.
- Saberwal G, Nagaraj R. 1994. Cell-lytic and antibacterial peptides that act by perturbing the barrier function of membranes: Facets of their conformational features, structure-function correlations and membrane-perturbing abilities. Biochimica et Biophysica Acta - Reviews on Biomembranes 1197:109–131. doi:10.1016/0304-4157(94)90002-7.
- Saelee N, Noonin C, Nupan B, Junkunlo K, Phongdara A, Lin X, Söderhäll K, Söderhäll I, Schönbach, C. 2013. β-thymosins and hemocyte homeostasis in a crustacean. PLoS One 8:e60974. doi:10.1371/journal.pone.0060974.
- Safer D, Chowrashi PK. 1997. β-thymosins from marine invertebrates: Primary structure and interaction with actin. Cell Motility and the Cytoskeleton 38:163–171. doi:10.1002/(SICI)1097-0169(1997)38:2<163::AID-CM5>3.0.CO;2-8.
- Schillaci D, Arizza V, Parrinello N, Di Stefano V, Fanara S, Muccilli V, Cunsolo V, Haagensen JJ, Molin S. 2010. Antimicrobial and antistaphylococcal biofilm activity from the sea urchin Paracentrotus lividus. Journal of Applied Microbiology 108:17–24. doi:10.1111/j.1365-2672.2009.04394.x.
- Schillaci D, Cusimano MG, Cunsolo V, Miccilli V, Russo D, Vazzana M, Vitale M, Arizza V. 2013. Immune mediators of sea-cucumber Holothuria tubulosa (Echinodermata) as source of novel antimicrobial and anti-staphylococcal biofilm agents. AMB Express 3:1–10.
- Spížek J, Novotná J, Řezanka T, Demain AL. 2010. Do we need new antibiotics? The search for new targets and new compounds. Journal of Industrial Microbiology Biotechnology 37:1241–1248. doi:10.1007/s10295-010-0849-8.
- Spoering AL, Gilmore MS. 2006. Quorum sensing and DNA release in bacterial biofilms. Current Opinion in Microbiology 9:133–137. doi:10.1016/j.mib.2006.02.004.
- Stabili L, Canicattì C. 1994. Antibacterial activity of the seminal plasma of Paracentrotus lividus. Canadian Journal of Zoology-Revue Canadienne de Zoologie 72:1211–1216. doi:10.1139/z94-162.
- Stabili L, Licciano M, Pagliara P. 1994. Evidence of antibacterial and lysozyme like activity in different planktonic larval stages of Paracentrotus lividus. Marine Biology 119:501–505. doi:10.1007/BF00354311.
- Stabili L, Pagliara P, Roch P. 1996. Antibacterial activity in the coelomocytes of the sea urchin Paracentrotus lividus. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 113:639–644. doi:10.1016/0305-0491(95)02080-2.
- Strominger JL. 2009. Animal antimicrobial peptides: Ancient players in innate immunity. The Journal of Immunology 182:6633–6634. doi:10.4049/jimmunol.0990038.
- Tang YQ, Yeaman MR, Selsted ME. 2002. Antimicrobial peptides from human platelets. Infection and Immunity 70:6524–6533. doi:10.1128/IAI.70.12.6524-6533.2002.
- Tennessen JA. 2005. Molecular evolution of animal antimicrobial peptides: Widespread moderate positive selection. Journal of Evolutionary Biology 18:1387–1394. doi:10.1111/j.1420-9101.2005.00925.x.
- Thomas S, Karnik S, Barai RS, Jayaraman VK, Idicula-Thomas S. 2010. CAMP: A useful resource for research on antimicrobial peptides. Nucleic Acids Research 38:D774–780. doi:10.1093/nar/gkp1021.
- Tseng BS, Zhang W, Harrison JJ, Quach TP, Song JL, Penterman J, Singh PK, Chopp DL, Packman AI, Parsek MR. 2013. The extracellular matrix protects Pseudomonas aeruginosa biofilms by limiting the penetration of tobramycin. Environmental Microbiology 15:2865–2878.
- Viljakainen L, Pamilo P. 2008. Selection on an antimicrobial peptide defensin in ants. Journal of Molecular Evolution 67:643–652. doi:10.1007/s00239-008-9173-6.
- Wade D, Boman A, Wahlin B, Drain CM, Andreu D, Boman HG, Merrifield RB. 1990. All-D amino acid-containing channel-forming antibiotic peptides. Proceedings of the National Academy of Sciences USA 87:4761–4765. doi:10.1073/pnas.87.12.4761.
- Wentland EJ, Stewart PS, Huang CT, McFeters GA. 1996. Spatial variations in growth rate within Klebsiella pneumoniae colonies and biofilm. Biotechnology Progress 12:316–321. doi:10.1021/bp9600243.
- Wenzel RP. 2007. Health care-associated infections: Major issues in the early years of the 21st century. Clinical infectious diseases 45:SS85–88. doi:10.1086/518136.
- Wimpenny J, Manz W, Szewzyk U. 2000. Heterogeneity in biofilms. FEMS Microbiology Reviews 24:661–671. doi:10.1111/j.1574-6976.2000.tb00565.x.
- Yount NY, Bayer AS, Xiong YQ, Yeaman MR. 2006. Advances in antimicrobial peptide immunobiology. Biopolymers 84:435–458. doi:10.1002/bip.20543.
- Zasloff M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389–395. doi:10.1038/415389a.
- Zhang FX, Shao HL, Wang JX, Zhao XF. 2011. β-thymosin is upregulated by the steroid hormone 20-hydroxyecdysone and microorganisms. Insect Molecular Biology 20:519–527. doi:10.1111/j.1365-2583.2011.01082.x.
