Abstract
A compilation of our present knowledge of the water mites (Acari: Hydrachnidia) adapted to life in standing waters on the three large islands in the western Mediterranean (Corsica, Sardinia and Sicily) is provided. In addition to published data, this study deals with a rich volume of new material from recent field work, mostly deriving from intermittent ponds and pools, an extremely poorly investigated yet peculiar habitat type in the Mediterranean area. Species richness of water mites reported for the standing waters of the three islands amounts to 91 species. Out of the 47 species for which we present new distributional data, Hydrachna incisa Halbert, 1903, Hydrachna leegei Koenike, 1895, Piersigia limophila Protz, 1896, Hydryphantes crassipalpis Koenike, 1914 and Piona laminata (Thor, 1900) have not been recorded previously from the Mediterranean area. Most of these species were believed to have typical North European distributions. In addition to these, a further 13 species are recorded for the first time from the area covered. In total, 11 species are new for Italy, seven more are new for Sicily, three for Sardinia and seven for Corsica. Redescriptions are given of Axonopsis complanata (Müller, 1776) (A. graeca, nov. syn), Brachypoda baderi (reported for the first time after the original description from Abruzzo, Italy, synonymization with B. mutila rejected) and B. mutila (recorded for the first time outside Algeria with certainty). For each species, information is given on habitat preference and geographical distribution; the significance of the data is discussed under perspectives of zoogeography and nature protection. The completeness of our knowledge for the three investigated island is assessed using rarefaction curves and non-parametric estimators of species richness; while Sicily can be considered fairly well known, Corsica and Sardinia require further sampling to assess their water mite diversity.
Introduction
Available knowledge on the diversity and distribution of certain “less charismatic” aquatic invertebrate groups is far from being satisfactory even in Europe; indeed, it has often been stressed that species distribution currently reflects taxonomists’ distribution (Ruffo & Stoch Citation2006). This scenario is further worsened by to the so-called taxonomy crisis (Boero Citation2001), and there is an undeniable risk that much biological diversity might in fact disappear even before being known and described. Furthermore, certain geographical areas or habitat typologies are often overlooked due to historical, cultural or economical reasons; this is the case for the biota of the smaller wetlands of the circum-Mediterranean countries which, in spite of its outstanding diversity and conservation value, has been nearly neglected till recent times (Alvarez Cobelas et al. Citation2005). This is unfortunate, as ponds and pools have been shown to contribute as much to the regional biodiversity of Mediterranean countries as rivers or lakes; they host a distinguishing fauna, and provide stepping-stones and increased connectivity between other freshwater habitats (Boix et al. Citation2012).
Notwithstanding their particular significance for understanding and investigating the ecology of inland water invertebrate assemblages (Smit & Van der Hammen Citation1992; Dohet et al. Citation2008), water mites are not included in the “European Water Framework Directive” (Directive 2000/60/EC of the European Parliament). Updated faunal works and taxonomical keys for Northern and Central European faunas are nowadays available (Gerecke Citation2007, Citation2010), while our knowledge of the circum-Mediterranean fauna is to date unsatisfactory. We are thus aiming in this paper to contribute to fill this gap on the circum-Mediterranean water mite fauna, focusing on the lentic inland water bodies of the three larger western Mediterranean islands (Corsica, Sardinia and Sicily).
Materials and methods
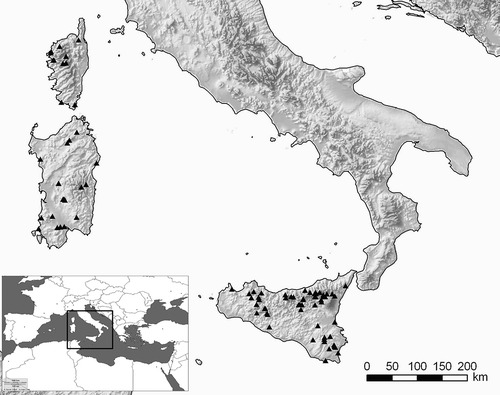
Most samplings were carried out from 2004 to 2013. In addition, we add also earlier, unpublished data from collecting activities during the last two decades of the 20th century. Each sampled site was unambiguously identified through its WGS84 geographical coordinates; some notes on the habitat typology and hydroperiod of each sampling site were also recorded ().
Figure 1. A map of the three largest western Mediterranean islands, showing the location of sites where new water mite material was collected.
Invertebrate samples were collected using 150-μm mesh-sized hand- and towing nets. The material from the 1980s and 1990s was sorted out in the field from the living material and stored in Koenike’s fluid; samples collected in the 21st century were fixed in situ in 80% ethanol and sorted in the laboratory under a stereomicroscope. All material was stored in separate tubes; when necessary, selected specimens were dissected and slide-mounted as described by Davids et al. (Citation2007). If no other bibliography is mentioned, species identification was based on K. Viets (Citation1936), Lundblad (Citation1956) and Gerecke (Citation2007, Citation2010).
A total of about 1000 water mite specimens were studied. Several locations were investigated only once; others were visited several times as indicated in .
Table I. Water mites of the three largest western Mediterranean islands; list of the new collecting sites. Codes within brackets refer to Italian provinces.
Species were classified as standing water dwellers (lenitobionts) based on the presence of swimming setae. This rather simple character works surprisingly well and, in many cases, all members of selected genera are lenitobionts, while other genera include species that avoid standing waters. However, the following restrictions were necessary: in Hydryphantes, H. handschini Walter, 1925 and H. armentarius Gerecke, Citation1996, both characterized by very low numbers of swimming setae, have a clear preference for helocrenic springs (Gerecke Citation1996). They were therefore excluded from this paper, but a few new records of the latter are included, which was collected in some (groundwater dependent?) pools of the Nebrodi mountains (Sicily). In the genus Arrenurus, accounting for the highest number of water mites species restricted to standing waters in Europe, several species have evolved special adaptations for other types of aquatic habitats. We excluded therefore three spring-dwelling species (crenobionts: A. fontinalis K. Viets, 1920, A. refractariolus Biesiadka, 1978, and A. scourfieldi Soar, 1913) and four interstitial species (A. corsicus E. Angelier, 1951, A. haplurus K. Viets, 1925, A. pygmaeus E. Angelier, Citation1954 and A. troglobius E. Angelier, 1951), which are all recorded from the study area (Smit et al. Citation2000).
Sample-based rarefaction curves and non-parametric estimators of species richness were calculated to measure and compare sampling efficiency across sites. Analyses were done using the EstimateS software version 9.1.0 (http://viceroy.eeb.uconn.edu/EstimateS), which adopts the simple analytical formulas of Colwell et al. (Citation2004) for accumulation curve and estimator calculation. Analyses were performed for each of the three islands.
The traditional methods of estimating overall species richness assume that all species are able, up to a certain limit, to colonize the whole area sampled; for this reason, analysis of similarities (ANOSIM: Clarke Citation1993) was used as a way to test statistically whether there was a significant difference between water mite assemblages in the sampling units of Corsica, Sardinia and Sicily. A presence/absence species × sites matrix was used and a Jaccard index was used to create a resemblance matrix. Subsequently, one-way ANOSIM was applied. The analyses were performed with the PRIMER v. 6.1 statistical package (Primer-E 2006) (Clarke & Gorley Citation2006).
Results
Species checklist
Ecological and biogeographical information for the 91 standing water-dwelling water mite species known from Corsica (41 species), Sardinia (39) and Sicily (70) is reported below. Out of the 47 species collected during this survey, 13 are recorded for the first time from the area covered. In total, 11 species are new to Italy, a further seven are new to Sicily, three are new to Sardinia, and seven are new to Corsica. Unpublished records are reported with a locality abbreviation (for details, see ) and differentiated as (males/females/deutonymphs). If not otherwise indicated, information on habitat preference and geographical distribution is based on Gerecke (Citation2007, Citation2010) and the Limnofauna Europaea update in http://www.watermite.org.
The species account follows the (super)family order in K.O.Viets (Citation1987); genera and species are treated in alphabetical order. An asterisk (*) is given to species for which previously unstudied material is treated; two asterisks (**) mark species previously unrecorded from the Tyrrhenian islands.
Superfamily Hydrachnoidea
Family Hydrachnidae
1. Hydrachna geographica Müller, 1776
New record: Italy: Sicily, I 1543 (1/0/0).
Habitat and biology: temporary and permanent standing waters. Larvae parasitic in the subelythral space of large dytiscid beetles.
Distribution: Holarctic, rarely found in the Mediterranean.
Note: first record from Italy.
*2. Hydrachna globosa (De Geer, 1778)
New records: Italy: Sicily, I 1430 (1/0/0); I 1431 (0/1/0); I 1433 (1/2/0); I 1436 (0/0/1).
Published records: Sardinia, Sicily (Gerecke Citation1991).
Habitat and biology: oligo- to eutrophic standing waters. Larvae parasitic on water bugs.
Distribution: Palaearctic.
**3. Hydrachna goldfeldi Thor, 1916
New record: Italy: Sardinia, I 1390 (1/0/0).
Habitat and biology: temporary and permanent standing waters. Larvae parasitic on water bugs of the families Nepidae and Notonectidae.
Distribution: Western Palaearctic; previously recorded only from scattered sites in Central, Western and Eastern Europe.
Note: first record from Italy.
**4. Hydrachna incisa Halbert, 1903
New records: Italy: Sardinia, I 1392 (0/1/0); Sicily, I 1546 (0/0/2).
Habitat and biology: standing waters. Life cycle unknown.
Distribution: Europe. Previously recorded from the British Isles, The Netherlands and Germany.
Note: first records from the Mediterranean area.
**5. Hydrachna leegei Koenike, 1895
Italy: Sicily, I 1410 (4/4/0); I 1420 (0/0/3); I 1421 (0/2/0); I 1422 (1/7/0); I 1423 (1/1/0); I 1437 (0/1/0); I 1441 (0/0/8); I 1442 (0/0/1); I 1444 (0/0/1); I 1445 (0/0/10); I 1455/3 (2/0/0).
Habitat and biology: temporary and permanent standing waters. Larvae parasitic on palpicorn water beetles (genera Helophorus and Laccobius).
Distribution: Palaearctic.
Note: first record from the Mediterranean area.
6. Hydrachna processifera Koenike, 1903
Published records: Sardinia, Sicily (Gerecke Citation1991), Sardinia (Davids et al. Citation2005).
Habitat and biology: temporary and permanent standing waters.
Distribution: Palaearctic.
*7. Hydrachna skorikowi (Piersig, 1900)
New records: Italy: Sicily, I 1407 (0/1/0); I 1542 (0/1/0).
Published records: Sicily (Gerecke Citation1991).
Habitat and biology: most kinds of standing waters, also man-made lakes; in Sicily frequently in remnant pools of summer-dry streams at high salinity. The fact that this species was found only once in this investigation could indicate that the species avoids temporary standing waters. Larvae parasitic on water bugs of the family Corixidae. In Central Europe, hibernation as parasitic larva.
Distribution: Palaearctic, Asia.
Hydrachna sp.
Records: Italy: Sardinia, I 1387 (0/0/1); Sicily, I 1413 (0/0/1).
Remark: deutonymphs could not be identified to species level.
Superfamily Eylaoidea
Family Eylaidae
*8. Eylais extendens (Müller, 1776)
New records: Italy: Sicily, I 1408 (0/1/0); I 1413 (0/0/1); I 1454 (0/0/1); I 1455/1 (1/1/0); I 1455/2 (0/1/0).
Published records: Sicily (Gerecke Citation1991).
Habitat and biology: all types of standing and slowly flowing waters, but sensitive to high electrolyte concentrations. Larvae parasitic on adult water beetles of the families Haliplidae, Dytiscidae, Laccophilidae and Hydrophilidae.
Distribution: Western Palaearctic.
*9. Eylais hamata Koenike, 1897
New records: Italy: Sicily: I 1393 (1/1/0); I 1410 (3/2/0); I 1413 (1/0/0); I 1418 (0/1/0); I 1419 (1/1/0); I 1421 (0/1/12); I 1422 (4/3/0); I 1423 (4/0/0); I 1424 (5/0/0); I 1426 (0/1/0); I 1440 (0/0/2); I 1441 (0/0/1); I 1442 (1/1/0); I 1446 (1/0/0); I 1455/1 (3/1/0); I 1455/3 (0/1/0); I 1455/4 (1/2/0); I 1469 (0/0/5).; I 1547 (0/2/0).
Published records: Sicily (Gerecke Citation1991).
Habitat and biology: all types of small standing waters. Larvae parasitic on beetles of the families Dytscidae and Helophoridae. A typical spring species, adults disappearing in early summer.
Distribution: Palaearctic.
**10. Eylais planipons Walter, 1924
New records: France: Corsica, F 151 (1/0/0); Italy: Sardinia, I 1461 (1/0/0); I 1463 (2/1/0); I 1549 (1/0/0); I 1550 (0/0/1); I 1551 (0/1/0); Sicily, I 1471 (1/0/0).
Habitat and biology: small ponds and ditches. Life cycle unknown.
Distribution: Mediterranean area, Iran.
Note: first record from Italy.
Eylais sp.
Records: Italy: Sardinia, I 1390 (0/0/3); I 1391 (0/0/2); Sicily, I 1403 (0/1/0).
Remark: deutonymphs and a female could not be identified to species level.
Family Limnocharidae
11. Limnochares aquatica (Linnaeus, 1758)
Published records: Corsica (Santucci Citation1965, Citation1971; Gerecke & Di Sabatino Citation2013), Sicily (Davids et al. Citation2005).
Habitat and biology: lakes and ponds, pools of shaded streams. Larvae parasitic on Heteroptera.
Distribution: Holarctic.
Family Piersigiidae
**12. Piersigia limophila Protz, 1896
New record: Italy: Sicily, I 1403 (0/1/0).
Habitat and biology: semiaquatic habitats at the border of marshes and swamps, in Sicily in a helocrenic spring area bordering a small lake. Larvae parasitic on beetles of the family Hydrophilidae (Enochrus).
Distribution: Holarctic. In Europe, previously recorded only from northern and eastern parts; recently discovered also in Central Europe, north of the Alps.
Note: first record from the Mediterranean area.
Superfamily Hydryphantoidea
Family Hydrodromidae
*13. Hydrodroma despiciens (Müller, 1776)
New records: Italy: SiciIy, 1417 (2/7/0); I 1425 (1/1/0); I 1427 (0/1/0); I 1428 (2/0/0); I 1438 (1/0/0).
Published records: Corsica (Angelier Citation1954; Santucci Citation1965, Citation1971–uncertain records); Corsica, Sardinia, Sicily (Gerecke Citation1991).
Habitat and biology: standing waters, with a preference for little alkaline waters. Life cycle unknown.
Distribution: previously considered cosmopolitan, but many older records are in need of revision. The separation between H. despiciens and H. pilosa has been ascertained only recently (Wiles Citation1985; Gerecke Citation1991) and H. reinhardi Pešić Citation2002 was described as an additional species from pool habitats in the Central Mediterranean area.
*14. Hydrodroma pilosa Besseling, 1940
New records: Italy: Sardinia, I 1462 (1/1/0); Sicily, I 1407 (0/1/0); I 1544 (0/1/0).
Published records: Sardinia, Sicily (Gerecke Citation1991).
Habitat and biology: standing waters and pools of streams, also in waters with elevated electrolyte concentration and high trophic levels (Gerecke Citation1991). Larvae parasitic on Diptera (Chaoboridae, Chironomidae, Tipulidae) and Trichoptera.
Distribution: Western Palaearctic.
15. Hydrodroma reinhardi Pešić, Citation2002
Published records: Corsica, Sardinia (Pešić Citation2002; Gerecke & Di Sabatino Citation2013).
Habitat and biology: pools of streams. Life cycle unknown.
Distribution: central Italy, Corsica and Sardinia, Montenegro.
Family Hydryphantidae
16. Diplodontus scapularis Dugès, 1834
Published records: Sicily (Gerecke Citation1991).
Habitat and biology: small standing water bodies and pools of streams, halotolerant. Larvae parasitic on palpicorn water beetles.
Distribution: Mediterranean area, coastal areas of Northern France, The Netherlands and Germany.
17. Diplodontus semiperforatus Walter, 1925
Published records: Sicily (Gerecke Citation1991).
Habitat and biology: small standing water bodies and pools of streams, also at very high electrolyte concentrations. Larvae parasitic on palpicorn water beetles.
Distribution: Mediterranean area, Sahara.
18. Georgella koenikei Maglio, 1906
Published record: Corsica (Angelier Citation1954).
Habitat and biology: small standing water bodies. Life cycle unknown.
Distribution: presence in the area questionable and in need of being confirmed. Ascertained records from the French Alps and Lombardia (Italy) only (Di Sabatino et al. Citation2009).
*19. Hydryphantes (s. str.) armentarius Gerecke, Citation1996
New records: Italy: Sicily, I 1403 (0/1/11); I 1435 (0/2/0); I 1449 (1/0/0); I 1451 (0/1/0).
Published records: Sicily (Gerecke Citation1996).
Habitat and biology: crenobiont. Often in weakly flowing farmland rheohelocrenes. Life cycle unknown.
Distribution: central Mediterranean area.
**20. Hydryphantes (s. str.) crassipalpis Koenike, 1914
New record: Italy: Sicily, I 1427 (0/1/1).
Habitat and biology: permanent and temporary standing waters. Life cycle unknown.
Distribution: Palaearctic. The detection of this species in Turkey (Özkan Citation1988) and the new record given here suggest that, in the SW Palaearctic, this species could be much more widely distributed than previously known.
Note: first record from the Mediterranean area.
*21. Hydryphantes (s. str.) dispar (Schaub, 1888)
New records: Italy: Sicily, I 1425 (0/1/0); I 1428 (1/0/0); I 1429 (0/0/1); I 1433 (0/0/1).
Published records: Sardinia, Sicily (Gerecke Citation1996).
Habitat and biology: permanent and temporary pools and ditches. Larvae parasitic on Diptera of the family Ephydridae.
Distribution: Palaearctic.
Note: only rarely found in the Mediterranean area.
22. Hydryphantes (Polyhydryphantes) flexuosus Koenike, 1885
Published record: Sicily (Gerecke Citation1996).
Habitat and biology: standing waters and pools of higher order streams, halotolerant. Life cycle undescribed.
Distribution: Palaearctic.
23. Hydryphantes (s. str.) parmulatus Koenike, 1912
Published record: Sicily (Gerecke Citation1996).
Habitat and biology: temporary pools. Life cycle undescribed.
Distribution: Central-Western Europe, Mediterranean area, rare.
24. Hydryphantes (s. str.) placationis Thon, 1899
Published records: Corsica (Angelier Citation1954), Sicily (Gerecke Citation1996).
Habitat and biology: temporary pools. Life cycle undescribed.
Distribution: Central-Western Europe, Mediterranean area, rare.
*25. Hydryphantes (s. str.) ruber (Geer, 1778)
New records: Italy: Sicily, I 1403 (0/0/1); I 1416 (0/1/0); I 1436 (1/2/2); I 1437 (0/3/9); I 1438 (0/2/3); I 1441 (8/6/0); I 1443 (2/4/0); I 1444 (4/5/1); I 1445 (6/18/0); I 1446 (1/4/1); I 1448 (1/4/5); I 1449 (0/6/6); I 1450 (0/2/4); I 1451 (1/1/0); I 1452 (0/0/1); I 1453 (3/34/15); I 1454 (0/0/1).
Published records: Sardinia (Costa Citation1884), Sicily (Gerecke Citation1996).
Habitat and biology: temporary and permanent standing waters, limnocrenes. Larvae parasitic on Diptera (family Culicidae – uncertain association, only 19th century bibliographic data).
Distribution: Holarctic (? – North American records are questionable). Widespread in Europe. The record from Sardinia stems from a time when the species was not well defined, but presence of the species is well possible.
Hydryphantes sp.
Record: Sicily, I 1439 (0/0/1).
Remark: deutonymph could not be identified to species level.
*26. Parathyas barbigera (K. Viets, 1908)
New record: Italy: Sicily, I 1416 (1/1/0).
Published record: Sicily (Gerecke Citation1996).
Habitat and biology: temporary vernal pools. Larvae parasitic on Diptera of the families Culicidae, Limoniidae and Tipulidae.
Distribution: Holarctic.
*27. Parathyas palustris (Koenike, 1912)
New record: Italy: Sicily, I 1403 (0/2/0).
Published records: Corsica (Gerecke Citation1996).
Habitat and biology: this species is on the habitat preference borderline of the species treated in this paper, with records both from helocrenes, but mostly in Northern Europe, also from forest swamps. The site from which the new record derives is a groundwater-influenced small lake. Larvae parasitic on Diptera of the families Chironomidae and Limoniidae.
Distribution: Holarctic.
Note: first record from Sicily.
28. Pseudohydryphantes parvulus (K. Viets, 1908)
Published records: Corsica (Gerecke & Di Sabatino Citation2013); Sardinia (Gerecke Citation1991).
Habitat and biology: standing waters and pools of streams. Life cycle unknown.
Distribution: Central and Southern Europe; extremely rare.
Superfamily Lebertioidea
Family Lebertiidae
29. Lebertia pilosa Maglio, 1924
Published record: Corsica (Gerecke Citation2009).
Habitat and biology: standing waters and pools of streams. Life cycle unknown.
Distribution: Europe, scattered records.
30. Lebertia porosa Thor, 1900
Published records: Corsica (Angelier Citation1954; Santucci Citation1965, Citation1971; Giudicelli Citation1970; Gerecke Citation2009; Gerecke & Di Sabatino Citation2013); Sicily (Ferrito Citation1994); Sardinia, Sicily (Gerecke Citation2009).
Habitat and biology: standing waters, pools and slow flowing sectors of streams. Larvae parasitic on chironomid Diptera.
Distribution: Western Palaearctic, widespread.
Family Oxidae
31. Oxus longisetus (Berlese, 1885)
Published record: Corsica (Angelier Citation1954).
Habitat and biology: standing waters. Life cycle unknown.
Distribution: Europe, widespread.
**32. Oxus ovalis (Müller, 1776)
New records: France: Corsica, F 23 (0/1/0); F 88 (1/1/0); Italy: Sardinia, I 373 (0/3/0); I 381 (1/1/0); Sicily, I 4 (0/1/0); I 100 (2/3/0); I 312 (0/1/0).
Habitat and biology: standing waters. Life cycle unknown.
Distribution: Western Palaearctic.
**33. Oxus setosus (Koenike, 1898)
New records: France: Corsica, F 42e (0/1/0); Italy: Sicily, I 36 (1/2/0); I 98 (4/1/0); I 100 (0/1/0); I 192 (0/1/0); I 193 (3/2/0); I 534 (1/1/0).
Habitat and biology: standing waters, pool areas of streams. Life cycle unknown.
Distribution: Palaearctic, widespread.
Family Teutoniidae
34. Limnolegeria longiseta Motaş, 1928
Published records: Sicily (Gerecke Citation1991).
Habitat and biology: cold lakes, pools of shaded streams. Life cycle unknown.
Distribution: Mediterranean, a few scattered areas.
*35. Teutonia cometes (Koch, 1837)
New record: Italy: Sardinia, I 1464 (0/0/1).
Published records: Corsica (Angelier Citation1954; Santucci Citation1965, Citation1971; Gerecke Citation1991; Gerecke & Di Sabatino Citation2013); Sardinia, Sicily (Gerecke Citation1991).
Habitat and biology: cold lakes, in the Mediterranean pools of shaded streams, limnocrenes.
Distribution: Palaearctic.
Family Limnesiidae
36. Limnesia acuminata Walter, 1925
Published records: Sardinia, Sicily (Gerecke Citation1991).
Habitat and biology: standing waters, pools of higher order streams. Life cycle unknown.
Distribution: west Mediterranean area.
37. Limnesia arevaloi arevaloi K. Viets, 1918
Published records: Sicily (Gerecke Citation1991).
Habitat and biology: small standing waters, also in fountains and temporary ponds, pools of low order streams. Life cycle unknown.
Distribution: west Mediterranean area.
38. Limnesia arevaloi ambulatoria Gerecke, Citation1991
Published records: Corsica, Sardinia (Gerecke Citation1991).
Habitat and biology: small standing waters, pools of low order streams. Life cycle unknown.
Distribution: Corsica, Sardinia, endemic.
39. Limnesia fulgida Koch, 1836
Published records: Corsica (Angelier Citation1954).
Habitat and biology: larger and smaller permanent standing waters.
Distribution: Palaearctic.
*40. Limnesia maculata (Müller, 1776)
New record: France: Corsica, F 153 (3/8/2). Italy: Sicily, I 1412 (4/1/1).
Published records: Sicily, Sardinia (Gerecke Citation1991).
Habitat and biology: permanent standing waters, pools of larger streams. Larvae parasitic on Diptera of the family Chironomidae.
Distribution: Palaearctic.
Note: first record from Corsica.
41. Limnesia manubriata Walter, Citation1928
Published records: Sicily (Gerecke Citation1991).
Habitat and biology: smaller standing waters, pools of streams.
Distribution: Africa south of Sahara, Mediterranean area.
42. Limnesia marmorata Neuman, 1870
Published records: Sardinia (‘L. maculata’ partim Gerecke Citation1991).
Habitat and biology: larger and smaller permanent standing waters.
Distribution: Europe. Detailed distribution unclear due to long-term taxonomic confusion with L. maculata. Based on the revision of Van Haaren and Tempelman (Citation2009), specimens published as L. maculata from Lago Baratz (Sardinia, I 351, Gerecke Citation1991) represent L. marmorata.
43. Limnesia walteri Migot, 1926
Published records: Corsica (Angelier Citation1954; Gerecke & Di Sabatino Citation2013), Sardinia, Sicily (Gerecke Citation1991).
Habitat and biology: smaller standing waters, pools of streams.
Distribution: Africa south of Sahara, Mediterranean area.
Superfamily Hygrobatoidea
Family Hygrobatidae
44. Hygrobates longipalpis (Hermann, 1804)
Published records: Corsica (Angelier Citation1954; Gerecke Citation1991; Gerecke & Di Sabatino Citation2013); Sardinia, Sicily (Gerecke Citation1991).
Habitat and biology: standing waters, pools of higher order streams. Larvae parasitic on Diptera (preferably Chironomidae, also Culicidae and Chaoboridae).
Distribution: Holarctic.
45. Hygrobates longiporus Thor, 1898
Published records: Corsica (Angelier Citation1954; Giudicelli Citation1970; Santucci Citation1971; Gerecke Citation1991; Gerecke & Di Sabatino Citation2013); Sardinia, Sicily (Gerecke Citation1991); Sicily (Ferrito Citation1994).
Habitat and biology: standing waters, pools of higher order streams. Without a parasitic larval stage.
Distribution: Palaearctic.
46. Hygrobates trigonicus Koenike, 1895
Published records: Corsica (Angelier Citation1954; Santucci Citation1971).
Habitat and biology: standing waters, pools of higher order streams. Larvae parasitic on chironomid diptera.
Distribution: Western Palaearctic.
Family Pionidae
*47. Piona alpicola Neuman, 1880
New records: Italy: Sicily, I 1496 (0/1/0); I 1500 (0/1/0); I 1501 (0/4/0); I 1502 (2/2/0); I 1504 (0/1/0).
Published record: Corsica (Smit & Gerecke Citation2010).
Habitat and biology: permanent standing waters. Larvae parasitic on Diptera of the family Chironomidae.
Distribution: Holarctic.
Note: first record from Sicily.
**48. Piona carnea (Koch, 1836)
New records: France: Corsica, F 156 (5/7/2).Italy: Sicily, I 1406 (0/6/0); I 1409 (2/6/3); I 1427 (0/1/0); I 1439 (1/0/0); I 1466 (1/0/0); I 1467 (1/2/0).
Habitat and biology: permanent standing waters, in Central Europe often in shaded habitats. Larvae parasitic on Diptera of the family Chironomidae.
Distribution: Holarctic.
Note: first records from Corsica and Sicily.
*49. Piona coccinea (Koch, 1836)
New records: Italy: Sicily, I 1414 (0/2/0).
Published records: Sicily (Gerecke Citation1991).
Habitat and biology: permanent standing waters. Larvae parasitic on Diptera of the family Chironomidae.
Distribution: Holarctic.
50. Piona conglobata (Koch, 1836)
Published record: Sicily (Moniez Citation1889).
Habitat and biology: permanent standing waters. Larvae parasitic on Diptera of the family Chironomidae.
Distribution: Holarctic.
**51. Piona damkoehleri K. Viets, 1930
New record: Italy: Sardinia, I 1389 (1/2/0).
Habitat and biology: standing waters. Life cycle unknown.
Distribution: Mediterranean area. Known from a few sites only.
Note: first record from Italy.
**52. Piona laminata (Thor, 1900)
New records: Italy: Sardinia, I 1548 (0/1/0); Sicily, I 1448 (0/1/0).
Habitat and biology: permanent standing waters. Life cycle unknown.
Distribution: Previously recorded from Northwestern, Central, Northern and Eastern Europe – often confused with P. ambigua (Piersig, 1894).
Note: first record from the Mediterranean.
Remark: a species with diagnostic characters restricted to the female, and therefore probably often confused with P. nodata (of which it was first described as a subspecies). Wainstein (Citation1976) ranked it at species level.
*53. Piona nodata (Müller, 1776)
New records: Italy: Sardinia, 1389 (0/2/0); I 1465 (0/2/0); Sicily, I 1403 (0/1/0); I 1545 (0/17/0).
Published records: Sicily (Gerecke Citation1991).
Habitat and biology: permanent standing waters. Larvae not parasitic.
Distribution: Holarctic.
Note: first record from Sardinia.
**54. Piona rotundoides (Thor, 1897)
New records: France: Corsica, F 154 (0/1/0); F 155 (0/1/0); F 156 (1/0/0).
Habitat and biology: permanent standing waters, preferably in larger water bodies. Life cycle unknown.
Distribution: widespread in Europe, but in the Mediterranean area previously only recorded from northern Italy (Lombardia, Piemonte, Ramazzotti Citation1947; Nocentini Citation1963).
Note: first record from Corsica.
55. Piona variabilis (Koch, 1836)
Published records: Corsica (Angelier Citation1954).
Habitat and biology: permanent standing waters. Life cycle unknown.
Distribution: Palaearctic.
Piona sp.
New records: Italy: Sicily, I 269 (0/0/11); I 1413 (0/0/1).
Remark: deutonymph not identifiable to species level.
*56. Pionopsis lutescens (Herrmann, 1804)
New records: Italy: Sardinia, I 1388 (0/2/0); Sicily, I 1437 (0/2/0); I 1438 (0/1/0); I 1468 (1/0/0); I 1544 (0/1/0).
Published records: Sicily (Gerecke Citation1991).
Habitat and biology: permanent standing waters. Larvae parasitic on chironomid Diptera.
Distribution: Palaearctic.
Note: first record from Sardinia.
Family Unionicolidae
57. Neumania elliptica Walter, 1925
Published records: Corsica (‘N. vietsi’, Angelier Citation1954); Corsica, Sardinia, Sicily (Pešić et al. Citation2007).
Habitat and biology: pools of streams. Life cycle unknown.
Distribution: west Mediterranean area.
**58. Neumania limosa (Koch, 1836)
New record: France: Corsica, F 156 (0/16/0).
Habitat and biology: permanent standing waters of various size. Life cycle unknown.
Distribution: widespread in Europe.
Note: first record from Corsica.
59. Neumania spinipes (Müller, 1776)
Published records: Sicily (Gerecke Citation1991).
Habitat and biology: permanent standing waters of various size. Life cycle unknown.
Distribution: widespread in Europe.
60. Neumania uncinata Walter, 1927
Published records: Corsica, Sardinia, Sicily (Pešić et al. Citation2007).
Habitat and biology: permanent standing waters of various size. Life cycle unknown.
Distribution: Southern and Central Europe.
61. Unionicola aculeata (Koenike, 1890)
Published record: Sardinia (Gerecke Citation1991).
Habitat and biology: permanent standing and slowly flowing waters. Larvae parasitic on chironomid midges; postlarval stages for transformation and oviposition associated with molluscs.
Distribution: widespread in Europe.
*62. Unionicola crassipes (Müller, 1776)
New records: Italy: Sicily, I 1411 (1/10/0); I 1415 (0/9/0).
Published record: Sardinia (Gerecke Citation1991).
Habitat and biology: permanent standing waters and lentic areas of streams. Deutonymphs and adults inhabiting sponges and therefore bound to equilibrated conditions in nutrient-rich, but well oxygenated waters. Larvae parasitic on Diptera of the family Chironomidae. Under Mediterranean climate conditions, this species is obviously very rare and was not found during a large-scale inventory of Sicilian streams 1985/86 (Gerecke Citation1991).
Distribution: Holarctic.
Note: first record from Sicily. The detection of two populations of Unionicola crassipes highlights the significance of the Nature Reserves of “Ficuzza” and “Zingaro” as refuges for threatened or rare, stenoecious species.
63. Unionicola gracilipalpis (K. Viets, 1908)
Published record: Sardinia (Gerecke Citation1991).
Habitat and biology: permanent standing and slowly flowing waters. Larvae parasitic on chironomid midges; postlarval stages for transformation and oviposition associated with spongillids.
Distribution: widespread in Europe, not reported from Iberian peninsula.
*64. Unionicola minor (Soar, 1900)
New record: France: Corsica, F 156 (13/27/2).
Published record: Corsica (Angelier Citation1954); Sardinia (Gerecke Citation1991).
Habitat and biology: permanent standing and slowly flowing waters. Larvae parasitic on chironomid midges; postlarval stages for transformation and oviposition associated with spongillids.
Distribution: widespread in Europe except for Scandinavia.
Family Aturidae
**65. Axonopsis complanata (Müller, 1776)
Axonopsis graeca Pešić & Smit 2012, nov. syn.
New record: Italy: Sardinia, I 373 (1/0/0).
Material compared: The Netherlands, Slikkendam, coll. Besseling 898 (1/0/0).
Habitat and biology: previously known from permanent standing waters only, in the Mediterranean area also in pools of streams.
Description: colour dark blue with a whitish-yellow transverse line. Idiosoma length/width 430/320, dorsal shield 430/280 µm, at anterior margin broadly fused to ventral shield. Dorsal furrow () with two free slit organs, in the anterior and central part respectively, and two elongated glandular paletelets in the posterior part; a third slit organ fused to posterior margin of anterior glandular platelet. Tips of Cx-I pointed, on level of frontal margin (). Length/height IV-L-4-6, 80/27, 90/27, 95/25; number of swimming setae: II-L-4, 1, II-L-5, 3; III-L-4, 2; III-L-5, 3; IV-L-4, 3, IV-L-5, 3. Gnathosoma stout, mouth opening terminal, length 80 µm. Palp: ; P-1 centrally narrowed, with one dorsal seta; P-2 ventral margin straight, dorsal margin strongly convex, P-3 ventral margin weakly concave, dorsal margin curved, P-4 ventral margin slightly inflated in proximal part, distally equally narrowed, ventral setae (one long, one short) in distal third. P-5 slender, with three claws, two strong, ventral, one short, dorsal; length/height P-1, 23/24; P-2, 52/45; P-3, 27/30; P-4, 80/28; P-5, 28/14 µm.
Figure 2. Axonopsis complanata, specimen from coll. Besseling (The Netherlands). a, palp; b, venter; c, dorsum. Scale bars: 100 µm.
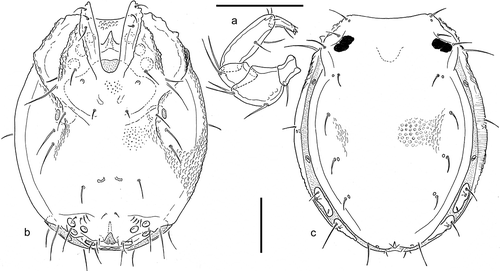
Remarks: since its early description from Denmark, Axonopsis complanata has been recorded from standing waters over a wide geographical range in Central and Northern Europe, including the perialpine lakes of northern Italy (Largaiolli Citation1907, Citation1910; Ramazzotti Citation1947; Nocentini Citation1960, Citation1979). However, as often in the earliest described water mites, little attention has been paid to a careful description of their diagnostic features. The specimen from Sardinia agrees from all points of view with the male from The Netherlands studied in comparison; the measurements agree with the few data available from bibliography. Furthermore, apart from differences in shape of the genital field, which are typical in the genus, the female described under the name Axonopsis graeca Pešić and Smit Citation2010 (in Pešić et al. Citation2010) from the island of Lesbos (Greece) is in perfect agreement with our descriptions given above. Obviously, A. graeca is a junior synonym of A. complanata.
Distribution: Europe; rare in the Mediterranean area.
Note: first record from Sardinia.
**66. Brachypoda baderi Di Sabatino & Cicolani Citation1990
Rejected synonymy: Brachypoda mutila (Pešić et al. Citation2006) – see discussion of B. mutila.
New records: France, Corsica, F 92 (1/4/0), Italy, Sardinia, I 378 (1/6/0); I 381 (0/2/0); I 383 (1/2/0), I 389 (0/5/0), I 849 (0/6/0); I 1155 (1/0/0), I 1162 (1/0/0); Sicily, I 1 (0/1/0); I 165 (1/1/0), I 166 (0/2/0), I 177 (0/1/0), I 308 (0/1/0), I 318 (0/8/0).
Description: colour yellow with a minor anterior, and a large posterior, dark violet mark. Excretory pore on dorsal shield, near posterior margin, directed posterodorsally. Tips of Cx-I distanced from frontal margin. Number of swimming setae: II-L-4, 1–2, II-L-5, 2–4; III-L-4, 2–3; III-L-5, 3–4; IV-L-5, 3. Palp relatively slender (L/H ratio P-2, 1.4-1.7; P-3, 1.20-1.4; P-4, 3,3–3, 9), P-1 curved, with one dorsal seta; P-2 ventral margin slightly concave, distally forming a blunt projection, dorsal margin convex, P-3 ventral margin concave, dorsal margin convex, P-4 proximally narrow, in the centre, near the insertion of two setae slightly elevated to form an obtuse projection near the insertion of the stronger ventral seta, a finer seta laterally on a further blunt elevation; distolaterally a fine peg seta, distoventrally a hair-like seta; dorsal margin equally convex, with several fine setae; P-5 slender with a long and strong ventral, and a fine dorsal claw. Male: Idiosoma posteriorly with obtuse lateral angles, length/width 400–450/320–360; IV-L-4–6: -; IV-L-4 with three stout setae (two of them slightly bifurcted) on a prominent ventral extension; IV-L-5 length/height 118–127/39–44 (ratio 2.9-3.1), bowed, with scattered ventral setae, two of them in distal part flattened, in lateral view appearing truncated; IV-L-6 length/height 104–112/21–23 (ratio 4.8–5.3), short and slightly curved, ventrally with a line of longer setae; claws modified as given in -; gonopore and flanking acetabula directed posteroventrally, distance tip Cx-I -gonopore 329–342 µm; excretory pore and flanking glandularia embedded in the ventral shield posterior to genital field. Gnathosoma length 75–81 µm; chelicera length 99–105 µm, length/height ratio 2.5–3.3, basal segment/claw ratio 1.8–1.9. Palp: ; length/height P-1, 29–31/16–18; P-2, 49–52/30–37; P-3, 30–32/23–24; P-4, 81–89/23–35; P-5, 29–30/9 µm. Female: Idiosoma length/width 491–563/392–432, distance tip Cx-I–gonopore 383–450 µm. Excretory pore platelet fused to posterior margin of dorsal shield. Length/height IV-L-5–6, 99–115/18–22, 81–95/17–18. Gnathosoma length 81–94 µm. Palp: ; length/height P-1, 31–35/18–20; P-2, 51–60/33–40; P-3, 32–36/24–29; P-4, 85–91/22–28; P-5, 30–31/9–10 µm.
Figure 3. Brachypoda baderi, appendages; a–d, male; a, palp; b–c, male IV-L-4-6 anterior, two different specimens; d, male IV-L-4-6 posterior; e, female palp. Scale bar: 100 µm.
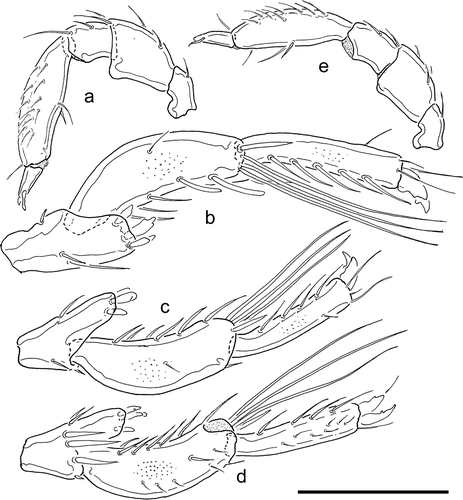
Remark: our measurements confirm that most specimens of Brachypoda baderi can be distinguished from B. mutila in both sexes by the more slender shape of the palp. Females differ furthermore in the excretory platelet fused to the dorsal shield (separate in B. mutila), males in the blunt posterolateral edges of idiosoma and a different shape and setation of IV-L-4–6.
The only further known species of the subgenus Hemibrachypoda, B. modesta Koenike, 1911 from Northern Germany, was described from a single female. A male from northern Bavaria attributed to that species by K.O.Viets (Citation1955), in shape of IV-L (all distal setae on IV-L-4 shortened and stout, IV-L-5 slender) and palp (P-4 slender), is very similar to B. baderi. The questions if B. baderi is present also north of the Alps, and which are the diagnostic features of B. modesta, require further study based on more populations from Central Europe.
Habitat and biology: in lentic areas of streams.
Distribution: previously known only from the type locality in Abruzzo (Italy), Central Mediterranean.
Note: first records from Corsica, Sardinia and Sicily.
**67. Brachypoda mutila Walter, Citation1928
New records: France: Corsica: F 48 (0/2/0); Italy: Sicily, I 37 (1/2/0); I 46 (0/2/0); I 98 (3/10/0), I 132 (2/6/0); I 133 (7/4/0); I 168 (4/0/0); I 177 (0/1/0); I 188 (1/0/0); I 193 (2/0/0); I 214 (0/1/0); I 250 (1/0/0); I 323 (0/1/0); I 544 (0/1/0); I 552 (2/5/0); I 563 (1/0/0); I 1067 (0/2/0); I 1076c (0/1/0).
Description: colour not reported, probably similar to B. baderi. Excretory pore with sexual difference. Tips of Cx-I and number of swimming setae as in B. baderi. Palp relatively stout (L/H ratio P-2, 1.2-1.4; P-3, 1.1-1.3; P-4, 3.0-3.5), in setation and shape of segments similar to B. baderi, but P-2 ventral margin nearly straight and ventral setae on P-4 more distally. Male: idiosoma length/width 450-495/360-369, distance tip Cx-I -gonopore 360–396 µm. IV-L-4–6: –; IV-L-4 three different setae (one fine and longer flanked by two stout setae, one thicker, the other thinner; IV-L-5 length/height 138–148/31–35 (ratio 4.0–4.8), only weakly curved and with subparallel dorsal and ventral margins, with numerous normal, not flattened, ventral setae; IV-L-6 length/height 129–135/21–23 (ratio 5.9–6.5), weakly curved, slender, ventrally with aline of longer setae; gnathosoma length 81–99 µm. Palp: –; length/height P-1, 33–35/20–21; P-2, 56–58/41–43; P-3, 30–35/26–29; P-4, 87–94/25–30; P-5, 28–29/20 µm. Female: idiosoma length/width 540–554/383–423, distance tip Cx-I -gonopore 419–441 µm. Length/height IV-L-5–6, 112–115/22–23, 84–92/16–20; gnathosoma length 90–98 µm. Palp: Length/height P-1, 34–35/20–21; P-2, 58–60/43–45; P-3, 32–36/28–29; P-4, 83–87/26–29; P-5, 28–30/10–12 µm.
Figure 4. Brachypoda mutila male, appendages. a–b, IV-L-4-6 anterior, two different specimens; c, male IV-L-4-6 posterior; d, palp medial; e, palp lateral. Scale bar: 100 µm.
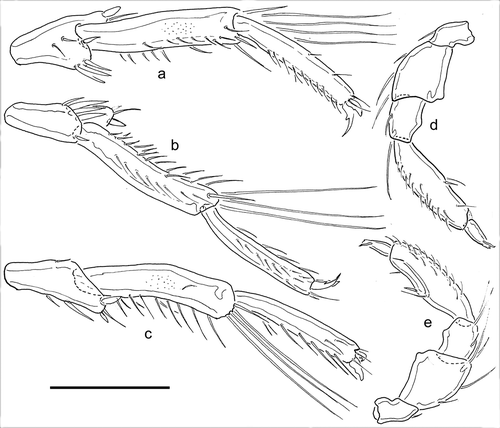
Remarks: the holo- and paratype from Algeria agree with the specimens from the Tyrrhenian islands in setation and proportions except for their slightly minor size (as is found also in a single female from Southern Spain, E 110). In particular, the holotype male shows the same differentiation of the apical setae on IV-L-4 and agrees in the slender shape and setation of IV-L-5. Males of populations under the name B. mutila from Crimea (Tuzovskij Citation1978, including also the reared larval stage) and Turkey (Pešić et al. Citation2006), recently assigned by Esen et al. (Citation2013) to B. orientalis Pešić & Esen, Citation2013, differ from typical B. mutila in: (1) apical setae on the extension of IV-L similar to each other in size and shape, all short and peg-like, and (2) IV-L-5 thickened and with more curved dorsal margin, more similar to B. baderi, ventral setae shorter, in proximal part peg-like. As the shape of male IV-L-4-6 and their setae is obviously less variable than assumed by Pešić et al. (Citation2006), there is no justification for their proposal to synonymize B. baderi with B. mutila.
Habitat and biology: in lentic areas of streams.
Distribution: west Mediterranean area.
Note: first records from Corsica and Sicily.
Superfamily Arrenuroidea
Family Mideopsidae
*68. Mideopsis roztoczensis Biesiadka & Kowalik, 1979
Published records: Corsica (“M. orbicularis” Angelier Citation1954; Santucci Citation1965, Citation1971); Sicily (“M. orbicularis” Ferrito Citation1994); Corsica, Sardinia, Sicily (Smit et al. Citation2000); Corsica (Gerecke & Di Sabatino Citation2013).
Habitat and biology: pools of streams. Life cycle unknown.
Distribution: Western, Central and Southern Europe. For a long time confused with M. orbicularis (Müller, 1776), a species preferably living in larger standing waters. All records under this name from the area covered refer to M. roztoczensis (Smit et al. Citation2000).
Family Momoniidae
69. Momonia falcipalpis Halbert, 1906
Published records: Sicily (Gerecke Citation1991).
Habitat and biology: lakes, ponds, pools of streams. Life cycle unknown.
Distribution: Palaearctic, rare scattered records.
Family Arrenuridae
70. Arrenurus (s.str.) abbreviator Berlese, 1888
Published records: Corsica (Angelier Citation1954); Sicily (Smit et al. Citation2000).
Habitat and biology: small lakes, ditches, ponds. Larvae parasitic on Odonata (Zygoptera).
Distribution: Western Palaearctic.
71. Arrenurus (s.str.) batillifer Koenike, 1896
Published records: Sicily (Smit et al. Citation2000).
Habitat and biology: lentic habitats of various types, sensitive to eutrophication. Larvae parasitic on Odonata (Zygoptera).
Distribution: Palaearctic.
**72. Arrenurus (s.str.) bicuspidator Berlese, 1885
New record: Italy: Sicily, I 1428 (2/2/0).
Habitat and biology: lakes, ponds. Larvae parasitic on Odonata (Zygoptera).
Distribution: Palaearctic.
Note: first record from Sicily.
73. Arrenurus (Micruracarus) bipapillosus Halbert, 1911
Published records: Corsica (Gerecke & Di Sabatino Citation2013); Corsica, Sicily (Smit et al. Citation2000).
Habitat and biology: lakes, ponds, remnant pools of temporary streams. Life cycle unknown.
Distribution: Western Palaearctic.
*74. Arrenurus (s.str.) bruzelii Koenike, 1885
New records: Italy: Sicily, I 1411 (2/2/0); I 1428 (10/4/0); I 1470 (0/1/0).
Published records: Sicily (Smit et al. Citation2000).
Habitat and biology: lakes, ponds; disappears at elevated eutrophication. Larvae parasitic on Odonata (Zygoptera).
Distribution: Palaearctic.
75. Arrenurus (s.str.) claviger Koenike, 1885
Published record: Corsica (Smit et al. Citation2000).
Habitat and biology: lakes, ponds, sensitive to eutrophication. Larvae parasitic on Odonata (Zygoptera).
Distribution: Western Palaearctic.
**76. Arrenurus (s.str.) compactus Piersig, 1894
New records: Italy: Sicily, I 1425 (0/25/0); I 1426 (0/8/0); I 1427 (23/30/0); I 1428 (1/4/0); I 1429 (0/3/0); I 1433 (1/0/0); I 1436 (0/3/0); I 1437 (0/3/0); I 1438 (0/9/0); I 1439 (4/0/0); I 1441 (1/4/0); I 1443 (0/3/0); I 1445 (0/2/0); I 1447 (0/2/1).
Habitat and biology: small, oligotrophic, often acidic standing waters. Larvae parasitic on Odonata (Zygoptera).
Distribution: Palaearctic.
Note: first records from Sicily.
*77. Arrenurus (s.str.) cuspidator (Müller, 1776)
New records: Italy: Sicily, I 1411 (9/10/0); I 1425 (0/3/0); I 1427 (3/3/0); I 1428 (1/0/0); I 1439 (15/13/0); I 1440 (2/0/0); I 1441 (1/1/0); I 1442 (0/1/0); I 1444 (0/4/0); I 1445 (1/2/0); I 1451 (0/3/0).
Published records: Sicily (Smit et al. Citation2000).
Habitat and biology: ponds, remnant pools of temporary streams. Larvae parasitic on Odonata (Zygoptera).
Distribution: Western Palaearctic.
*78. Arrenurus (s.str.) cuspidifer Piersig, 1894
New records: Italy: Sicily, I 1497 (0/1/0); I 1498 (1/2/0); I 1499 (0/1/0).
Published records: Corsica (Angelier Citation1954); Sardinia, Sicily (Smit et al. Citation2000).
Habitat and biology: ponds, remnant pools of temporary streams, tolerant to eutrophication, also in slightly brackish water. Larvae parasitic on Odonata (Zygoptera).
Distribution: Palaearctic.
79. Arrenurus (Megaluracarus) cylindratus Piersig, 1896
Published records: Corsica, Sardinia, Sicily (Smit et al. Citation2000).
Habitat and biology: ponds with groundwater influence, pools of springs and low order streams. Life cycle unknown.
Distribution: Europe.
80. Arrenurus (Micruracarus) detruncatus (C. & E: Angelier, 1953)
Published record: Corsica (Angelier Citation1954).
Habitat and biology: recorded from pools in a stream and an oxbow pond. Life cycle unknown.
Distribution: French Pyrenees, Corsica.
81. Arrenurus (s.str.) distans Walter, 1927
Published records: Sicily (Smit et al. Citation2000).
Habitat and biology: lakes, temporary and permanent ponds. Life cycle unknown.
Distribution: west Mediterranean area, Iran, rare.
82. Arrenurus (s.str.) furcillatus K. Viets, 1930
Published records: Sicily (Smit et al. Citation2000).
Habitat and biology: ponds and ditches. Larvae parasitic on Odonata (Zygoptera).
Distribution: Western Palaearctic. Scattered records from the Mediterranean area, Central Europe, Caucasus and Asia Minor, rare.
*83. Arrenurus (Megaluracarus) globator (Müller, 1776)
New records: Italy: Sicily, I 1439 (0/1/0); I 1441 (2/0/0); I 1471 (0/1/0).
Published records: Sicily (Smit et al. Citation2000).
Habitat and biology: ponds and lakes. Larvae with a wide host spectrum, including adult Diptera of the families Chironomidae, Culicidae and Dixidae and larval Coleoptera of the families Dytiscidae and Gyrinidae.
Distribution: Palaearctic.
84. Arrenurus (s.str.) latus Barrois & Moniez, 1887
Published records: Sicily (Smit et al. Citation2000).
Habitat and biology: ponds, remnant pools of temporary streams, tolerant to eutrophication and elevated electrolyte contents. Larvae parasitic on Diptera (Chaoboridae, Chironomidae, Culicidae).
Distribution: Western Palaearctic.
85. Arrenurus (Micruracarus) novus George, 1884
Published records: Sicily (Smit et al. Citation2000).
Habitat and biology: ponds, remnant pools of temporary streams, tolerant to eutrophication and terrestrialization, but not supporting total dessication. Life cycle unknown.
Distribution: Western Palaearctic to Afrotropical.
*86. Arrenurus (s.str.) papillator (Müller, 1776)
New record: Italy: Sardinia, I 1390 (1/0/0).
Published records: Sicily (Smit et al. Citation2000).
Habitat and biology: character species of temporary ponds, also in brackish habitats, deutonymphs and adults capable to survive drought in humid sediments. Larvae parasitic on Odonata (Zygoptera and Anisoptera).
Distribution: Palaearctic.
Note: first record from Sardinia.
*87. Arrenurus (s.str.) radiatus Piersig, 1894
New record: Italy: Sicily, I 1403 (0/1/0).
Published records: Corsica (Angelier Citation1954); Sicily (Smit et al. Citation2000).
Habitat and biology: ponds, pools in slowly running waters. Larvae parasitic on Odonata (Zygoptera).
Distribution: Palaearctic.
*88. Arrenurus (s.str.) robustus Koenike, 1894
New record: Italy: Sicily, I 1428 (1/0/0).
Published records: Corsica (Angelier Citation1954); Sicily (Smit et al. Citation2000).
Habitat and biology: ponds, pools in slowly running waters. Larvae parasitic on Odonata (Zygoptera).
Distribution: Western Palaearctic.
*89. Arrenurus (s.str.) rodrigensis Lundblad, 1954
New records: Italy: Sicily, I 1426 (0/1/0); I 1427 (1/2/0); I 1428 (5/6/0); I 1429 (0/1/0); I 1435 (0/4/0); I 1440 (0/0/1); I 1443 (0/2/0); I 1445 (0/2/0).
Published records: Sardinia, Sicily (Smit et al. Citation2000).
Habitat and biology: ponds, small lakes, pools of running waters. Larvae parasitic on Odonata (Zygoptera).
Distribution: Mediterranean area.
90. Arrenurus (Truncaturus) stecki Koenike, 1894
Published records: Sicily (Smit et al. Citation2000).
Habitat and biology: ponds, ditches, semiaquatic habitats (quagfens). Life cycle unknown.
Distribution: Western Palaearctic.
*91. Arrenurus (s.str.) virens Neuman, 1880
New record: Italy: Sicily, I 1440 (2/0/0).
Published records: Sicily (Smit et al. Citation2000).
Habitat and biology: ponds, ditches, lentic areas of high order streams. Larvae parasitic on Odonata (Zygoptera).
Distribution: Western Palaearctic.
Arrenurus sp.
New Records: Italy: Sicily, I 1427 (0/0/66); I 1428 (0/0/3); I 1434 (0/8/0); I 1439 (0/0/11).
Females and deutonymphs not identifiable to species level.
Statistical analysis
In spite of the high number of sampling sites, the rarefaction curves did not reach the asymptote in Corsica and Sardinia, while the sampling efficiency was higher and accurate in Sicily (). The estimated number of species, calculated using the classical Chao2 equation and the non-parametric incidence based estimator (ICE), gave similar results, demonstrating that sampling effort was insufficient in Corsica and Sardinia, where approximately 50% or less of total estimated species richness was collected; the steepness of the curves, including uniques (i.e. species present in a single site) indicates that the number of species is probably underestimated even applying Chao2 and ICE correction, and cannot be assessed accurately using the data in hand. On the contrary, the knowledge of Sicilian water mites seems quite accurate, and the curves tend to converge towards an asymptote, which is around 70 species.
Figure 5. Species rarefaction curves for water mites in the three islands (S) and estimated species richness using Chao2 and incidence based estimator (ICE) non-parametric estimators for increasing sampling effort; uniques (i.e. number of species present in a single site) and duplicates (i.e. species present in two sites) are reported as well.
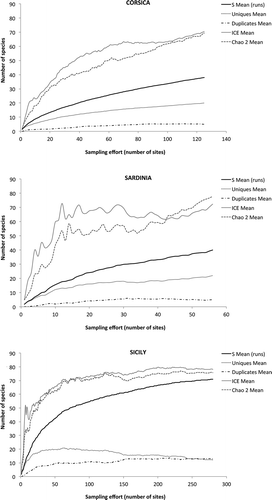
The investigated water mite assemblages from the three islands (Corsica, Sardinia and Sicily) investigated in ponds, pools and lakes during the present research (47 species) were not statistically different (ANOSIM, 9999 permutations, Global R = 0.055, p = 0.995). Pairwise differences between islands were never significant; Corsica and Sardinia (r = 0.236, p = 0.064) slightly differed from each other, while both were subsets of the well-sampled Sicily (Corsica – Sicily, r = 0.095, p = 0.223, Sardinia – Sicily, r = – 0.095, p = 0.933). Average similarities between sampled sites within Corsica (6.49), Sardinia (11.32) and Sicily (6.34) were extremely low, indicating a high heterogeneity between sites. The low within-islands similarities in water mite assemblages in standing waters, as well as the absence of statistically significant between-islands differences, may be due to a random distribution of the species pools among sites independently of their geographical location. The inadequacy of the sampling effort performed in Corsica and Sardinia, analyzed above, may influence the accuracy of these results.
Discussion
Due to their complex life cycle with a larval stage selectively bound to specific insect host taxa, water mites are particularly sensitive to environmental change and therefore important indicators in nature conservation (e.g. Martin & Gerecke Citation2009). During the past few decades, intense investigation has improved our knowledge of the water mite fauna of the Mediterranean but, with the exception of a study by Valdecasas and Camacho (Citation1986) in the Sierra de Guadarrama (Spain), interest has been focussed mostly on running waters and spring habitats. In part, this bias was induced by the observation that water mites restricted to these habitats tend to have smaller distribution areas (e.g. Smit et al. Citation2000; for aquatic beetles see Ribera Citation2000) and were therefore considered more interesting from a zoogeographical point of view. This tendency is confirmed also by the results of our statistical analysis. The results may be influenced by a sampling bias of the different habitat types, and must be taken with caution, but they suggest that standing water species colonize wider distribution areas, which often include extended parts of the West Palaearctic, occasionally the whole Palaearctic or even the Holarctic. As noted by Boix et al. (Citation2012), due to high rates of dispersal via phoresis and intensive habitat connectivity, the western Mediterranean area offers a large potential for species distribution.
However, in a preliminary study for a red list of the European water mites, Gerecke and Lehmann (Citation2005) reported that the highest numbers of taxa with scattered distributions, or species which were not recorded for more than 50 years, were found among inhabitants of small standing waters and temporary ponds. Habitats of this type, typical elements in the natural and traditional agricultural landscapes around the Mediterranean, were in sharp decline during the last few decades, and their rate of extinction was dramatic due to reclamation, groundwater management, transformation of natural pools into water reservoirs, and changes in precipitation frequency and intensity (Zacharias & Zamparas Citation2010; Céréghino et al. Citation2014). Consequently, the water mite fauna described herein, including the numerous species documented for the first time from the Mediterranean islands, must be considered at high risk of extinction at a population level: it is not the species as a whole which is endangered, but its genetic diversity, which is expressed by the presence of isolated local populations on the investigated islands.
Our data derive from a variety of projects with different aims and which were conducted with different methodologies; the fauna of Sicily is documented in much detail (a transverse line of low collecting site density crossing the centre of the island in a SW–NE direction is due to elevated electrolyte concentration in inland waters caused by evaporite outcrops), while records from Corsica and Sardinia are rather scattered and the faunal scenario is quite incomplete. For this reason, it is not surprising that attempts at a statistical analysis of distribution patterns or zoogeographical gradients were unsuccessful.
A series of interesting results emerges, concerning topics which deserve to be highlighted in future research:
With five species recorded for the first time from the Mediterranean area, and, in addition, six first records for Italy, seven for Sicily, three for Sardinia and seven for Corsica, our new results are an important contribution to a more complete knowledge of the biodiversity in the area. For several species, the southern distribution limit is considerably extended (e.g. members of the family Piersigiidae appeared so far restricted to Northwestern, Northern and Central Europe with a southern limit in the German pre-Alps, Gerecke et al. Citation2011). Our data suggest that intense investigations in upper montane areas on the islands will produce records of additional species previously believed to be restricted to Central and Northern Europe. At our present state of knowledge, the standing water mite fauna under the cold-temperate climate in Southern Europe is not characterized by own endemic elements, but represents a “diluted northern fauna”. However, such isolated outposts at the border of distribution areas are of particular biogeographical interest. Standing water habitats in mountain forests and subalpine meadows are refugial habitats for the remnants of a highly threatened relict fauna that has probably just disappeared from several parts of the Mediterranean.
In the western Mediterranean, pool areas of permanent streams and residual pools of temporary running waters are often the only refugial areas suitable for the survival of limnic water mite species. The fact that many species, known from lakes and ponds in Central and Northern Europe, in the south are recorded exclusively from streams is most probably not the consequence of a local stenotopy, but simply the result of a large-scale destruction of suitable standing water habitats. In this context, ‘suitable’ means that the water should be unpolluted, available year-round and with a stable or only slightly changing water level or, if temporary, with diversified riparian sediment structures allowing the survival of mites and/or their hosts under wet conditions (Wiggins et al. Citation1980; Gerecke Citation1991). Where such kinds of habitat disappeared from the landscape, the importance of streams increased under the aspects of nature conservation: here, a responsible water management becomes crucial for the survival of pond water fauna in oasis-like refugial habitats in summer dry streams. For instance, the fact that standing water-dwelling species of the genera Brachypoda, Hygrobates and Lebertia on the western Mediterranean islands have so far been found exclusively in streams is probably best explained by the lack of other suitable habitats at middle and low elevations.
The data assembled herein, with a total of 91 species, collected during a variety of investigations with different aims, where water mite collection was often a marginal aspect, highlight the important contribution of this taxonomic group to the diversity of lakes, ponds, ditches and pools in the Mediterranean area. In the study area, from a proper research project covering the maximum possible diversity of habitats, and following a well-defined field work protocol, we would expect a further significant increase of our knowledge on water mite diversity, especially in the more incompletely known Corsican and Sardinian areas.
Acknowledgements
Francesco Mascia is gratefully acknowledged for the help provided in the frame of the samplings realised in Sardinia. The investigation of the aturid mites was made possible by a grant from Deutsche Forschungsgemeinschaft to RG; Nerina Ferrito (Catania), Astrid Schwarz (Darmstadt) and Martina Pusch (Schöneiche) contributed mites collected during their field work. Harry Smit (Alkmaar) made available material for comparison from Naturalis, Leiden, The Netherlands. The sampling activities carried out in Sicily by FM were partially supported by the “Fondi di Ateneo” (60%).
References
- Alvarez Cobelas M, Rojo C, Angeler DG. 2005. Mediterranean limnology: Current status, gaps and the future. Journal of Limnology 64:13–29. doi:10.4081/jlimnol.2005.13.
- Angelier E. 1954. Contribution à l’étude de la faune d’eau douce de Corse. Acariens (Hydrachnellae et Porohalacaridae) des eaux superficielles. Vie et Milieu 5:74–148.
- Boero F. 2001. Light after dark: The partnership for enhancing expertise in taxonomy. TRENDS in Ecology & Evolution 16:266. doi:10.1016/S0169-5347(01)02133-4.
- Boix D, Biggs J, Céréghino R, Hull AP, Kalettka T, Oertli B. 2012. Pond research and management in Europe: “Small is Beautiful”. Hydrobiologia 689:1–9. doi:10.1007/s10750-012-1015-2.
- Céréghino R, Boix D, Cauchie HM, Martens K, Oertli B. 2014. The ecological role of ponds in a changing world. Hydrobiologia 723:1–6. doi:10.1007/s10750-013-1719-y.
- Clarke KR. 1993. Non-parametric multivariate analyses of changes in community structure. Austral Ecology 18:117–143. doi:10.1111/j.1442-9993.1993.tb00438.x.
- Clarke KR, Gorley RN. 2006. PRIMER v6.1: User Manual/Tutorial. Plymouth, UK:PRIMER-E.
- Colwell RK, Mao CX, Chang J. 2004. Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology 85:2717–2727. doi:10.1890/03-0557.
- Costa A. 1884. Notizie ed osservazioni sulla geo-fauna sarda, memoria IV. Atti della Accademia di Scienze fisiche, matematiche di Napoli s. 2. 13:1–31.
- Davids C, Di Sabatino A, Gerecke R, Gledhill T, Smit H. 2005. On the taxonomy of water mites (Acari: Hydrachnidia) described from the Palaearctic, part 1: Hydrachnidae, Limnocharidae and Eylaidae. Zootaxa 1061:36–64.
- Davids C, Di Sabatino A, Gerecke R, Gledhill T, Smit H. 2007. Acari, Hydrachnidia I. In: Gerecke R, editor. Süßwasserfauna von Mitteleuropa. Vol. 7. 2–1. Heidelberg: Spektrum-Elsevier-Verlag. pp. 241–388.
- Di Sabatino A, Cicolani B. 1990. Brachypoda (Hemibrachypoda) baderi, (Acari, Hydrachnellae, Aturidae): A new species from running waters of Central Italy. Acarologia 30:373–379.
- Di Sabatino A, Gerecke R, Gledhill T, Smit H. 2009. On the taxonomy of water mites (Acari: Hydrachnidia) described from the Palaearctic, part 2: Hydryphantoidea and Lebertioidea. Zootaxa 2266:1–34.
- Ector L, Dohet A, Hoffmann L, Cauchie H. 2008. Identification of benthic invertebrate and diatom indicator taxa that distinguish different stream types as well as degraded from reference conditions in Luxembourg. Animal Biology 58:419–472. doi:10.1163/157075608X383719.
- Esen Y, Pešić V, Erman O. 2013. Water mites of the genus Brachypoda (Acari: Hydrachnidia: Aturidae) in Turkey. Zootaxa 3686:326–334. doi:10.11646/zootaxa.3686.3.2.
- Ferrito V. 1994. Les macroinvertébrés benthiques de la rivière Simeto (Sicile) et de quelques-uns de ses affluents. Annales de Limnologie - International Journal of Limnology 30:33–56. doi:10.1051/limn/1994003.
- Gerecke R. 1991. Taxonomische, faunistische und ökologische Untersuchungen an Wassermilben aus Sizilien, unter Berücksichtigung anderer aquatischer Invertebraten. Lauterbornia 7:1–303.
- Gerecke R. 1996. Untersuchungen über die Wassermilben der Familie Hydryphantidae (Acari, Actinedida) in der Westpalaearktis II. Die Wassermilben der Familie Hydryphantidae in den Mittelmeerländern - Systematik, Faunistik, Zoogeographie. Archiv für Hydrobiologie Suppl 77:337–513.
- Gerecke R (editor). 2007. Chelicerata: Araneae, Acari I. Süßwasserfauna von Mitteleuropa 7/2-1. Heidelberg: Spektrum-Elsevier-Verlag. pp. 1–388.
- Gerecke R. 2009. Revisional studies on the European species of the water mite genus Lebertia Neuman, 1880 (Acari: Hydrachnidia: Lebertiidae). Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft 566:1–144.
- Gerecke R (editor). 2010. Chelicerata: Acari II. Süßwasserfauna von Mitteleuropa 7/2-2. Heidelberg: Spektrum-Verlag. pp. 1–236.
- Gerecke R, Di Sabatino A. 2013. The water mites (Hydrachnidia and Halacaridae) of the collection Daniele Benfatti at the Museo Civico di Storia Naturale Verona. Bollettino del Museo Civico di Storia Naturale di Verona 37:67–112.
- Gerecke R, Heckes U, Hess M, Mauch E. 2011. Limnologische Untersuchungen von Fließgewässern und Quellen am Hohen Trauchberg, Ostallgäu/Bayerische Alpen. Lauterbornia 73:23–148.
- Gerecke R, Lehmann EO. 2005. Towards a long term monitoring of Central European water mite faunas (Acari: Hydrachnidia and Halacaridae) - considerations on the background of data from 1900 to 2000. Limnologica - Ecology and Management of Inland Waters 35:45–51. doi:10.1016/j.limno.2004.09.001.
- Giudicelli J. 1970. Les biocénoses zonales d’un réseau hydrographique. Annales de la Faculté des Sciences, Marseille 43B:107–125.
- Largaiolli V. 1907. Idracne del Trentino. 6° Contributo allo studio delle idracne italiane. Rivista mensile di Pesca 9:173–180.
- Largaiolli V. 1910. Ricerche biolimnologiche sui laghi trentini. 6. Il lago di Loppio. Rivista mensile di pesca e idrobiologia 5:193–200.
- Lundblad O. 1956. Zur Kenntnis süd- und mitteleuropäischer Hydrachnellen. Arkiv för zoologi 10:1–306.
- Martin P, Gerecke R. 2009. Diptera as hosts of water mite larvae - an interesting relationship with many open questions. Lauterbornia 68:95–103.
- Moniez RL. 1889. Note sur la faune des eaux douces de la Sicilie. La Feuille des jeunes naturalistes 20:17–19.
- Nocentini AM. 1960. Hydrachnellae del Lago di Mergozzo. Memorie dell’Istituto Italiano di Idrobiologia 12:245–287.
- Nocentini AM. 1963. Strutture differenziali della fauna macrobentonica litorale del Lago Maggiore. Memorie dell’Istituto Italiano di Idrobiologia 16:189–274.
- Nocentini AM. 1979. Variazioni temporali e spaziali della fauna macrobentonica litorale del Lago di Mergozzo. Memorie dell’Istituto Italiano di Idrobiologia 37:277–327.
- Özkan M. 1988. Hydryphantes (s. str.) crassipalpis KOENIKE, 1914 (Hydryphantidae, Hydrachnellae, Acari) üzerine bir arastirma. Doga - TU Zooloji D.C 12:86–110.
- Pesic V. 2002. Hydrodroma reinhardi sp.n., a new species of water mites (Acari, Actinedida, Hydrodromidae) from the Mediterranean area. Aquatic Insects 24: 317–323. doi:10.1076/aqin.24.4.317.8239.
- Pešić V, Erman O, Esen Y. 2006. New records of water mites (Acari: Hydrachnidia) from Turkey. Acta entomologica Serbica 11:95–99.
- Pešić V, Gerecke R, Cîmpean M. 2007. Water mites of the genus Neumania Lebert (Acari, Hydrachnidia: Unionicolidae: Pionatacinae) in the Mediterranean area. Annales de Limnologie - International Journal of Limnology 43:187–198. doi:10.1051/limn:2007013.
- Pešić V, Smit H, Gerecke R, Di Sabatino A. 2010. The water mites (Acari: Hydrachnidia) of the Balkan peninsula, a revised survey with new records and descriptions of five new taxa. Zootaxa 2586:1–100.
- Ramazzotti G. 1947. Gli idracnidi del bacino delle Isole Borromee (Lago Maggiore). Memorie dell’Istituto Italiano di Idrobiologia 3:323–298.
- Ribera I. 2000. Biogeography and conservation of Iberian water beetles. Biological Conservation 92:131–150. doi:10.1016/S0006-3207(99)00048-8.
- Ruffo S, Stoch F. editors. 2006. Checklist and distribution of the Italian fauna. 10,000 terrestrial and inland waters species. Memorie del Museo Civico di Storia Naturale di Verona, 2.serie, Sezione Scienze della Vita 17:1–307.
- Santucci J. 1965. Hydracariens (Hydrachnellae) des eaux superficielles du Porto (Corse). Rapporti et Procés-verbaux de réunions de la Commission Internationale sur le Mer Méditerraneen 18:545–548.
- Santucci J. 1971. Contribution à l’étude de la répartition des Hydracariens (Hydrachnellae) des eaux superficielles d’un torrent de Corse ˗ Le Porto. Annales de la Faculté des Sciences, Marseille 45:81–99.
- Smit H, Gerecke R. 2010. A checklist of the water mites of France (Acari: Hydrachnidia). Acarologia 50:21–91. doi:10.1051/acarologia/20101952.
- Smit H, Gerecke R, Di Sabatino A. 2000. A catalogue of water mites of the superfamily Arrenuroidea (Acari: Actinedida) from the Mediterranean. Archiv für Hydrobiologie 121:201–267.
- Smit H, Van der Hammen H. 1992. Water mites as indicators of natural aquatic ecosystems of the coastal dunes of the Netherlands and northwestern France. Hydrobiologia 231:51–64. doi:10.1007/BF00008530.
- Tuzovskij PV. 1978. K diagnozu Brachypoda (Hemibrachypoda) mutila (WALTER, 1928) (Axonopsidae, Acariformes). (On the diagnosis of Brachypoda (Hemibrachypoda) mutila (WALTER, 1928) [Axonopsidae, Acariformes]). Naucnye Doklady Vyssei Skoly; Biologicheskie Nauki, Zool 4:47–52.
- Valdecasas A, Camacho AI. 1986. Las Hidracnelas leniticas de la Sierra de Guadarrama (Acari, Parasitengona, Hydrachnellae). Graellsia 42:149–160.
- Van Haaren T, Tempelman D. 2009. The Dutch species of Limnesia, with ecological and biological notes (Acari: Hydrachnidia: Limnesiidae). Nederlandse Faunistische Mededelingen 30:53–74.
- Viets K 1936. Wassermilben oder Hydracarina (Hydrachnellae und Halacaridae). In: Dahl F.: Tierwelt Deutschlands, Jena (G. Fischer) 31:1–288., 32:289–574.
- Viets KO. 1955. Wassermilben aus Nordbayern (Hydrachnellae und Porohalacaridae, Acari). Abhandlungen der Bayerischen Akademie der Wissenschaften, Mathematisch-Naturwissenschaftliche Klasse, Neue Folge 73:1–106.
- Viets KO. 1987. Die Milben des Süßwassers (Hydrachnellae und Halacaridae [part.], Acari). II.: Katalog. Sonderbände des Naturwissenschaftlichen Vereins in Hamburg 8:1–1012.
- Wainstein BA. 1976. Licinki i sistema vodjanych klescej podsemejstva Pioninae (Hygrobatidae, Acariformes). (The larvae and system of the water mites of the subfamily Pioninae [Hygrobatidae, Acariformes]). Institut Biologiya Vnutrennikh Vod; Trudy 34:29–69.
- Walter C. 1928. Hydracariens de l’Algérie et de la Tunisie. (Collections de M. H. Gauthier) (Deuxième liste). Bulletin de la Société d’histoire naturelle d’Afrique du Nord, Alger 19:280–336.
- Wiggins GB, Mackay RJ, Smith IM. 1980. Evolutionary and ecological strategies of animals in annual temporary pool. Archiv für Hydrobiologie Supplement 58:97–206.
- Wiles PR. 1985. The systematics of the British Hydrodromidae VIETS, 1936. Archiv für Hydrobiologie Suppl 70:365–403.
- Zacharias I, Zamparas M. 2010. Mediterranean temporary ponds. A disappearing ecosystem. Biodiversity Conservation 19:3827–3834. doi:10.1007/s10531-010-9933-7.
