Abstract
In this study, measurements of morphological parameters, sizes and frequencies of peripheral blood cells (erythrocytes, leukocytes, thrombocytes) on blood smear preparation devices stained with May-Grünwald stain were evaluated for both sexes in 20 Emys trinacris (Testudines: Emydidae) specimens. Erythrocytes were higher in male than in female specimens. The leukocyte of E. trinacris contains eosinophil, basophil, monocyte, heterophil and lymphocyte. The eosinophil was higher in males than in females whereas lymphocytes were higher in females than in males. The erythrocyte morphological parameters (EL [erythrocyte length], EW [erythrocyte width], L/W [length/width], ES [erythrocyte size]) were compared with the same data from Emys orbicularis s.l, and from species belonging to other chelonian genera. The erythrocyte size did not vary within the studied Palearctic Emys taxa, whereas it proved to differ from that observed in other chelonians.
Introduction
Emys trinacris (Fritz et al. Citation2005) (Testudines: Emydidae) is a Sicilian endemic pond turtle (Fritz et al. Citation2005); although it is morphologically close to Emys orbicularis s.l. (Fritz et al. Citation2006), molecular taxonomic studies have unambiguously revealed the presence of significant differences between the two species, which are adelphotaxa (Fritz et al. Citation2007; Pedall et al. Citation2011; Stuckas et al. Citation2014). Considering the biogeographical and evolutionary importance of this species and the drastic reduction of its populations caused by habitat destruction, pollution, and pathogens, in 2013, E. trinacris was listed in the International Union for Conservation of Nature (IUCN) Red List as “Endangered” (EN) (Rhodin et al. Citation2009).
To date, few studies have focused on the biology of this endemic species, and nothing is known about the hematologic blood characterization (HBC) of the species and on the health status of the wild populations of E. trinacris.
In the literature, HBC has been used successfully to diagnose chelonian diseases and to assess the physiological status of wild turtle populations (Duguy Citation1967; Dessauer Citation1970; Frye Citation1991; Campbell Citation1996; Stein Citation1996). This approach is widely used because the HBC is a minimally invasive tool that allows health evaluations, especially in relation to determining potential effects associated with stress factors such as pollution, disease, invasion by exotic species, etc. In order to be soundly usable, the reference evaluations have to be performed on healthy animals (Nagy & Medica Citation1986; Deem et al. Citation2006).
Blood cell parameters of reptiles may be influenced by several factors, such as age, sex, seasonality, reproduction, nutritional status and environmental parameters such as temperature, salinity, oxygen and light (Dessauer Citation1970; Duguy Citation1970; Frye Citation1991; Wilkinson Citation2003; Tavares-Dias et al. Citation2009; Yilmaz & Tosunoglu Citation2010; Gu et al. Citation2011; Scheelings & Jessop Citation2011; Tosunoglu et al. Citation2011; Scheelings & Rafferty Citation2012); these parameters can vary through the annual cycle and throughout the life of the individuals. Studies that describe chelonian blood cells are rare and the data are often in contradiction because of the lack of standard criteria used to categorize blood cells (Work et al. Citation1998). Various authors described circulating blood cells of different amphibian and reptile species; moreover, there are many chelonian species for which blood cell morphology and reference values are still unknown or imprecise. Descriptions of the morphologic characteristics of blood cells of pond turtles are limited and fail to standardize the parameters of the HBC (Metin et al. Citation2006; Rossini et al. Citation2012).
In order to perform conservation activities for the populations of E. trinacris, reference studies on biological parameters are necessary. Moreover, as a good practice, any action taken for the protection and the conservation of species in danger of extinction cannot ignore the full knowledge of their biology. For this reason, the blood cell parameters of the Sicilian pond turtle E. trinacris have been documented by analysing blood samples from free-living males and females. Moreover, blood cell parameter data obtained from E. trinacris were compared with those available from E. orbicularis s.l. Other comparisons were performed with those of the American emydid Trachemys scripta elegans (Wied, 1839).
The present study describes for the first time the blood cell parameters in E. trinacris obtained from the turtles under natural conditions, and aims to establish the blood cell parameter reference values necessary for the evaluation of the health status of individuals from wild populations.
Materials and methods
Sampling area
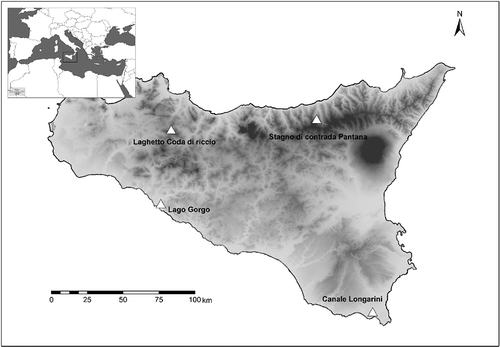
Collection was performed in four sites located in the Sicilian mainland as shown in and .
Table I. Geographical coordinates of the sampled sites and synopsis of the studied Emys trinacris specimens.
The pond turtles were caught by hand or with hoop net traps (Ream & Ream Citation1966). Caught pond turtles were weighed and measured: measurements included the length and the width of the carapace, the length and the width of the plastron, the carapace height, the total and cloaca-apex tail length in mm (with a caliper, to the nearest 0.5 mm) (Fritz et al. Citation2005), and the body mass in grams for each specimen (Zuffi & Gariboldi Citation1995; Zuffi et al. Citation2006). Ten blood samples of Trachemys scripta elegans were obtained in September 2013 from specimens housed in the Botanical Garden of the department of STEBICEF, University of Palermo.
Blood sampling, cell morphology and counts
Blood samples were obtained from the dorsal coccygeal vessel via heparinized glass capillaries (Hutchison & Szarski Citation1965; Szarski Citation1968). After obtaining blood samples, the animals were immediately released to their natural environments. For each individual, one to three capillaries were collected.
Blood smears were prepared in situ. A blood drop was smeared on a glass slide and air-dried. The sample was then fixed in methanol and stained by the Pappenheim method (May-Grünwald + Giemsa-Romanowsky staining diluted 1:10 in buffered water, pH 7) for 20 min, and washed in running tap water for 2 minutes. One hundred erythrocytes and 30 each of thrombocytes, eosinophils, basophils, lymphocytes and monocytes were measured under a microscope (Leica DMRE) equipped with a digital camera (Leica DCF420 C). In each smear were recorded lengths (L) and widths (W) of 100 randomly chosen erythrocytes as well as nuclear lengths (NL) and nuclear widths (NW). Erythrocyte sizes (ES) and their nucleus sizes (NS) were computed from the following equations (Arikan & Cicek Citation2010):
Statistical analyses
Hematological variables (number of cells or dimensions) were summarized as mean, standard deviation (SD), standard error of the mean (SE) and range. We used analysis of the t test for a comparison of the sexes.
Results
Sampling and measurements
A total of 20 wild pond turtles Emys trinacris, 16 male and four female, were sampled in Sicily from 2012 to 2013 (). The average body weights for the male and female turtles used for the study were 355.31 ± 154.78 g and 504.33 ± 75.5 g, respec-tively. The measured carapace lengths were 12.77 ± 2.15 cm and 14.08 ± 1.11 cm for male and female specimens, respectively.
Those samples which showed wounds or epiphytes or possessed parasites in the blood were not included in the analyses.
Emys trinacris blood cells
Differential blood cell count of the peripheral blood of E. trinacris was carried out using blood smears stained with May-Grünwald Giemsa observed under a light microscope equipped with a digital camera. The leukocyte types recognized in Emys trinacris correspond with those found in other species of the family Emydidae (). In particular, seven cell types were identified: (a) nucleated erythrocytes, (b) eosinophils, (c) basophils, (d) monocytes, (e) thrombocytes, (f) heterophils and (g) lymphocytes (). Significant differences in cell size were not observed in either sex. Males possessed significantly higher numbers of red blood cells (p < 0.05) and eosinophils (p < 0.01), while females showed a significantly (p < 0.05) higher percentage of lymphocytes (). For other cell types, although their number varied between the genders, no significant differences were found.
Figure 2. Blood smears of Emys trinacris stained with May-Grünwald Giemsa. Pictures were taken with an optical microscope equipped with a digital camera. a, erythrocyte; b, eosinophil; c, basophil; d, monocyte; e, heterophil; f, lymphocyte; g, thrombocyte. Scale bars: 10 µm.
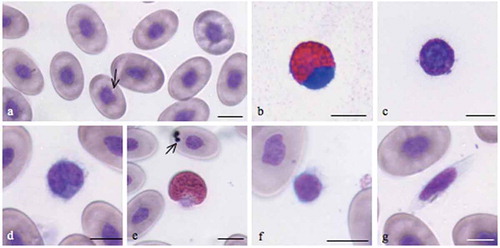
Table II. Leukocytes in turtle species of the Emydidae family.
Erythrocyte morphology
Mature erythrocytes were homogeneous in size, shape and color (). They were nucleated cells with elliptical shape and abundant pale pink cytoplasm like those of the other turtle species (Kassab et al. Citation2009; Orós et al. Citation2010). The violet-blue oval or round nucleus with rounded or irregular poles was centrically positioned and had condensed deeply basophilic chromatin. Its major axis was parallel with the long diameter of the cell. Some erythrocytes had small intracytoplasmic inclusions (arrow ) or might contain vacuoles (arrow ). Although the erythrocytes of males compared with females were generally larger in size, a significant difference was not recorded. Parasites were not detected. The results of erythrocyte measurements are summarized in .
Table III. Differential blood cells count and size in peripheral blood of Emys trinacris. (*) = Significant differences p < 0.05; (**) = significant differences p < 0.01. The erythrocyte size was reported as ratio between measurements (µm) of length and width (L/W). The size of the thrombocyte was the length of the cells (L) expressed in µm.
White blood cell morphology
Eosinophils
The eosinophils () were easily distinguished by their round eosinophilic cytoplasmic granules, which fill the whole cytoplasm. They were the smallest among the granulocytes, ranging between 13.5 ± 5.67 and 12.6 ± 1.3 µm for males and females respectively (). The nucleus contains coarse, clumped chromatin and strongly stained blue. It was round to oval, single or bilobed and eccentrically placed near the membrane. The cytoplasm was filled with granules measuring approximately 1.15 ± 0.035 µm. Males had a significantly (p < 0.01) higher number of eosinophils (20.2 ± 1.2) than females (17.5 ± 1.09).
Basophils
The basophils () were present with a percentage of 5.8 ± 0.45 and 5.4 ± 0.77 respectively for male and female turtles. They were small cells, about 9.49 µm, without significant differences between the genders. They are easily identified by their deeply stained cytoplasm filled with very dense, dark purple granules. Their large nuclei (7.09 ± 0.25) were round and centrically placed ().
Monocytes
The monocyte () contained a large amount of light blue-gray, finely granular or vacuolated cytoplasm and an oval or kidney-shaped nucleus with a dense chromatin pattern near the membrane. The mean diameter in observed monocytes ranged between 11.3 ± 4.81 and 11.7 ± 1.75 µm and did not differ significantly between males and females (). The presence of this cell in both males and females was the same.
Heterophils
Heterophils contained large, eosinophilic and fusiform cytoplasmic granules. The cytoplasm, which can be difficult to visualize, was light blue or clear (). The nucleus is segmented and frequently displaced toward the edge of the cell and appeared basophilic with dense chromatin. No significant differences were found between males and females for size; the diameter ranged from 13.9 ± 6.71 in males to 13.3 ± 1.27 in females, and the frequency was 15.4 ± 0.8% in males and 15.7 ± 1.2% in females.
Lymphocytes
The lymphocytes of E. trinacris were easily recognizable because they differed greatly from thrombocytes (). They were round cells with a diameter of 6.7 ± 2.12 µm in males and 6.5 ± 1.26 µm in females. They contained a small amount of blue-stained cytoplasm and a round nucleus with a fine reticular pattern. The lymphocyte showed a nuclear to cytoplasmic ratio greater than one.
Thrombocytes
The thrombocytes () were observed as spindle-shaped cells (25.0 ± 1.23 × 5.0 ± 0.89 µm for males and 22.9 ± 0.63 × 4.8 ± 0.54 for females) that contained a central, ellipsoidal, densely stained nucleus of about 12.9 ± 1.3 × 4.11 ± 0.74 µm for both males and females. The cytoplasm was hyaline and had no granules.
Discussion
The comparison of the red blood cells of E. trinacris with those of E. orbicularis s.l. showed a high variability in size (). In particular, E. orbicularis data in the literature show mean values of L/W ranging from 1.6 to 1.8 µm, with cell average areas ranging from 110.9 to 225.1 μm2. The red blood cells of E. trinacris were larger compared with the erythrocytes of the other two taxa. They were long on average, 22.5 µm and 14.1 µm wide, with a cell area of 249 μm2. The nuclei of the red blood cells of E. trinacris were smaller when compared with those of the other two species (). A further comparison was made between the sizes of the erythrocytes of E. trinacris with those of Trachemys scripta (Schoepff, 1792) (). The data show that the sizes of EL, EW, their ratio (L/W) and the cell areas were significantly larger than those of T. scripta. However, the size of the nuclei of the erythrocytes of E. trinacris did not differ significantly when compared with those of T. scripta ().
Table IV. Erythrocyte dimensions from male and female individual of Emys trinacris compared with E. orbicularis galloitalica and E. orbicularis hellenica (µm ± standard deviation, SD). EL: erythrocyte length, EW: erythrocyte width, L/W: ratio between measurements (µm) of length and width, ES: erythrocyte size, NL: nucleus length, NW: nucleus width, NS: nucleus size. ES and NS were estimated with the respective formulas ELEWπ/4 and NLNWπ/4.
Table V. Erythrocyte dimensions of Trachemys scripta elegans (µm ± standard deviation, SD). EL: erythrocyte length, EW: erythrocyte width, L/W: ratio between measurements (µm) of length and width, ES: erythrocyte size, NL: nucleus length, NW: nucleus width, NS: nucleus size. ES and NS were esteemed with the respective formulas: ELEWπ/4 and NLNWπ/4. * = p < 0.05.
Hematological and biochemical parameters are useful tools in measuring the physiological status of turtles because they may provide information on the health and general condition of individuals and populations (Campbell Citation1998; Oliveira-Júnior et al. Citation2009). Moreover, such tools have been used as physiological disturbance indicators of diseases, stress or exposure to contaminants, as well as to assess degrees of dehydration (Peterson Citation2002; Christopher et al. Citation2003; Tavares-Dias et al. Citation2008).
Since data on the hematology of the Sicilian endemic Emys trinacris are currently lacking, the main aim of this study was to characterize, for the first time, the blood cells of E. trinacris and, in particular, to classify its leukocytes. The classification criteria of chelonian leukocytes pose many problems, partly because these cells show morphological variation among the species and partly because several different nomenclatures have been used to describe them. Moreover, some cells are not easily identified based on their morphological differences, e.g.: small lymphocytes may be morphologically similar to thrombocytes. Most authors agree that reptiles do not have neutrophils, whereas they do have heterophils and eosinophils, which both show acidophilic granules (Canfield Citation1998). Some studies classify acidophils (i.e., heterophils and eosinophils) as a single cell type at different stages of maturation (Azevedo & Lunardi Citation2003). Neutrophils have been reported only in some reports (Wood & Ebanks Citation1984; Pitol et al. Citation2007). Some authors (Christopher et al. Citation1999; Dickinson et al. Citation2002; Knotková et al. Citation2002) refer to the presence of azurophils in the peripheral blood of chelonians, and the very existence of azurophils is still in dispute (Rosskopf Citation2000). Studies involving other species aiming to study leukocytes under light microscopy analysis showed, in the turtle species Podocnemis expansa (Schweigger, 1812) and Emys orbicularis s.l., the presence of basophiles, eosinophils, lymphocytes, monocytes and heterophils (Metin et al. Citation2006).
Furthermore, another difficulty lies in determining the proportions of these cells within a particular species since these vary with individual physiologic status and the method of investigation (Campbell & Ellis Citation2007).
Erythrocytes
Mature erythrocytes of E. trinacris proved to be morphologically similar to those of various species of turtles and tortoises and in particular to those of E. orbicularis s.l. (Ugurtas et al. Citation2003; Metin et al. Citation2006). They were ellipsoidal cells with a centrally positioned, ovoid nucleus and cytoplasmic inclusions, observed in over 30% of erythrocytes. For other reptiles, these inclusions have been reported to be degenerated organelles (Alleman et al. Citation1992; Clark et al. Citation2001; Chung et al. Citation2009) and may be related to the aging of erythrocytes (Heard et al. Citation2004). Others have postulated that the basophilic inclusions could be micronuclei and, therefore, they could be biomarkers for chromosomal damage from genotoxic environmental pollutants (Matson et al. Citation2005; Metin et al. Citation2006). The erythrocyte mean sizes did not differ significantly from those of E. orbicularis s.l. (p > 0.05), failing in an attempt to use the erythrocyte size as a discriminator between species of the same Emydinae subfamily. The higher number of red blood cells observed in males of E. trinacris than in females is similar to the findings in other turtles such as E. orbicularis (Duguy Citation1967) and Kinixys erosa (Schweigger, 1812) (Oyewale et al. Citation1998). The higher number of red blood cells found in males may depend on testosterone hormone levels. In fact, testosterone, when present in chelonians (Paitz & Bowden Citation2013), is able to increase the number of erythrocytes (Fried & Gurney Citation1965; Pati & Thapliyal Citation1984; Oyewale et al. Citation1998).
White blood cell morphology
Our findings conform to the basic morphological description for other Emydidae turtle species such as Emys orbicularis, Graptemys gibbonsi (Lovich & McCoy, 1992), Pseudemys rubriventris (LeConte, 1830) and Clemmys muhlenbergii (Schoepff, 1801) ().
Eosinophils
In turtles, the same authors showed two types of eosinophils distinguishable by the shape of cytoplasmic granules. Azevedo and Lunardi (Citation2003) observed in the blood of Chrysemys dorbigni (Duméril & Bibron, 1835) two types of granulocytes that exhibit eosinophilia, one of them with round cytoplasmic granules and the other with elongated cytoplasmic granules. It has been suggested that these cells may be eosinophils in different stages of maturation, but they also may be distinct cell types, i.e. eosinophils and heterophils (Azevedo & Lunardi Citation2003). In E. trinacris, most leukocytes were heterophils, basophils and eosinophils. Similar findings have also been reported by (Oliveira-Júnior et al. Citation2009) for Podocnemis expansa. This result was not confirmed for other species of turtles. In fact, only captive female Clemmys muhlenbergii had a higher absolute eosinophil count and a higher percentage of eosinophils compared with captive males; conversely, wild females were not significantly different from wild males (Brenner et al. Citation2002).
Basophils
The numbers of basophils in turtles vary greatly. In Graptemys gibbonsi, basophils were found to be the most abundant leukocyte type, about 40% of total leukocytes (Perpiñán et al. Citation2008). Higher percentages of basophils (50–63%) have been found in other chelonians, such as Chelydra serpentina (Linnaeus, 1758) (Mead et al. Citation1983). In contrast, moderate basophil percentages have been found in other species, such as 5.7% in Gopherus polyphemus (Daudin, 1802) (Taylor & Jacobson Citation1982) and 8% in Geochelone radiata (Shaw, 1802) (Marks & Citino Citation1990). Basophil numbers were almost nonexistent (~0.8 for both sexes) in Clemmys muhlenbergii (Brenner et al. Citation2002). However, care must be taken when analyzing published works; as an example, basophil counts in E. orbicularis varied widely: 0–4% was reported by Duguy (Citation1970) and about 34% was reported by Javanbakht et al. (Citation2013). This variation of basophil density in various species of turtles is difficult to explain. Many factors can affect the number of basophils and the leukocytic formula such as age, health status, ecological factors and the seasons (for a review see Duguy Citation1970). We found in E. trinacris that the percentage of basophils did not vary significantly between sexes, ranging between 20.5 ± 2.58 for males and 17.3 ± 1.58 for females.
Monocytes
This leukocyte type is not present in all species of turtles. Indeed, in Chelonia mydas (Linnaeus, 1758), authors did not identify monocytes (Wood & Ebanks Citation1984; Aguirre et al. Citation1995). Often, monocytes are not visible if the blood smears are performed with blood that was taken eight or more hours before (Work et al. Citation1998). Monocytes from E. trinacris were similar to the monocytes from E. orbicularis described by Metin et al. (Citation2006) or Ocadia sinensis (Gray, 1870) described by Chung et al. (Citation2009). In E. trinacris, monocytes were round cells and had a similar size in both males and females, (11.3 and 11.7 µm respectively). Also, their frequency was similar for both sexes (~4.2%).
Heterophils
The heterophils of chelonians are analogous to mammalian neutrophils (Montali Citation1988) and can be easily distinguished by the fusiform red granules contained in the cytoplasm. They had the same percentage for both sexes (~15%). These frequencies correspond with those found in Graptemys gibbonsi (Perpiñán et al. Citation2008), but differ from those in Pseudemys rubriventris (~26.9%) (Innis et al. Citation2007) and Clemmys muhlenbergii and Chrysemys picta (Schneider, 1783) (both about 9.3% (Brenner et al. Citation2002; Schwanz et al. Citation2011). Furthermore, the percentage value is included within the range indicated for E. orbicularis (Duguy Citation1970). The number and size of heterophils have been observed to be influenced by individual and seasonal factors (Duguy Citation1970).
Lymphocytes
The lymphocytes were the smallest cells, with a diameter on average about 6.6 µm for both sexes. Female had a significantly (p < 0.05) higher percentage of lymphocytes (27.3 ± 1.24) compared with males (22.5 ± 0.69). The same result was reported by Brenner et al. (Citation2002) for C. muhlenbergii, where female and male lymphocyte percentages were 1.8 and 1.5%, respectively. The percentages of lymphocytes found in both genders of E. trinacris were coherent with values reported for other Emydidae turtles such as G. gibbonsi and C. muhlenbergii (Brenner et al. Citation2002; Perpiñán et al. Citation2008) but differ from those for P. rubriventris, in which these cells represent about 50% of the white blood cell differential count (Innis et al. Citation2007).
Thrombocytes
Although the similarity of thrombocytes and leukocytes in reptiles is known (Frye Citation1991), in the case of E. trinacris, thrombocytes differ greatly from those of other pond turtles. In E. orbicularis, the thrombocytes are round cells with a nucleus round to oval and dark (Metin et al. Citation2006), whereas in Pseudemys rubriventris these were elliptical, with central ovoid basophilic nuclei, lightly basophilic cytoplasm, and were often noted in small clusters (Innis et al. Citation2007). The thrombocytes of E. trinacris have an elongated cell shape with a central ovoid nucleus. The size and number do not differ between the sexes.
The comparison of the erythrocyte size parameters with those of Emys orbicularis s.l. showed no important differences even in comparison between the two sexes (). The data reported by Javanbakht et al. (Citation2013) had the lowest values, which probably derived from different environmental conditions (e.g. temperature, air pressure) (Ruiz et al. Citation1983, Citation1989) or different activity levels (e.g. healthy, breeding, hibernating, foraging and daily activity) (Sykes & Klaphake Citation2008; Tosunoglu et al. Citation2011; Yu et al. Citation2013).
However, significant differences were found when the erythrocyte parameters (EL, EW, L/W, ES) of E. trinacris were compared with those of T. scripta elegans, a tortoise belonging to the same family but to a different genus (). The morphology of E. trinacris erythrocytes was similar to that of T. scripta elegans, but the size was greater. The L/W ratio was about 1.6 for E. trinacris and about 1.4 for T. scripta elegans; consequently, erythrocyte shape was more ellipsoidal in E. trinacris. No significant differences were found in the nucleo-cytoplasmic ratio.
The results of our analysis show that the morphology of erythrocytes within the family of Emydidae does not change greatly and, in a comparison between species from different genera, only a few differences in size can be found.
The findings of this study present for the first time data on the cytomorphological structure and numbers of peripheral blood cells in both sexes of wild-caught, healthy E. trinacris. Since dates were derived from specimens in good health, the hematological profile here reported could be used as reference values for studies on E. trinacris, and could be beneficial to future clinical and conservation work on the endangered Sicilian pond turtle.
Acknowledgements
We thank the Director of Orto botanico of the University of Palermo for permission to sample the Trachemys scripta elegans specimens, the President of Parco dei Nebrodi and the Italian Ministry of the Environment and Protection of Land and Sea for granting the authorization U. prot. PNM-2011- 0022035 25/10/2011 to sample Emys.
Research partially funded by the “Fondi di Ateneo” (60%) of the University of Palermo.
References
- Aguirre AA, Balazs GH, Spraker TR, Gross TS. 1995. Adrenal and hematological responses to stress in juvenile green turtles (Chelonia mydas) with and without fibropapillomas. Physiological Zoology 68:831–854.
- Alleman AR, Jacobson ER, Raskin RE. 1992. Morphologic and cytochemical characteristics of blood cells from the desert tortoise (Gopherus agassizii). American Journal of Veterinary Research 53:1645–1651.
- Arikan H, Cicek K. 2010. Morphology of peripheral blood cells from various species of Turkish herpetofauna. Acta Herpetologica 5:179–198.
- Azevedo A, Lunardi LO. 2003. Cytochemical characterization of eosinophilic leukocytes circulating in the blood of the turtle (Chrysemys dorbignih). Acta Histochemica 105:99–105. doi:10.1078/0065-1281-00693.
- Brenner D, Lewbart G, Stebbins M, Herman DW. 2002. Health survey of wild and captive bog turtles (Clemmys muhlenbergii) in North Carolina and Virginia. Journal of Zoo and Wildlife Medicine 33:311–316.
- Campbell TW. 1996. Clinical pathology. In: Mader DR, editor. Reptile medicine surgery. Philadelphia, Pennsylvania, USA: Saunders Company Ltd. pp. 248–257.
- Campbell TW. 1998. Interpretation of the reptilian blood profile. Exotic Pet Practice 3:33–36.
- Campbell TW, Ellis C. 2007. Avian and exotic animal hematology and cytology. 3rd ed. Ames, Iowa, USA: Blackwell Pub.
- Canfield PJ. 1998. Comparative cell morphology in the peripheral blood film from exotic and native animals. Australian Veterinary Journal 76:793–800. doi:10.1111/j.1751-0813.1998.tb12328.x.
- Christopher MM, Berry KH, Henen BT, Nagy KA. 2003. Clinical disease and laboratory abnormalities in free-ranging desert tortoises in California (1990–1995). Journal of Wildlife Diseases 39:35–56. doi:10.7589/0090-3558-39.1.35.
- Christopher MM, Berry KH, Wallis IR, Nagy KA, Henen BT, Peterson CC. 1999. Reference intervals and physiologic alterations in hematologic and biochemical values of free-ranging desert tortoises in the Mojave Desert. Journal of Wildlife Diseases 35:212–238. doi:10.7589/0090-3558-35.2.212.
- Chung CS, Cheng CH, Chin SC, Lee AH, Chi CH. 2009. Morphologic and cytochemical characteristics of Asian yellow pond turtle (Ocadia sinensis) blood cells and their hematologic and plasma biochemical reference values. Journal of Zoo and Wildlife Medicine 40:76–85. doi:10.1638/2008-0023.1.
- Clark P, Johnstone AC, Ellison R, Goold M. 2001. Inclusions in the erythrocytes of eastern water dragons (Physignathus lesueurii). Australian Veterinary Journal 79:61–62. doi:10.1111/j.1751-0813.2001.tb10643.x.
- Colagar H, Jafari N. 2007. Red blood cell morphology and plasma proteins electrophoresis of the European pond terrapin Emys orbicularis. African Journal of Biotechnology 6:1578–1581.
- Deem SL, Dierenfeld ES, Sounguet GP, Alleman AR, Cray C, Poppenga RH, Norton TM, Karesh WB. 2006. Blood values in free-ranging nesting leatherback sea turtles (Dermochelys coriacea) on the coast of the Republic of Gabon. Journal of Zoo and Wildlife Medicine 37:464–471. doi:10.1638/05-102.1.
- Dessauer HC. 1970. Blood chemistry of reptiles: Physiological and evolutionary aspects. In: Gans C, Parson T, editors. Biology of the reptilia. London, UK: Academy Press. pp. 1–72.
- Dickinson VM, Jarchow JL, Trueblood MH. 2002. Hematology and plasma biochemistry reference range values for free-ranging desert tortoises in Arizona. Journal of Wildlife Diseases 38:143–153. doi:10.7589/0090-3558-38.1.143.
- Duguy R. 1967. Le cycle annuel des elements figures dus sang chez Emys orbicularis L., Lacerta muralis Laur., et Natrix maura L. Bulletin de la Societe Zoologique de France 92:15.
- Duguy R. 1970. Numbers of blood cells and their variation. In: Gans C, Parson T, editors. Biology of the reptilia. Vol. 3. London, UK: Academy Press. pp. 93–110.
- Fried W, Gurney CW. 1965. Use of mild plethora to demonstrate an erythropoietic effect from small amounts of androgens. Experimental Biology and Medicine 120:519–521. doi:10.3181/00379727-120-30577.
- Fritz U, d’Angelo S, Pennisi MG, Lo Valvo M. 2006. Variation of Sicilian pond turtles, Emys trinacris - What makes a species cryptic? Amphibia-Reptilia 27:513–529. doi:10.1163/156853806778877095.
- Fritz U, Fattizzo T, Guicking D, Tripepi S, Pennisi MG, Lenk P, Joger U, Wink M. 2005. A new cryptic species of pond turtle from southern Italy, the hottest spot in the range of the genus Emys (Reptilia, Testudines, Emydidae). Zoologica Scripta 34:351–371. doi:10.1111/j.1463-6409.2005.00188.x.
- Fritz U, Guicking D, Kami H, Arakelyan M, Auer M, Ayaz D, Fernandez CA, Bakiev AG, Celani A, Dzukic G, Fahd S, Havaš P, Joger U, Khabibullin VF, Mazanaeva LF, Široky' P, Tripepi S, Vélez AV, Antón GV, Wink M. 2007. Mitochondrial phylogeography of European pond turtles (Emys orbicularis, Emys trinacris) - an update. Amphibia-Reptilia 28:418–426. doi:10.1163/156853807781374737.
- Frye FL. 1991. Hematology as applied to clinical reptile medicine. In: Frye FL, editor. Biomedical surgical aspects of captive reptile husbandry. Vol. 1. Malabar, Florida, USA: Krieger Publishing Co. pp. 209–280.
- Gu HX, Zhang FY, Li PP. 2011. A Review of chelonian hematology. Asian Herpetological Research 2:12–20. doi:10.3724/SP.J.1245.2011.00012.
- Heard D, Harr K, Wellehan J. 2004. Diagnostic sampling and laboratory tests. In: Girling SJ, Raiti P, editors. Manual of reptiles. 2nd ed. Quedgeley, Gloucester, UK: BSAVA Publisher. pp. 70–86.
- Hutchison HV, Szarski H. 1965. Number of erythrocytes in some amphibians and reptiles. Copeia 1965:373–376. doi:10.2307/1440807.
- Innis CJ, Tlusty M, Wunn D. 2007. Hematologic and plasma biochemical analysis of juvenile head-started northern red-bellied cooters (Pseudemys rubriventris). Journal of Zoo and Wildlife Medicine 38:425–432. doi:10.1638/1042-7260(2007)38[425:HAPBAO]2.0.CO;2.
- Javanbakht H, Vaissi S, Parto P. 2013. The morphological characterization of the blood cells in the three species of turtle and tortoise in Iran. Research in Zoology 3:38–44.
- Kassab A, Shousha S, Fargani A. 2009. Morphology of blood cells, liver and spleen of the Desert tortoise (Testudo graeca). The Open Anatomy Journal 1:1–10. doi:10.2174/1877609400901010001.
- Knotková Z, Doubek J, Knotek Z, Hájková P. 2002. Blood cell morphology and plasma biochemistry in Russian tortoises (Agrionemys horsfieldi). Acta Veterinaria Brno 71:191–198. doi:10.2754/avb200271020191.
- Marks SK, Citino SB. 1990. Hematology and serum chemistry of the radiated tortoise (Testudo radiata). Journal of Zoo and Wildlife Medicine 21:342–344.
- Matson CW, Palatnikov G, Islamzadeh A, McDonald TJ, Autenrieth RL, Donnelly KC, Bickham JW. 2005. Chromosomal damage in two species of aquatic turtles (Emys orbicularis and Mauremys caspica) inhabiting contaminated sites in Azerbaijan. Ecotoxicology 14:513–525. doi:10.1007/s10646-005-0001-0.
- Mead KF, Borysenko M, Findlay SR. 1983. Naturally abundant basophils in the snapping turtle, Chelydra serpentina, possess cytophilic surface antibody with reaginic function. Journal of Immunology 130:334–340.
- Metin K, Türkozan O, Kargin F, Koca YB, Taskavak E, Koca S. 2006. Blood cell morphology and plasma biochemistry of the captive European pond turtle Emys orbicularis. Acta Veterinaria Brno 75:49–55. doi:10.2754/avb200675010049.
- Montali RJ. 1988. Comparative pathology of inflammation in the higher vertebrates (reptiles, birds and mammals). Journal of Comparative Pathology 99:1–26. doi:10.1016/0021-9975(88)90101-6.
- Nagy KA, Medica PA. 1986. Physiological ecology of desert tortoises in southern Nevada. Herpetologica 42:73–92.
- Oliveira-Júnior AA, Tavares-Dias M, Marcon JL. 2009. Biochemical and hematological reference ranges for Amazon freshwater turtle, Podocnemis expansa (Reptilia: Pelomedusidae), with morphologic assessment of blood cells. Research in Veterinary Science 86:146–151. doi:10.1016/j.rvsc.2008.05.015.
- Orós J, Casal A, Arencibia A. 2010. Microscopic studies on characterization of blood cells of endangered sea turtles. In: Méndez-Vilas A, Díaz J, editors. Microscopy: Science, technology, applications and education. Vol. 1. Badajoz, Spain: Formatex Research Center. pp. 75–84.
- Oyewale J, Ebute C, Ogunsanmi A, Olayemi F, Durotoye L. 1998. Weights and blood profiles of the West African hinge backed tortoise, Kinixys erosa and the desert tortoise, Gopherus agassizii. Journal of Veterinary Medicine Series A 45:599–605. doi:10.1111/j.1439-0442.1998.tb00864.x.
- Paitz RT, Bowden RM. 2013. Sulfonation of maternal steroids is a conserved metabolic pathway in vertebrates. Integrative and Comparative Biology 53:895–901. doi:10.1093/icb/ict027.
- Pati A, Thapliyal J. 1984. Erythropoietin, testosterone, and thyroxine in the erythropoietic response of the snake, Xenochrophis piscator. General and Comparative Endocrinology 53:370–374. doi:10.1016/0016-6480(84)90264-8.
- Pedall I, Fritz U, Stuckas H, Valdeón A, Wink M. 2011. Gene flow across secondary contact zones of the Emys orbicularis complex in the Western Mediterranean and evidence for extinction and re-introduction of pond turtles on Corsica and Sardinia (Testudines: Emydidae). Journal of Zoological Systematics and Evolutionary Research 49:44–57. doi:10.1111/j.1439-0469.2010.00572.x.
- Perpiñán D, Hernandez-Divers SM, Latimer KS, Akre T, Hagen C, Buhlmann KA, Hernandez-Divers SJ. 2008. Hematology of the Pascagoula map turtle (Graptemys Gibbonsi) and the southeast Asian box turtle (Cuora amboinensis). Journal of Zoo and Wildlife Medicine 39:460–463. doi:10.1638/2007-0044.1.
- Peterson CC. 2002. Temporal, population, and sexual variation in hematocrit of free-living desert tortoises: Correlational tests of causal hypotheses. Canadian Journal of Zoology-Revue Canadienne De Zoologie 80:461–470. doi:10.1139/z02-021.
- Pitol DL, Issa JPM, Caetano FH, Lunardi LO. 2007. Morphological characterization of the leukocytes in circulating blood of the turtle (Phrynops hilarii). International Journal of Morphology 25:6. doi:10.4067/S0717-95022007000400002.
- Ream C, Ream R. 1966. The Influence of sampling methods on the estimation of population structure in painted turtles. American Midland Naturalist 75:325–338. doi:10.2307/2423395.
- Rhodin AGJ, Parham JF, van Dijk PP, Iverson JB. 2009. Turtles of the world: annotated checklist of taxonomy and synonymy, 2009 update, with conservation status summary. Conservation biology of freshwater turtles and tortoises: A compilation project of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group. Chelonian Research Monographs 5:000.39–000.84.
- Rossini M, Blanco PA, Marín E, Comerma-Steffensen S, Zerpa H. 2012. Haematological values of post-laying Arrau turtle (Podocnemis expansa) in the Orinoco River, Venezuela. Research in Veterinary Science 92:128–131. doi:10.1016/j.rvsc.2010.10.026.
- Rosskopf WJ. 2000. Disorders of reptilian leucocytes and erythrocytes. In: Fudge AM, editor. Laboratory medicine: Avian and exotic pets. Philadelphia, USA: Saunders, W.B. pp. 198–204.
- Ruiz G, Rosenmann M, Veloso A. 1983. Respiratory and hematological adaptations to high altitude in Telmatobius frogs from the Chilean Andes. Comparative Biochemistry and Physiology Part A: Physiology 76:109–113. doi:10.1016/0300-9629(83)90300-6.
- Ruiz G, Rosenmann M, Veloso A. 1989. Altitudinal distribution and blood values in the toad, Bufo spinulosus Wiegmann. Comparative Biochemistry and Physiology Part A: Physiology 94:643–646. doi:10.1016/0300-9629(89)90609-9.
- Scheelings TF, Jessop TS. 2011. Influence of capture method, habitat quality and individual traits on blood parameters of free ranging lace monitors (Varanus varius). Australian Veterinary Journal 89:360–365.
- Scheelings TF, Rafferty AR. 2012. Hematologic and serum biochemical values of gravid freshwater Australian chelonians. Journal of Wildlife Diseases 48:314–321. doi:10.7589/0090-3558-48.2.314.
- Schwanz L, Warner DA, McGaugh S, Di Terlizzi R, Bronikowski A. 2011. State-dependent physiological maintenance in a long-lived ectotherm, the painted turtle (Chrysemys picta). The Journal of Experimental Biology 214:88–97. doi:10.1242/jeb.046813.
- Stein G. 1996. Hematologic and blood chemistry values in reptiles. In: Mader DR, editor. Reptile medicine and surgery. Philadelphia, Pennsylvania, USA: Saunders, W B. Company Ltd. pp. 473–483.
- Stuckas H, Velo-Antón G, Fahd S, Kalboussi M, Rouag R, Arculeo M, Marrone F, Sacco F, Vamberger M, Fritz U. 2014. Where are you from, stranger? The enigmatic biogeography of North African pond turtles (Emys orbicularis). Organisms Diversity and Evolution. doi:10.1007/s13127-014-0168-4.
- Sykes JM, Klaphake E. 2008. Reptile hematology. Veterinary Clinics of North America: Exotic Animal Practice 11:481–500. doi:10.1016/j.cvex.2008.03.005.
- Szarski H. 1968. Evolution of cell size in lower vertebrates. In: Orvig T, editor. Current problems of lower vertebrate phylogeny. Stockhol, SE: Almqvist and Wiksell. pp. 445–453.
- Tavares-Dias M, Oliveira-Júnior AA, Marcon JL. 2008. Methodological limitations of counting total leukocytes and thrombocytes in reptiles (Amazon turtle, Podocnemis expansa): An analysis and discussion. Acta Amazonica 38:351–356. doi:10.1590/S0044-59672008000200020.
- Tavares-Dias M, Oliveira-Junior AA, Silva MG, Marcon JL, Barcellos JFM. 2009. Comparative hematological and biochemical analysis of giant turtles from the Amazon farmed in poor and normal nutritional conditions. Veterinarski Arhiv 79:601–610.
- Taylor RW, Jacobson ER. 1982. Hematology and serum chemistry of the gopher tortoise, Gopherus polyphemus. Comparative Biochemistry and Physiology Part A: Physiology 72:425–428. doi:10.1016/0300-9629(82)90241-9.
- Tosunoglu M, Yilmaz N, Gul C. 2011. Effects of varying ecological conditions on the blood parameters of freshwater turtles in Canakkale (Turkey). Ekoloji 20:7–12. doi:10.5053/ekoloji.2011.782.
- Ugurtas IH, Sevinc M, Yildirimhan HS. 2003. Erythrocyte size and morphology of some tortoises and turtles from Turkey. Zoological Studies 42:173–178.
- Wilkinson R. 2003. Clinical pathology. In: McArthur S, Wilkinson R, Meyer J, editors. Medicine and surgery of tortoises and turtles. Oxford, UK: Blackwell Publishing. pp. 141–186.
- Wood FE, Ebanks GK. 1984. Blood cytology and hematology of the green sea turtle, Chelonia mydas. Herpetologica 40:6.
- Work TM, Raskin RE, Balazs GH, Whittaker SD. 1998. Morphologic and cytochemical characteristics of blood cells from Hawaiian green turtles. American Journal of Veterinary Research 59:1252–1257.
- Yilmaz N, Tosunoglu M. 2010. Hematology and some plasma biochemistry values of free living freshwater turtles (Emys orbicularis and Mauremys rivulata) from Turkey. North-Western Journal of Zoology 6:109–117.
- Yu PH, Yang PY, Chiu YS, Chi CH. 2013. Hematologic and plasma biochemical reference values of the yellow pond turtle Mauremys mutica and the effects of sex and season. Zoological Studies 52:24–29. doi:10.1186/1810-522X-52-24.
- Zuffi MAL, Gariboldi A. 1995. Geographical patterns of Italian Emys orbicularis: A biometrical analysis. In: Llorente GA, Montori A, Santox X, Carretero MA, editors. Scientia Herpetologica. Barcelona: Agal. pp. 120–123.
- Zuffi MAL, Odetti F, Batistoni R, Mancino G. 2006. Geographic variation of sexual size dimorphism and genetics in the European pond turtle, Emys orbicularis and Emys trinacris, of Italy. Italian Journal of Zoology 73:363–372. doi:10.1080/11250000600971323.