Abstract
Pterostichus rhaeticus is widely distributed in northern and central Europe and has now been recorded for the first time in Croatia, and this record represents its southeasternmost distribution point. This species was only recently separated from the very common and widespread Pterostichus nigrita Paykull, 1790. These two sibling species differ in a few morphological features and can be certainly distinguished by the form of male and female genitalia. Therefore, we measured a few morphometric features, e.g. body length, elytrae length and width, male right paramere and female eighth abdominal sternite. In general, P. rhaeticus is significantly smaller and narrower than P. nigrita. However, in mixed populations, the differences in body size, length and width of elytrae were not observed and the overlap in sizes is considerable. Additionally, we noticed that the range edge individuals of P. rhaeticus are larger compared to individuals in central Europe. Most of the individuals of both species can be clearly and immediately identified by the shape of their genitalia, but in mixed populations these differences are possibly not so obvious, and measurements are necessary. P. rhaeticus is a tyrphophilous species that was exclusively found at three peatlands in Croatia. It was found in the open peat bog centre and adjacent shaded habitats, in both acidic peaty and alkaline soils with high soil humidity. According to present knowledge, this species can be considered an indicator species of peatland habitats in Croatia. These distinctive habitats are highly endangered in the Western Balkans, and therefore the survival of this and other bog-specific species depends on active conservation and protection measures.
Keywords:
Introduction
Pterostichus rhaeticus was only recently separated from the very common and widespread Pterostichus nigrita Paykull, 1790 (Koch & Thiele Citation1980; Koch Citation1986). Previously, only one species was recognised, P. nigrita (Erichson Citation1860; Apfelbeck Citation1904); however, in some older literature, P. rhaeticus was listed as a variety (Ganglbauer Citation1892) or a subspecies of P. nigrita (Csiki Citation1946). Nevertheless, recent studies have shown that the Pterostichus nigrita species complex comprises four distinct species: P. nigrita, P. rhaeticus, P. fuscicornis Reiche & Saulcy, 1855 and, most recently, P. carri Angus, Galián, Wrase & Chaladze 2008 (Koch Citation1986; Angus et al. Citation2009). The question of the P. nigrita/P. rhaeticus relationship could not have been answered using a molecular genetic approach, i.e. previously used markers (mtCOI and rDNA genes) did not provide enough resolution either to support or to reject the hypotheses of the sibling species or speciation in progress (Raupach et al. Citation2010).
Thus, P. rhaeticus and P. nigrita are mainly considered sibling species that differ in few morphological features (Koch Citation1986; Angus et al. Citation2000, Citation2009). According to Koch (Citation1986), males can be usually distinguished by the form of the right aedeagal paramere and the internal sac of the copulatory organ. The right paramere of P. nigrita is apically larger and narrower and has a shallow incision, while the right paramere of P. rhaeticus is shorter and broader, having a deeper incision. This morphological feature gives a clear identification of most individuals that have typical parameres (Koch Citation1986; Luff Citation1990). However, Luff (Citation1990) and Angus et al. (Citation2000) showed that there are considerable variations in the shape of the right parameres of P. rhaeticus, and identification of males based only on the latter character (especially in mixed populations) is not reliable. Therefore, in order to distinguish these two species, measurements of the right paramere should be obtained (Luff Citation1990). Koch (Citation1986) also showed that the internal sac of the aedeagus of P. nigrita males has sclerotized spines, while P. rhaeticus males usually have no spines on their internal sac. However, Angus et al. (Citation2000) proved that both species show variations in the chaetotaxy of the internal sac. Females can be unmistakably distinguished by the form of the coxostylus and the sclerotized part of the eighth abdominal sternite (Koch Citation1986; Angus et al. Citation2000).
P. rhaeticus and P. nigrita are both Palaearctic species that are widely distributed in northern and central Europe (Hůrka Citation1996; Löbl & Smetana Citation2003; Freude et al. Citation2006). However, P. rhaeticus does not occur in southern Europe (Angus et al. Citation2009), although it was found in the Far East and Kamchatka (Löbl & Smetana Citation2003). P. nigrita has the widest distribution range of all four species of this species complex and it occurs not only in Europe (except the Iberian Peninsula), but also in the Caucasus region, across Siberia to Kamchatka and Japan, Turkey and Syria, and in North Africa (Löbl & Smetana Citation2003; Angus et al. Citation2009). Therefore, the distribution range of P. rhaeticus is more restricted than the range of P. nigrita. P. fuscicornis is known from the Middle East (Löbl & Smetana Citation2003; Angus et al. Citation2009), whereas P. carri occurs in the Iberian Peninsula and Morocco (Angus et al. Citation2009).
In northern and central Europe, P. rhaeticus and P. nigrita frequently occur together in the same habitats (e.g. Främbs Citation1990; Spitzer et al. Citation1999; Luff Citation2007; Sushko Citation2007). According to Müller-Motzfeld and Hartmann (Citation1985), ecological differences between these two species are still insufficiently known. They are both hygrophilous species that prefer wet habitats (Müller-Motzfeld & Hartmann Citation1985; Koch Citation1986). P. rhaeticus is more often found in peatlands (Müller-Motzfeld & Hartmann Citation1985; Hůrka Citation1996) and it can be classified as a tyrphophilous species – a peat bog-characteristic species (Peus Citation1928; Roubal Citation1934). It was recorded from various peatlands in Europe (e.g. Främbs et al. Citation2002; Mossakowski et al. Citation2003; Sushko Citation2007; Buchholz et al. Citation2009; Colombetta Citation2012). Even though it is usually more abundant in peat bogs than in other wetland habitats, it is not restricted to bogs exclusively (Peus Citation1928; Roubal Citation1934; Spitzer et al. Citation1999). In northern Europe, for instance, it also can be found in marshes and wet grasslands (Grandchamp et al. Citation2005; Luff Citation2007), upland grazed meadows (Cole et al. Citation2006), managed forest stands (de Warnaffe & Lebrun Citation2004) and moist deciduous forests (Bukejs & Telnov Citation2007). P. nigrita is a euritopic species occurring in a wider range of habitats, e.g. from different types of forests (de Warnaffe & Lebrun Citation2004; Gaublomme et al. Citation2005; Skłodowski Citation2006), agricultural soils (Schwerk et al. Citation2006), wet meadows and wetlands (Grandchamp et al. Citation2005; Zahn et al. Citation2007) and peatlands (Spitzer et al. Citation1999) to margins of water with vegetation (Hůrka Citation1996).
Therefore, the aims of this paper are as follows: (1) to assess the distribution of P. rhaeticus at its southern edge in the Balkans, (2) to get a deeper insight into the ecology and reproductive biology of P. rhaeticus and (3) to evaluate its conservation status in the studied area.
Material and methods
Study area
The study was carried out at three distinct peatlands that differ in size, type, vegetation and location.
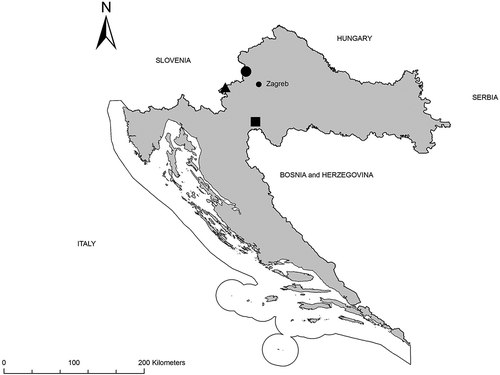
Peat bog Dubravica is located in northwestern Croatia near the village Dubravica (N45°57'51.48”, E15°45'15.48”; 24.5 km from Zagreb) at an altitude of l60 m above sea level (a.s.l.). During the last 50 years, this acidophilous bog has drastically decreased in size, due to changes of water level and natural process of vegetation succession (Hršak Citation1996). Nowadays, the bog covers an area of 605 m2 and the main and most widespread vegetation type historically belonged to the community of white-beaked rush (association Rhynchosporetum albae W. Koch 1926) (Horvat Citation1939; Hršak Citation1996). However, the present remnants of the bog vegetation can be associated with an impoverished community of star-headed sedge and round-leaved sundew (association Drosero-Caricetum echinatae Horvat (1950) 1962, alliance Rhynchosporion albae W. Koch 1926), usually developed in drier conditions compared to Rhynchosporetum albae. The bog is completely surrounded by sessile oak and hornbeam forest (association Epimedio-Carpinetum betuli (Ht.1938) Borh. 1963). It has been protected as a Botanical Reserve since 1966 and it is a NATURA 2000 site.
Đon močvar peat bog is situated in central Croatia, near the Blatuša village (N45°19'4.33”, E15°54'32.83”; 47.5 km from Zagreb), at an altitude of 130 m a.s.l. It covers a 10-ha area, representing one of the oldest and largest peat bogs in Croatia (Gigov & Nikolić Citation1960). This acidophilous bog is a complex ecosystem, characterised by a variety of different habitats from open woodless Sphagnum sites with hummocks, deep hollows and small ponds (with main association Drosero-Caricetum echinatae), to more swampy areas characterised by associations of Rhynchosporetum albae, Caricetum lasiocarpae W. Koch 1926 and Caricetum acutiformis Eggl. 1933, dominated by Rhynchospora alba (L.) Vahl., Eriophorum angustifolium Honck., Carex lasiocarpa Ehrh., Carex acutiformis Ehrh. and Phragmites australis (Cav.) Trin. ex Steud. On the peat bog borders, forest habitats dominated by Alnus glutinosa (L.) Gaertn. are developed and characterised by a high abundance of Frangula alnus Mill. and several Salix species (mostly S. aurita L.). Abandonment of traditional grazing and mowing has caused changes in plant composition and vegetation structure. Due to the spreading of woody vegetation (dominated by Betula pendula Roth and F. alnus), and of the moor grass in particular (Molinia caerulea (L.) Moench), the bog area has decreased from 40 ha to 10 ha from the beginning of the 20th century (Alegro & Šegota Citation2008). However, the bog and adjacent area have been protected as a Botanical Reserve and it is also a NATURA 2000 site (Alegro & Šegota Citation2008).
Basophilous fen Jarak is situated in northwestern Croatia near the village of Sošice in the Žumberak-Samoborsko gorje Nature Park, at an altitude of 690 m a.s.l. (N45°45'46.44”, E15°22'1.2”; 56 km from Zagreb). It covers an area of 0.24 ha. The fen vegetation belongs to association Eriophoro-Caricetum paniceae Horvat 1964. Typical fen-specific plant species Eriophorum latifolium Hoppe, E. angustifolium and four Carex spp. (C. flacca Schreb., C. caryophyllea Latourr., C. hostiana DC. and C. paniculata L.) are found in great abundance. The fen is surrounded by a forest dominated by Fagus sylvatica L. and plantations of Pinus sylvestris L. The fen vegetation is seriously threatened by rather aggressive P. australis, which is rapidly overgrowing the fen (Šoštarić et al. Citation2012).
Sampling
P. rhaeticus and P. nigrita were collected by pitfall traps together with other soil arthropods. Five traps (polythene pots 8.5 cm wide and 12.0 cm deep), 5 m apart, were placed per site. Due to the different sizes of the peatlands, the number of sites and pitfall traps varied among the studied peatlands (e.g. Dubravica bog – three sites, Jarak fen – four sites, Đon močvar bog – 12 sites). The traps were partially filled with saturated salt solution, and a drop of neutral-smelling detergent was added in order to reduce the surface tension of the liquid. A Styrofoam roof was placed a few centimetres above each trap to protect it from litter and rain. The trapping period covered the whole vegetation season of two years (2008–2009; with the exception of Đon močvar bog where the soil fauna was collected from 2008–2010), from the end of April to the beginning of December. Traps were emptied monthly.
Soil analysis
Soil humidity was measured monthly during the study period at each sampling site by taking a soil sample at approximately 10 cm depth, and average values were calculated. In substrate samples, water content was determined by the gravimetric method. The same soil sample was used to measure pH in water with a ratio of 1:2.5 (weight/volume) (10 g substrate/25 mL H2O) using a WTW pH 330i meter.
Identification and morphometric analyses
P. rhaeticus and P. nigrita were identified using Koch (Citation1986); Luff (Citation1990) and Angus et al. (Citation2000), and identifications were based on female and male genitalia. Male genitalia were removed from the abdomen, and the right aedeagal paramere was pulled away from the aedeagus and viewed from the inside. Parameres were measured as follows: L – length of apical portion of paramere, B1 – basal width, B2 – median width and A – apical angle (–). Female genitalia are usually retracted within the sclerotized segments of the abdomen, but become visible when the abdomen is gently pressed. Sclerotized parts of the eighth abdominal sternite were removed from the flesh and cleared. They were also measured and measurements included: L – length, B – breadth, M – length of membranous strip and S – distance from the strip to the basal lobe (–). All measurements were made in accordance with Luff (Citation1990), where they are described in detail. Altogether, 74 individuals were measured (39 males and 35 females) ().
Figure 1. Measurements of taxonomic characters: a, male genitalia – right paramere of Pterostichus rhaeticus; b, male genitalia – right paramere of Pterostichus nigrita: L – length of apical portion of paramere, B1 – basal width, B2 – median width, A – apical angle; c, female genitalia – half of the eighth sternite of P. rhaeticus; d, female genitalia – half of the eighth sternite of P. nigrita: L – length, B – breadth, M – length of membranous strip, S – distance from the strip to the basal lobe.
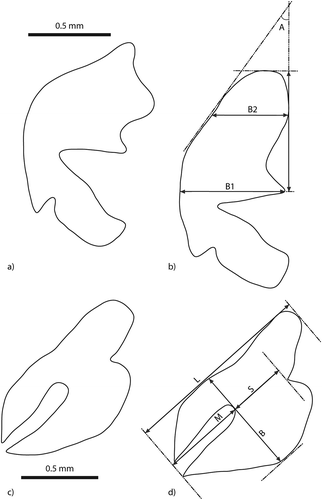
Table I. Overview of Pterostichus rhaeticus and Pterostichus nigrita individuals used in morphometric analysis with respect to their origin from Croatian peatlands.
Additionally, we measured body length (T – the distance from the front margin of the labrum to the apical part of the elytrae), length of elytrae (L – the distance from the top of the scutellum to the apical part of the elytrae), width of both elytrae 1 (W1 – the distance between the left and right lateral margins of the elytrae at the apical part of the scutellum), and width of both elytrae 2 (W2 – the distance between the left and right lateral margins of the elytrae at their widest part, usually the posterior third of the elytrae). We calculated the ratio between total body length and width of both elytrae (T/W1 and T/W2) and the ratio between length and width of both elytrae (L/W1 and L/W2). In total, 43 individuals of P. rhaeticus (17 males and 26 females) and 89 individuals of P. nigrita (39 males and 50 females) were measured. All proportions were measured using an eyepiece micrometer on a Zeiss Stemi 2000 microscope.
In addition, individuals of P. rhaeticus recorded at Croatian peatlands were compared with specimens collected in the Czech Republic, Šumava Mts., South Bohemia (leg. J. Blizek and det. V. Křivan). All collected individuals from Croatian peatlands and those from the Czech Republic are deposited in the first author’s collection (Croatia, Zagreb).
Data analyses
Data of morphometric features were tested for normality using a Shapiro-Wilk W test. Differences in each morphometric feature (e.g. body length, elytrae length and width, ratio between length and width of elytrae, etc.) between P. rhaeticus and P. nigrita were tested using a Mann-Whitney U test. The same test was applied to determine the differences of sexual dimorphism in both species. Data were analysed using Statistica 10.0 (Statsoft Inc. Citation2010).
Results and discussion
Distribution of Pterostichus rhaeticus at its southern edge
During this survey, P. rhaeticus was found at three peatlands in Croatia, at the peat bogs Dubravica and Đon močvar, and at the fen Jarak (). This species was also recorded in several neighbouring countries, e.g. Italy (Brandmayr et al. Citation2005; Colombetta Citation2012), Slovenia (Koch Citation1986) and Hungary (Löbl & Smetana Citation2003). However, it was not found in the former Yugoslavian countries (except Slovenia) (Drovenik & Peks Citation1999; Ćurčić et al. Citation2007), Bulgaria (Guéorguiev & Guéorguiev Citation1995), Romania (Máthé et al. Citation2005), Albania (Guéorguiev Citation2007) and Greece (Arndt et al. Citation2011). Therefore, this record represents its southeasternmost distribution point.
Morphological separation of Pterostichus rhaeticus and Pterostichus nigrita
P. rhaeticus is generally smaller and more slender than P. nigrita (), which is in accordance with Müller-Motzfeld and Hartmann (Citation1985), Huber and Marggi (Citation1986) and Koch (Citation1986). Total body and elytrae lengths, and also elytrae width, of both females and males of P. rhaeticus are significantly smaller and narrower than in P. nigrita. However, there is no significant difference in the ratio of elytrae length and width (L/W1 and L/W2) and in the ratio of total length and elytrae width (T/W2) between these two species (). Additionally, comparing these morphometric features between both males and females separately resulted in the same trend ().
Table II. Differences in morphological features of Pterostichus rhaeticus and Pterostichus nigrita (means ± standard deviation, SD; minimum and maximum values). Mann-Whitney U test results are shown and asterisks show level of significance; ns – non-significant.
Table III. Differences in morphological features between males and females of Pterostichus rhaeticus and Pterostichus nigrita. Mann-Whitney U test results are shown and asterisks show level of significance; ns – non-significant.
However, the overlap in sizes is considerable, especially at sites where mixed populations occur, e.g. at the Đon močvar bog. Consequently, there were no significant differences in the size of the body (U = 56.0, p > 0.05), elytrae length (U = 58.5, p > 0.05), elytrae width (1: U = 77.5, p > 0.05 and 2: U = 77.0, p > 0.05) and ratio between length of elytrae and width of elytrae (L/W1: U = 65.0, p > 0.05; L/W2: U = 66.5, p > 0.05) of these two species at the Đon močvar bog. The sexes of both species differ considerably in size, i.e. females are longer and wider compared to males (), with the exception of elytrae width measured between the left and right lateral margins of the elytrae at the apical part of the scutellum of P. rhaeticus, where no significant difference between males and females was observed (). In general, individuals of P. rhaeticus in Croatia are longer and wider compared to the individuals of this species in central Europe, e.g. Germany (Müller-Motzfeld & Hartmann Citation1985), but comparable to Swiss populations at lower altitudes (from 200–600 m a.s.l.) (Huber & Marggi Citation1986). This might be caused by a longer period of postembryonic development and more favourable climate conditions (Huber & Marggi Citation1986).
Table IV. Differences in morphological features between males and females of Pterostichus rhaeticus and Pterostichus nigrita (means ± standard deviation, SD; minimum and maximum values). Mann-Whitney U test results are shown and asterisks show level of significance; ns – non-significant.
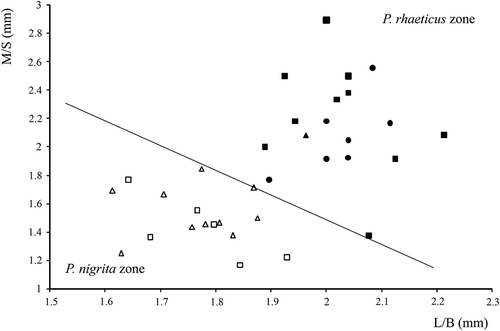
In the studied peatlands, both males and females of P. rhaeticus and P. nigrita were identified. – shows typical right parameres of male P. rhaeticus and P. nigrita, and – shows the specific shape of the eighth abdominal sternite of P. nigrita females. The scatter plot of length/median width of the male right paramere is plotted against the apical angle and shown in . The line that separates the P. rhaeticus and P. nigrita zones is taken from Luff (Citation1990). As can be seen from , the apical angle of the right paramere of P. rhaeticus is less acute compared to that of P. nigrita, ranging from 55° to 87° in the present study. On the contrary, the apical angle of P. nigrita is sharp and usually ranged from 39° to 49°, which is in accordance with Luff (Citation1990). However, three individuals of P. rhaeticus from the Jarak fen fall near the line of separation within the P. nigrita zone, as the result of a narrow apical angle and a less broad transverse apical part of the paramere. This indicates that the apical angle does not absolutely discriminate these two species, as was previously stated by Luff (Citation1990). These three individuals can still be attributed to P. rhaeticus due to the shape of the parameres, the lack of spines on their internal sac and the size of the individuals. Parameres of P. rhaeticus vary, especially in mixed populations (e.g. Jarak fen) as shown in . In such cases, discrimination of males between these two species could be subtle and measurements of parameres are required. shows length/breadth (L/B) ratio plotted against the ratio of the length of the membranous strip/length from the strip to the basal lobe (M/S). The eighth abdominal sternite in P. nigrita is much broader than in P. rhaeticus and this difference is obvious in the majority of cases. Therefore, Luff (Citation1990) and Angus et al. (Citation2000) concluded that this morphological character is reliable for the identification and separation of P. rhaeticus and P. nigrita females.
Figure 3. Male genitalia (right paramere) – Pterostichus rhaeticus: a, Dubravica bog; b, Jarak fen; Pterostichus nigrita: c, Jarak fen.
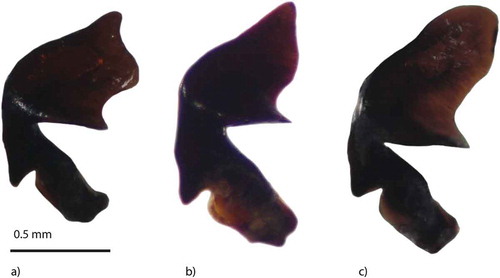
Figure 4. Female structure of eighth abdominal sternite – Pterostichus rhaeticus: a, Đon močvar bog; Pterostichus nigrita: b, Jarak fen.

Figure 5. Male right parameres of Pterostichus rhaeticus and Pterostichus nigrita. Lengths over median width (mm) are plotted against the apical angle (°). Line that separates P. rhaeticus and P. nigrita zones and measurements are based on Luff (Citation1990). P. rhaeticus: Dubravica bog (black circle), Đon močvar bog (black square), Jarak fen (black triangle); P. nigrita: Jarak fen (white triangle), Đon močvar bog (white square).
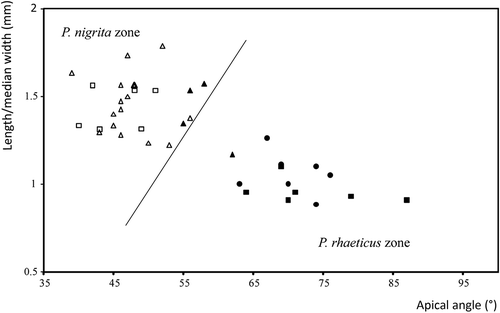
Figure 6. Female eighth abdominal sternite of Pterostichus rhaeticus and Pterostichus nigrita. Length of membranous strip/distance from membranous strip to start of basal lobe (M/S) plotted against length/breadth (L/B). Measurements are based on Luff (Citation1990). P. rhaeticus: Dubravica bog (black circle), Đon močvar bog (black square), Jarak fen (black triangle); P. nigrita: Jarak fen (white triangle), Đon močvar bog (white square).
Biology of Pterostichus rhaeticus
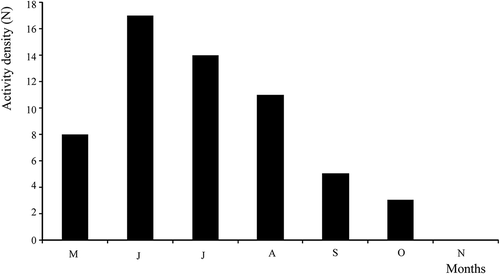
Activity-density was expressed as the total number of beetles trapped each sample month (all studied years included) and plotted against time (). The maximum seasonal activity of P. rhaeticus was observed in June. During the summer, the number of individuals decreased, while no individual was collected in November. In Switzerland, the maximum seasonal activity was observed in May (Luka et al. Citation2009). It seems that P. rhaeticus is a spring breeder, which hibernates as an adult. Similar seasonal activity is observed for P. nigrita, which is also a spring breeder (Thiele Citation1977a,b; Thiele & Fiedler Citation1981; Lindroth Citation1992a; Luka et al. Citation2009). Furthermore, all collected individuals of both species have fully developed wings (macropterous), while in north and central Europe, both macropterous and brachypterous individuals occur (Hůrka Citation1996; Freude et al. Citation2006). According to Gray’s hypothesis, flight ability should be more developed in individuals from more disturbed environments (Gray Citation1989). Therefore, macropterous carabid species are generally associated with disturbed habitats, environmental heterogeneity and low water stability, while brachypterous species are positively correlated with highly stable environments (Brandmayr Citation1983; Venn Citation2007). Additionally, Lindroth (Citation1992b) and den Boer (Citation1971) showed that an old, stable population is likely to be composed of almost entirely brachypterous individuals and, correspondingly, a less stable population should contain more macropterous individuals. As only macropterous individuals were collected in peatlands in Croatia, we can assume that these are fragmented and isolated populations, probably because they are situated on the edge of the species’ distribution range.
Ecology and habitat requirements of Pterostichus rhaeticus
Identifications based on male and female genitalia showed that the population from Dubravica bog is entirely P. rhaeticus ( and ). It is interesting to point out that mixed populations of P. rhaeticus and P. nigrita were found at the Đon močvar bog and at the Jarak fen. Nevertheless, as can be seen from and , both species can be clearly distinguished (with the exception of three individuals of P. rhaeticus at the Jarak fen). Interestingly, P. rhaeticus was not found in recent surveys of wetlands in continental Croatia, e.g. Lonjsko polje (Vujčić-Karlo & Durbešić Citation2004; Brigić et al. Citation2014) or at the Plaški fen (Brigić A., unpublished data). Furthermore, it does not occur in wetland habitats in Dalmatia, e.g. Vransko jezero Nature Park area (Vujčić-Karlo Citation2007), around ponds of the Islands of Dugi otok and Kornat (Vujčić-Karlo Citation2006, Citation2010) or the Neretva River floodplain (Kučinić et al. Citation1996; Durbešić et al. Citation1998). Additionally, neither species was recorded in various flooded forests along the Drava River in the Baranja Region (Tallósi Citation2008).
In the present study, P. rhaeticus was recorded at the open bog centre, in the adjacent forests and at the bog edges. It appears that this species is indifferent to shade, which is in accordance with Hůrka (Citation1996). At the Đon močvar bog, it was found only in the adjacent forest of common alder with elongated sedge (Carici elongatae-Alnetum glutinosae Koch 1926) that surrounds the bog from one side. The soil humidity of this forest is quite high over the whole year (season mean ± standard deviation, SD: 65.70% ± 14.22) and frequently stagnant water was on the ground. At the Dubravica bog, it was recorded in the open bog area and at the bog’s edge and in the adjacent forest in particular. However, at the Jarak fen, it was sampled exclusively in an adjacent young forest dominated by Salix spp. Overall, soil humidity was exceedingly high in the bog and at its edge, but lower in the forests (season mean ± SD: Dubravica bog: centre – 80.44% ± 10.19; edge – 74.47% ± 4.64; forest – 24.81% ± 4.75; Jarak fen: young forest – 52.87% ± 8.45). P. rhaeticus was generally found mainly on acidic soils having low pH values (mean pH values ± SD, Đon močvar bog: 4.82 ± 0.58; forest – 4.68 ± 0.42; Dubravica bog: centre – 4.47 ± 0.09; edge – 4.06 ± 0.12; forest – 4.09 ± 0.12). The only exception is the Jarak fen, where soil pH values are higher (mean pH values ± SD, 7.17 ± 0.03).
Conservation of Pterostichus rhaeticus in the Western Balkans
P. rhaeticus is a very common and abundant species in northern and central Europe (Anderson et al. Citation2000; Freude et al. Citation2006; Luff Citation2007). In the Czech Republic, it is sporadic, but could be locally common (Hůrka Citation1996); however, in Hungary, it is a rare species (Sághy et al. Citation2005). According to present knowledge, this species is rare in Croatia (and most likely in the rest of the Western Balkans) and its distribution is exclusively limited to peatlands and their adjacent habitats. Furthermore, it should be emphasised that populations of P. rhaeticus are rather small in Croatian peatlands, since during this study, only 58 individuals were collected in total. On the contrary, abundance of this species in north European peat bogs is significantly higher (e.g. Främbs et al. Citation2002; Mossakowski et al. Citation2003). These results are in accordance with the abundant-centre hypothesis, according to which species are most abundant in the centre of their geographic range, while they decline in abundance towards the range edges (Hengeveld & Haeck Citation1982). Accordingly, this tyrphophilous species can be considered a true indicator species of peatlands in Croatia. Peatlands are rare, small in size and exceedingly localised habitats in the Western Balkans. Also, they are critically endangered, mainly due to abandonment of traditional land use and changes in water level (meliorations) resulting in rapid vegetation succession (Horvat Citation1939; Hršak Citation1996; Topić & Stančić Citation2006; Šoštarić et al. Citation2012). Therefore, P. rhaeticus should be included in the Red List of the ground beetles of Croatia (Vujčić-Karlo et al. Citation2007) in the category of endangered species, and special attention should be given to the protection of all of its habitats. Conservation of peatland habitats is essential not only for this species, but also for some other rare and endangered plant and animal species, such as Drosera rotundifolia L., Rhynchospora alba (L.) Vahl., peat moss species (Sphagnum L.), Araneus alsine (Walckenaer, 1802), Carabus nodulosus Creutzer, 1799, etc. (Hršak Citation1996; Alegro & Šegota Citation2008; Brigić et al. Citation2009). It should include active measures, such as mowing the M. caerulea grass, removing woody vegetation and preserving the hydrological conditions of peatlands.
Acknowledgements
The authors would like to thank Zorana Sedlar, Renata Šoštarić, Denis Križanić and Stjepan Križanić for their help in the field sampling. We are grateful to Dietrich Mossakowski for providing valuable old literature on peat bogs and to Aleš Bezděk for providing P. rhaeticus specimens from the Czech Republic. Additionally, we would like to thank Sanja Gottstein for her help in measuring morphological features and Ana Previšić for her comments on this manuscript. The study was partially supported by the State Institute for Nature Protection, Republic of Croatia (Grant No. 888/08-3) and Žumberak-Samoborsko gorje Nature Park (PI: R. Šoštarić).
References
- Alegro A, Šegota V. 2008. Florističke i vegetacijske značajke botaničkog rezervata „Đon močvar” u Blatuši. Zagreb: Prirodoslovno-matematički fakultet Sveučilišta u Zagrebu. pp. 1–33.
- Anderson R, McFerran D, Cameron A. 2000. The ground beetles of Northern Ireland (Coleoptera - Carabidae). Belfast: Ulster Museum. pp. 1–246.
- Angus RB, Brown RE, Bryant LJ. 2000. Chromosomes and identification of the sibling species Pterostichus nigrita (Paykull) and P. rhaeticus Heer (Coleoptera: Carabidae). Systematic Entomology 25:325–337. doi:10.1046/j.1365-3113.2000.00108.x.
- Angus RB, Galián J, Wrase DW, Chaladze G. 2009. The western Palaearctic species of the Pterostichus nigrita (Paykull) complex, with the description of a new species from Spain and a new subspecies of P. nigrita from Anatolia (Coleoptera, Carabidae). Nouvelle Revue d’Entomologie 4:297–316.
- Apfelbeck V. 1904. Die Käferfauna des Balkanhalbinsel, mit Berücksichtigung Klein-Asiens und der Insel Kreta. I Band. Berlin: Friedländer und Sohn Verlag. 1–422.
- Arndt E, Schnitter P, Sfenthourakis S, Wrase DW. 2011. Ground Beetles (Carabidae) of Greece. Sofia-Moscow: Pensoft Publishers, Pensoft Series Faunistica (No. 100). pp. 1–393.
- Brandmayr P. 1983. The main axes of the coenoclinal continuum from macroptery to brachyptery in carabid communities of the temperate zone. In: Brandmayr P, Den Boer PJ, Weber F, editors. Ecology of carabids: The synthesis of field study and laboratory experiment. Westphalia: University of Münster. pp. 147–169.
- Brandmayr P, Zetto T, Pizzolotto R. 2005. I Coleotteri Carabidi per la valutazione ambientale e la conservazione della biodiversità. Manuale operativo 34:1–240.
- Brigić A, Alegro A, Šegota V. 2009. Araneus alsinae (Walckenaer, 1802), (Araneidae, Araneae, Arachnida) - a rare and likely threatened spider of the Croatian fauna. Natura Croatica 18:39–48.
- Brigić A, Vujčić-Karlo S, Matoničkin Kepčija R, Stančić Z, Alegro A, Ternjej I. 2014. Taxon specific response of carabids (Coleoptera, Carabidae) and other soil invertebrate taxa on invasive plant Amorpha fruticosa in wetlands. Biological Invasions 16:1497–1514. doi:10.1007/s10530-013-0587-8.
- Buchholz S, Hannig K, Schirmel J. 2009. Ground beetle assemblages of peat bog remnants in Northwest Germany (Coleoptera: Carabidae). Entomologia Generalis 32:127–144.
- Bukejs A, Telnov D. 2007. Materials about the fauna of beetles (Insecta: Coleoptera) of Naujene rural municipality (Daugavpils district, Latvia), part 2. Acta Biologica Universitatis Daugavpiliensis 7:191–208.
- Cole JL, Pollock MG, Robertson D, Holland JP, McCracken DI. 2006. Carabid (Coleoptera) assemblages in the Scottish uplands: The influence of sheep grazing on ecological structure. Entomologica Fennica 17:229–240.
- Colombetta G. 2012. I Coleotteri Carabidi di ambienti umidi e pascoli magri (magredi) del Friuli Venezia Giulia (Italia nord-orientale). Gortania 33:69–96.
- Csiki E. 1946. Die Käferfauna des Karpaten-Beckens. I Band., Allgemeiner Teil und Caraboidea. Budapest: Naturwissenschaftliche Monographien-Kubascha. pp. 1–798.
- Ćurčić SB, Brajković MM, Ćurčić BPM. 2007. The Carabids of Serbia. Belgrade – Vienna: Institute of Zoology, Faculty of Biology, University of Belgrade; Committee for Karst and Speleology, Serbian Academy of Sciences and Arts in Belgrade; Department of Conservation Biology, Vegetation, and Landscape Ecology, Faculty of Life Sciences, University of Vienna; UNESCO MAB Committee Serbia in Belgrade. pp. 1–1083.
- den Boer PJ. 1971. On the dispersal power of carabid beetles and its possible significance. In: den Boer PJ, editor. Dispersal and dispersal power of carabid beetles. LH Wageningen: Miscellaneous papers 8, Agricultural University, pp. 119–137.
- Drovenik B, Peks H. 1999. Catalogus faune. Carabiden der Balkanlander, Coleoptera Carabidae. Schwanfeld: Heinz Peks. pp. 1–122.
- de Warnaffe GDB, Lebrun P. 2004. Effects of forest management on carabid beetles in Belgium: Implications for biodiversity conservation. Biological Conservation 118:219–234. doi:10.1016/j.biocon.2003.08.015.
- Durbešić P, Krulik I, Vujčić-Karlo S, Gjurašin B, Jalžić B, Perović F, Krčmar S, Merdić E. 1998. Faunističko - ekološka studija člankonožaca (Arthropoda) slatkovodnog dijela ušča Neretve. Zagreb: Hrvatsko ekološko društvo. pp. 1–64.
- Erichson WF. 1860. Insecten Deutschlands, I. Band. Berlin: Nicolaische Verlagsbuchhandlung. pp. 1–791.
- Främbs H. 1990. Changes in carabid beetle populations on a regenerating, excavated peat bog in Northest Germany. In: Stork NE, editor. The role of ground beetles in ecological and environmental studies. Andover: Intercept. pp. 157–169.
- Främbs H, Dormann W, Mossakowski D. 2002. Spatial distribution of carabid beetles on Zehlau bog. Baltic Journal of Coleopterology 2:7–13.
- Freude H, Harde K-W, Lohse GA, Klausnitzer B. 2006. Die Käfer Mitteleuropas, Band 2 Adephaga 1: Carabidae (Laufkäfer). Heidelberg/Berlin: Spektrum Verlag. pp. 1–521.
- Ganglbauer L. 1892. Die Käfer von Mitteleuropa I. Vienna: Carl Gerold’s Sohn. pp. 1–557.
- Gaublomme E, Dhuyvetter H, Verdyck P, Desender K. 2005. Effects of urbanisation on carabid beetles in old beech forests. In: Lövei GL, Toft S, editors. European Carabidology 2003. Århus: DIAS Report. pp. 111–123.
- Gigov A, Nikolić V. 1960. Rezultati analize polena na nekim tresavama u Hrvatskoj. Glasnik prirodnjačkog muzeja u Beogradu, Serija B 15:3–26.
- Grandchamp A-C, Bergamini A, Stofer S, Niemelä J, Duelli P, Scheidegger C. 2005. The influence of grassland management on ground beetles (Carabidae, Coleoptera) in Swiss montane meadows. Agriculture, Ecosystems and Environment 110:307–317. doi:10.1016/j.agee.2005.04.018.
- Gray JS. 1989. Effects of environmental stress on species rich assemblages. Biological Journal of the Linnean Society 37:19–32. doi:10.1111/j.1095-8312.1989.tb02003.x.
- Guéorguiev B. 2007. Annotated Catalogue of the Carabid Beetles of Albania (Coleoptera: Carabidae). Sofia-Moscow: Pensoft Publishers, Pensoft Series Faunistica (No. 64). pp. 1–243.
- Guéorguiev V, Guéorguiev B. 1995. Catalogue of the ground-beetles of Bulgaria (Coleoptera: Carabidae). Sofia-Moscow: Pensoft Publishers, Pensoft Series Faunistica (No. 2). pp. 1–279.
- Hengeveld R, Haeck J. 1982. The distribution of abundance. I. Measurements. Journal of Biogeography 9:303–316. doi:10.2307/2844717.
- Horvat I. 1939. Prilog poznavanju cretova u Hrvatskom zagorju. Geografski glasnik 8–10:67–79.
- Hršak V. 1996. Vegetation succession at acidic fen near Dubravica in the Hrvatsko zagorje region. Natura Croatica 5:1–10.
- Huber C, Marggi W. 1986. Verbreitung von Pterostichus nigrita (Payk.) und Pterostichus rhaeticus Heer (Coleoptera, Carabidae) in der Schweiz. Mitteilungen der Schweizerischen Entomologischen Gesellschaf 59:439–445.
- Hůrka K. 1996. Carabidae of the Czech and Slovak Republics. Zlin: Kabourek. pp. 1–565.
- Koch D. 1986. Morphological-physiological studies on “Pterostichus nigrita” (Col., Carab.), a complex of sibling species. In: den Boer PJ, Luff ML, Mossakowski D, Weber F, editors. Carabid beetles, their evolution, natural history and classification. Stuttgart/New York: Gustav Fischer. pp. 267–279.
- Koch D, Thiele HU. 1980. Zur ökologisch-physiologischen Differenzierung und Speziation der Laufkäfer-Art Pterostichus nigrita (Coleoptera: Carabidae. Entomologia Generalis 6:135–150.
- Kučinić M, Perović F, Vujčić-Karlo S. 1996. Entomološka istraživanja donjeg toka rijeke Neretve. Zagreb: Hrvatski prirodoslovni muzej. pp. 1–70.
- Lindroth CH. 1992a. Ground beetles (Carabidae) of Fennoscandia, specific knowledge regarding the species, Part I. New Delhi: Amerind Publishing Co. Pvt. Ltd. pp. 1–630.
- Lindroth CH. 1992b. Ground Beetles (Carabidae) of Fennoscandia, General analysis with a discussion on biogeographic principles, Part III. New Delhi: Amerind Publishing Co. Pvt. Ltd. pp. 1–814.
- Löbl I, Smetana A. 2003. Catalogue of Palaearctic Coleoptera. Vol. 1. Stenstrup: Apollo Books. pp. 1–819.
- Luff ML. 1990. Pterostichus rhaeticus Heer (Col., Carabidae), a British species previously with P. nigrita (Paykull). Entomologist’s Monthly Magazine 126:245–249.
- Luff ML. 2007. The Carabidae (ground beetles) of Britain and Ireland, Handbooks for the identification of British Insects. St. Albans: Royal Entomological Society. pp. 1–247.
- Luka H, Marggi W, Huber C, Gonseth Y, Nagel P. 2009. Coleoptera, Carabidae: ecology, atlas, Fauna Helvetica 24. Neuchâtel: Schweizerische Entomologische Gesellschaft. pp. 1–677.
- Máthé I, Urák I, Balog A, Balázs E. 2005. The community structure of the ground dwelling carabid beetles (Coleoptera: Carabidae) and spiders (Arachnida: Araneae) in peat bog “Mohos” (Transylvania, Romania. Entomologica Romanica 10:37–42.
- Mossakowski D, Främbs H, Lakomy W. 2003. The Carabid and Staphilinid fauna of raised bogs. A comparison of Northwest Germany and the Baltic region. Baltic Journal of Coleopterology 3:137–144.
- Müller-Motzfeld G, Hartmann M. 1985. Zur Trennung von Pterostichus rhaeticus HEER and P. nigrita PAYK. (Col., Carabidae). Entomologische Nachrichten und Berichte 29:13–17.
- Peus F. 1928. Beiträge zur Kenntnis der Tierwelt nordwestdeutscher Hochmoore. Eine ökologische Studie. Insekten, Spinnentiere (teilw.), Wirbeltiere. Zeitschrift für Morphologie und Ökologie der Tiere 12:533–683. doi:10.1007/BF00403122.
- Raupach MJ, Astrin JJ, Hannig K, Peters MK, Stoeckle MY, Wägele J-W. 2010. Molecular species identification of Central European ground beetles (Coleoptera: Carabidae) using nuclear rDNA expansion segments and DNA barcodes. Frontiers in Zoology 7:26. doi:10.1186/1742-9994-7-26.
- Roubal J. 1934. Die Coleopterenwelt (Tyrphobionte, Tyrphophile, Tyrphoxene, etc.) der Treboner (Wittingauer) Moore. Folia Zoologica Hydrobiologica 7:56–97.
- Sághy Z, Bérces S, Takács A. 2005. Long-term monitoring of ground beetles (Coleoptera, Carabidae) in a Hungarian wetland area. In: Lövei GL, Toft S, editors. European Carabidology 2003. Århus: DIAS Report. pp. 255–263.
- Schwerk A, Sałek P, Duszczyk M, Abs M, Szyszko J. 2006. Variability of carabidae in time and space in open areas. Entomologica Fennica 17:258–268.
- Skłodowski J. 2006. Anthropogenic transformation of ground beetle assemblages (Coleoptera: Carabidae) in Białowieża Primeval Forest, Poland: From primeval forests to managed woodlands of various ages. Entomolologica Fennica 17:296–314.
- Šoštarić R, Sedlar Z, Mareković S. 2012. An endangered rich fen habitat along the Jarak stream (Nature Park Žumberak-Samoborsko gorje, Croatia. Natura Croatica 21:335–348.
- Spitzer K, Bezděk A, Jaroš J. 1999. Ecological succession of a relict Central European peat bog and variability of its insect biodiversity. Journal of Insect Conservation 3:97–106. doi:10.1023/A:1009634611130.
- Statsoft Inc. 2010. Statistica (Date Analysis Software System), Version 10. http://www.statsoft.com/
- Sushko G. 2007. Beetles (Coleoptera) of raised bogs in North-Western Belarus (Belarusian Land O’Lakes). Baltic Journal of Coleopterology 7:207–214.
- Tallósi B. 2008. Population-level baseline surveying and preparative investigations for the monitoring of carabid beetles (Coleoptera, Carabidae) in areas along the Drava River and Baranja (Croatia). In: Purger JJ, editor. Biodiversity studies along the Drava River. Pécs: University of Pécs. pp. 165–220.
- Thiele HU. 1977a. Measurement of day-length as a basis for photoperiodism and annual periodicity in the carabid beetle Pterostichus nigrita F. Oecologia 30:331–348. doi:10.1007/BF00399765.
- Thiele HU. 1977b. Carabid beetles in their environments. Berlin: Springer-Verlag. pp. 1–369.
- Thiele HU, Fiedler EP. 1981. Long-term experiments on the development of the carabid beetle Pterostichus nigrita Paykull under simulated climatic conditions (photoperiod and temperature) of different latitudes. Zoologische Jahrbücher: Abteilung für Systematik, Geographie und Biologie der Tiere 108:441–461.
- Topić J, Stančić Z. 2006. Extinction of fen and bog plants and their habitats in Croatia. Biodiversity and Conservation 15:3371–3381. doi:10.1007/s10531-005-4874-2.
- Venn S. 2007. Morphological responses to disturbance in wing-polymorphic carabid species (Coleoptera: Carabidae) of managed urban grasslands. Baltic Journal of Coleopterology 7:51–59.
- Vujčić-Karlo S. 2006. Inventarizacija trčaka – Carabidae, strizibuba – Cerambycidae i belegara – Scarabaeidae (kornjaši – Coleoptera) u parku prirode “Telašćica. Zadar: Narodni muzej Zadar. pp. 1–39.
- Vujčić-Karlo S. 2007. Inventarizacija trčaka (Coleoptera, Carabidae) u Parku prirode Vransko jezero. Zadar: Narodni muzej Zadar. pp. 1–71.
- Vujčić-Karlo S. 2010. Inventarizacija trčaka – Carabidae (kornjaši - Coleoptera) na Tarcu u Nacionalnom parku “Kornati. Zadar: Narodni muzej Zadar. pp. 1–50.
- Vujčić-Karlo S, Brigić A, Šerić-Jelaska L, Kokan B, Hrašovec B. 2007. Crveni popis ugroženih vrsta trčaka (Coleoptera, Carabidae) u Hrvatskoj, Available: http://www.dzzp.hr/publikacije/crveni_popis_trchci.pdf. Accessed Oct 2013 15.
- Vujčić-Karlo S, Durbešić P. 2004. Ground beetle (Coleoptera: Carabidae) fauna of two oak woods with two different water balances. Acta Entomologica Slovenica 12:139–150.
- Zahn A, Juen A, Traugott M, Lang A. 2007. Low density cattle grazing enhances arthropod diversity of abandoned wetland. Applied Ecology and Environmental Research 5:73–86.