Abstract
The population structure and dynamics of Orchestia montagui Audouin, 1826 (Crustacea: Amphipoda: Talitridae) were studied from the supralittoral zone of Bizerte lagoon for 1 year. Monthly field surveys were performed from July 2006 to June 2007. Individuals sampled were categorized by sex, size and abundance on each sampling date. Abundance varied during the study period, with a noticeable decrease in November. Ovigerous females were found throughout the year. The sex ratio changed throughout the year but, overall, the population was male biased. Analysis of the size frequency polymodal distribution suggested the presence of six cohorts on the first sampling date and 10 new ones during the next 11 months. Lifespan was estimated at 6–7 months. Cohorts born in summer and autumn tend to live longer than those born in winter. At this study site, O. montagui appeared as a semiannual species, with iteroparous females producing at least two broods, and exhibited a bivoltine life cycle.
Introduction
Orchestia montagui Audouin, 1826, a talitrid amphipod species, commonly occurs on sandy and rocky Mediterranean coasts, within decaying seagrass, beach wrack or gravel (Pavesi et al. Citation2013). The species is generally associated with slow-drying sediments that are covered with plant debris, cobbles and/or boulders (Lowry & Fanini Citation2013). The association of the species and sediment type is similar for other talitrid species (Lowry & Fanini Citation2013) and reflects the ecological distribution, as described by Bousfield (Citation1984).
A number of studies have focused on the population genetic structure of O. montagui in relation to its characteristics within a colonized environment. However, previous studies based on allozymes have revealed a poor genetic structure of O. montagui populations (De Matthaeis et al. Citation1995, Citation2000a, Citation2000b; Angelini et al. Citation1996; Cervelli et al. Citation2006). More recent studies that were based on more variable and informative markers (cytochrome oxidase subunit I and microsatellites) showed signatures of local differentiation (Pavesi et al. Citation2012, Citation2013) and differed from previous hypotheses. Although O. montagui carries out short-distance dispersal related to its semi-terrestrial life style, surface marine currents with rafting via seagrass wrack may function as a mean of passive dispersal and can explain the species population genetic structure at a large geographical scale (Pavesi et al. Citation2013). These authors also suggested a phylogeographic species complex based predominately on marine forces. They hypothesized intrinsic temporal environment instability (e.g., due to the removal of wrack through beach cleaning, tourism, wave action) that could also play a role in the abundance of populations, eventually leading to frequent population crashes and genetic bottlenecks.
The distribution, orientation, locomotor rhythm and biology of many talitrid populations have been studied at several Tunisian sites including the Bizerte lagoon (Jelassi & Nasri-Ammar Citation2012, Citation2013; Jelassi et al. Citation2012, Citation2013), Bizerte Corniche (Ayari & Nasri-Ammar Citation2012a), Barkoukech (Nasri-Ammar & Morgan Citation2006), Zouaraa (Charfi-Cheikhrouha et al. Citation2000; Scapini et al. Citation2002; Marques et al. Citation2003; Bouslama et al. Citation2009), Korba (Nasri-Ammar & Morgan Citation2005; Bouslama et al. Citation2007) and Gabes (Ayari & Nasri-Ammar Citation2011, Citation2012b). In addition, the population dynamics and structure have focused on populations of Orchestia mediterranea (Louis Citation1977; Wildish Citation1979), Deshayesorchestia quoyana (Marsden Citation1991), Talitrus saltator (Williams Citation1978; Pavesi et al. Citation2007) Orchestia gammarellus (Persson Citation1999; Pavesi et al. Citation2007), Platorchestia platensis (Pavesi et al. Citation2007) and Macarorchestia remyi (Pavesi & De Matthaeis Citation2009).
Population size, reproductive strategies and potential for dispersal between habitats are usually considered to reflect evolutionary fitness (Marques et al. Citation2003). However, there is lack of knowledge regarding the reproductive biology and population dynamics of O. montagui.
The objective of this study was to investigate how O. montagui can adapt to harsh environments, specifically where animals have to cope continuously with the constraints of this changing habitat (Marques et al. Citation2003). Furthermore, we investigate the reproductive strategy of O. montagui compared to other sympatric species, and to also access the annual cycle of growth of local populations.
Material and methods
Study area
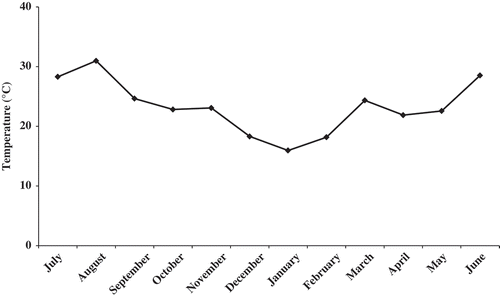
The Bizerte lagoon is a coastal lagoon, in northeastern Tunisia, between 37°8–14′N and 9°46–56′E. It is connected to the Mediterranean Sea by a canal that is 11.3 km in length, 300 m wide and 13 m deep. It is also connected to the Garaet Ichkeul Lagoon through oued Tinja. In winter, a surplus of fresh water is discharged from the Garaet Ichkeul to Bizerte Lagoon through oued Tinja, whereas during summer this oued allows seawater to enter Garaet Ichkeul across the Bizerte Lagoon. Specimens were sampled within the supralittoral zone of Bizerte Lagoon, at Menzel Jmil (37°13′8′′N 09°55′1′′E) (). The collection site was divided into five zones based upon plant associations that extend from the shoreline to a road. These five zones and respective alga associations were categorized as: zone 1 (Cymodocea), zone 2 (Suaeda maritima), zone 3 (Suaeda maritima, Salicornia arabica, Obione portulacoides), zone 4 (Obione portulacoides) and zone 5 (dry ground zone). Temperature was measured in situ using a thermo-hygrometer. It varied between 15.95°C in January and 30.98°C in August ().
Sampling and laboratory procedures
Monthly sampling of Orchestia montagui occurred from July 2006 to June 2007. Specimens were collected by hand in the morning, during a fixed period (2 h each time), under Cymodocea, wood remains, sheets and empty bottles, and between the roots of plants, in particular Suaeda maritima. They were collected with the respective substratum and kept in polystyrene boxes that had been perforated to provide ventilation.
In the laboratory, specimens were preserved in 70% ethanol. Cephalic length (CL) was measured using a binocular microscope equipped with a micrometrical ocular lens, calibrated with objective micrometers.
Individuals were then sexed (male, female, or immature) by the presence of copulatory appendages in males, or the presence of oostegites, with or without setae, in females. Females were then divided into non-reproductive (without setose oostegites) and reproductive, the latter including ovigerous females (with setose oostegites or with eggs/embryos) and females with an empty marsupium (assuming that they had just released eggs). In the absence of any secondary sexual dimorphic features, individuals were considered to be immature. Species identification was carried out using the keys of Chevreux and Fage (Citation1925) and Ruffo (Citation1993). It is worth noting that according to our previous results, different co-existing species occupy different niches; for example, O. montagui was generally found closer to the shoreline than the other species (Jelassi et al. Citation2012). In addition, because of the uncertainty of the determination of juveniles, which should be a challenge for further research, their number was estimated proportionally to the number of ovigerous females only. Despite the fact that this is a rough estimation to which the risk of a misidentification has to be added, in our opinion, this is the most appropriate procedure.
Eggs present in the marsupium were counted in females that did not undergo any egg loss. For the sex ratio, the hypothesis of 1:1 was tested with a Chi square test.
Field growth rates were estimated by tracking recognizable modal distributions in the population using size–frequency of distributions (0.04-mm length classes) in successive sample dates, following a methodology previously described (Pardal et al. Citation2000; Marques et al. Citation2003). Modal distributions are assumed to result from pulses in recruitment, conventionally referred to as cohorts. Size–frequency analysis was carried out using the probability paper method (Harding Citation1949), as performed by Cassie (Citation1954, Citation1963). Computations used the ANAMOD software (Nogueira Citation1992), and the reliability of the distribution separation method was tested with both χ2 and G tests (p ≤ 0.05).
Results
Population structure
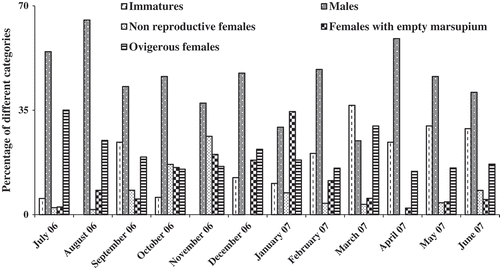
A total of 2946 specimens of Orchestia montagui were collected. The number of animals collected monthly ranged between 99 in November 2006 and 388 in July 2006. The monthly change in population structure showed that immature individuals were present throughout the year except in August and in November. The highest percentage of immature individuals occurred in March 2007 (36.63%). The overall percentage of immature individuals was 17.99% (N = 530). For males, the greatest percentage was found in April (58.99%) with an overall percentage of 46.78% (N = 1378) (, ).
Table I. Categories and sex ratio of the Orchestia montagui population in the supralittoral zone of Bizerte lagoon, Tunisia.
Ovigerous females were present throughout the year, with one peak of abundance observed in July (35.05%). The percentage of females with an empty marsupium varied between 2.21% and 34.55% in April and in January, respectively. Whereas non-reproductive females were absent in December and April, their greatest percentage (26.26%) was recorded in November. For females that were either ovigerous, with an empty marsupium, or non-reproductive, their overall percentages were 20.74% (N = 611), 8.93% (N = 263) and 5.57% (N = 164), respectively (, ).
Relationship between cephalic length and body length
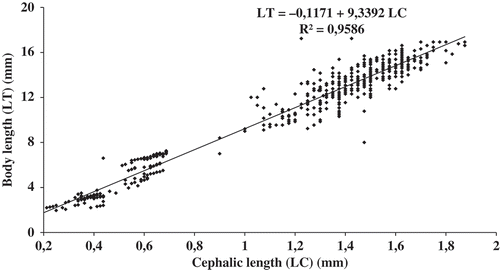
Since CL is a more reliable measurement than body length (LT) due to the curvature of the body, CL was measured for each specimen. However, the body and cephalic lengths of approximately 150 individuals were measured accurately to enable all CL to be converted to LT. For O. montagui, the relationship of LT to CL was: LT = –0.1171 + 9.3392 CL (). The mean cephalic length of males, ovigerous females, females with an empty marsupium and non-reproductive females was 1.52 ± 0.13 mm (0.75–1.95 mm), 1.35 ± 0.15 mm (0.93–1.92 mm), 1.29 ± 0.17 mm (0.88–1.90 mm) and 1.20 ± 0.20 mm (0.75–1.80 mm), respectively.
Reproductive activity and sex ratio
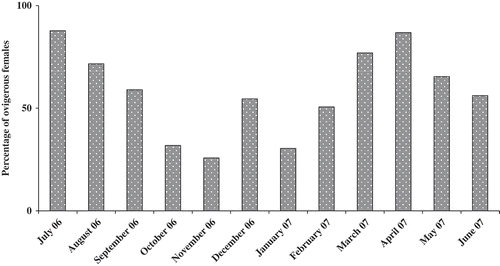
Breeding activity was assessed by estimating the percentage of ovigerous females (number of ovigerous females divided by total female number × 100). Breeding occurred throughout the study period, with two peaks recorded in July 2006 and April 2007 (87.7 and 86.79%, respectively), suggesting a continuous reproduction of O. montagui during the study period ().
Figure 5. Monthly variation of ovigerous females (%) of Orchestia montagui in the supralittoral zone.
The overall sex ratio was 1.33♂:1♀ (57% were males and 43% females). A χ2 test showed a highly significant difference between the sexes (χ2 = 95.70, df = 1, p < 0.0001). Seasonal analysis of the sex ratio revealed that it was equal in winter and autumn, but was highly male biased in spring (1.9:1) and summer (1.5:1). Furthermore, except for October and December, χ2 tests revealed highly significant differences in the monthly sex ratios ().
Fecundity
Fecundity was estimated from the number of eggs inside the marsupium. The lowest value, one egg, was found in a female whose body length was 10.50 mm, whereas the highest value, 17 eggs, was observed in a female with a body length of 12.50 mm. The mean number of eggs was estimated at nine. In addition, no significant correlation between fecundity and the body length was found (R2 = 0.0074).
Population dynamics
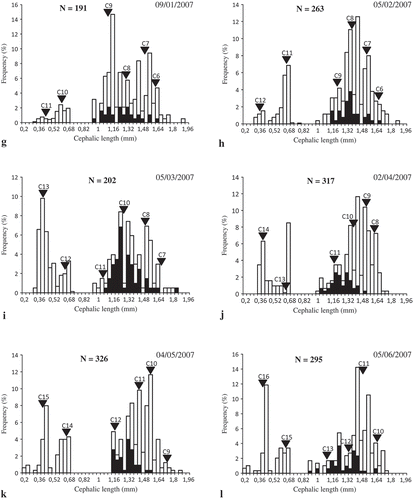
Size–frequency distributions were calculated to determine cohorts in the O. montagui population. Six cohorts were recognized in July 2006 and 10 new cohorts were observed during the rest of the study period (). These new cohorts appeared on 3 August (cohort 7), 6 September (cohort 8), 4 October (cohort 9), 4 December (cohort 10), 9 January (cohort 11), 5 February (cohort 12), 5 March (cohort 13), 2 April (cohort 14), 4 May (cohort 15) and 5 June (cohort 16).
Figure 6. Size-frequency distribution of Orchestia montagui from the supralittoral zone. Inverted triangle indicates average cephalic length (CL) of the numbered cohorts or groups of cohorts; black bar indicates ovigerous females; a-l represent the size-frequency distribution of O. montagui in each month.
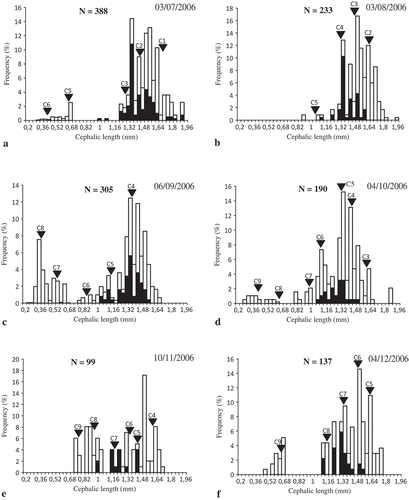
The minimum CL varied from 0.37 to 0.42 mm (LT from 3.34 to 3.81 mm). The maximum CL ranged between 1.62 and 1.73 mm (LT between 15.01 and 16.04 mm).
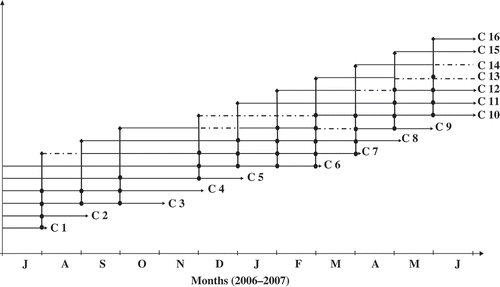
Growth was found to be continuous throughout the lifespan and varied with season. Growth rate was quicker in the early phases, and appeared to be higher during summer and autumn. Moreover, cohorts born during the summer and autumn (cohorts 7, 8 and 9) tended to have a longer lifespan than those born in winter (cohort 10). Taking into consideration growth rate and the disappearance of cohorts, lifespan was estimated to be 6–7 months ().
Figure 7. Estimated growth and life span of Orchestia montagui cohorts or groups of cohorts (average cephalic length, CL ± standard deviation) in the supralittoral zone. Broken lines indicate probable cohort merging or cohort evolution in time.
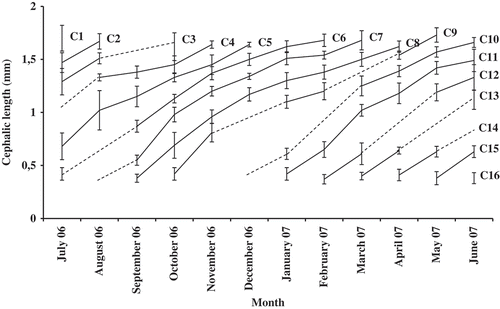
Size frequency analyses identified cohorts to which ovigerous females belonged, as well as their contribution to recruitment (). Females of C1, C2, C3 and C4 gave birth to C7, whereas only females of C3 and C4 gave birth to C8 and also to C9, along with females of C5. Then, C3, C4 and C5 disappeared in October, November and December, respectively.
Cohort C10 was the outcome of ovigerous females of C5, C6 and C7, whereas cohort C11 was the offspring of C6, C7 and C8. Ovigerous females of these cohorts (6, 7 and 8) along with those of C9 and C10, gave birth to C12 and C13. Females of cohorts C7, C8, C9 and C10 gave birth to C14. Cohort C15 was the offspring of C9, C10, C11 and C12. Cohort C16 was the outcome of C10, C11, C12 and C13.
Hence, the life cycle characteristics of O. montagui living in the supralittoral zone of Bizerte lagoon may be summarized as follows: (a) semiannual species – females appear to produce two to five broods per year; (b) iteroparous females – females appear to reproduce twice or more in their lifetime; (c) bivoltine life cycle – the population produces two generations per year; (d) variable life span – the cohorts born in summer and autumn live longer than those born in winter.
Discussion
The temporal abundance for a Tunisian population of Orchestia montagui living in the supralittoral zone of Bizerte lagoon was variable. Abundance was lowest in November and December (winter), with abiotic conditions, especially the decrease of temperature, contributing to it.
O. montagui showed continuous reproduction, which is similar to what was reported for O. mediterranea in the estuary of Bou Regreg in Morocco (Elkaïm et al. Citation1985), and for Deshayesorchestia capensis (Van Senus Citation1988), P. platensis (Ciavatti Citation1989), O. gammarellus and O. mediterranea all occurring also in the supralittoral zone of Bizerte lagoon (unpublished data).
Orchestia gammarellus, collected from the Smir lagoon in Morocco, offers a good example of intraspecific plasticity. This population showed, for the first time, a seasonal pattern of reproduction (Aksissou & Elkaïm Citation1996) as well as continuous reproduction throughout the year, especially after the construction of a dam within Smir and the harbour of Kabila (Aksissou et al. Citation1998). Other talitrid species such as T. saltator (Marques et al. Citation2003; Bouslama et al. Citation2007), Britorchestia brito (Gonçalves et al. Citation2003) and Deshayesorchestia deshayesii (Ayari-Akkari et al. Citation2014 in press) reproduced following a seasonal pattern. Seasonal variation was also observed in a population of T. saltator on the Atlantic coast (Williams Citation1978), in O. gammarellus in the Baltic Sea (Persson Citation1999) and in O. mediterranea in the Medway estuary in the North of Europe. The non-reproductive rest period was observed from October to mid-April (Wildish Citation1979), but a population from Languedoc ponds (southern Europe) had its rest period from November to mid-March (Louis Citation1977). The study of Italian and Portuguese populations by Marques et al. (Citation2003) showed that recruitment occurs over seven months from early March to late September. Seasonal reproduction occurred from February to November for D. deshayesii in the Ionian Sandy Beach in Southern Italy (Prato et al. Citation2009). Williams (Citation1978) showed that populations of T. saltator from Britannic Island had a much shorter reproductive period, from May to the end of August.
The duration of reproduction may be correlated with geographic gradient, temperature and photoperiod (Williamson Citation1951; Marques et al. Citation2003). The latter authors showed that in populations of T. saltator, recruitment periods were shorter in the Atlantic and northern Mediterranean and longer in the southern coasts of Mediterranean.
According to Morritt and Stevenson (Citation1993), temperature is the critical factor controlling reproduction in O. gammarellus, which starts when temperature reaches 10°C. Furthermore, Dias and Sprung (Citation2003) showed that temperature must override day length in inducing reproduction. In the present study, breeding activity was observed throughout the year and was maintained at a minimum temperature of 15.95°C.
Populations that are either male- or female-biased may be affected by factors associated with their life cycle, including mortality, as well as longevity and the relative growth rates of the sexes (Hamilton Citation1967; Wenner Citation1972). Sex ratios of O. montagui revealed a clear male dominance. This result was similar to that found in a UK population of O. gammarellus (Wildish Citation1979), in the Atlantic (Lavos, Portugal) and Mediterranean (Collelungo, Italy) within populations of T. saltator (Marques et al. Citation2003), and in populations of B. brito occurring in the Atlantic (Gonçalves et al. Citation2003). In addition, populations of O. gammarellus, O. mediterranea and O. aestuarensis from the European Atlantic and Mediterranean coasts showed a male dominance (Ginsburger-Vogel & Magniette-Mergault Citation1981; Ginsburger-Vogel Citation1989, Citation1991). Reasons for a male-biased sex ratio remain unclear, although the presence of endoparasites has been hypothesized to explain deviation towards male dominance (Ginsburger-Vogel & Magniette-Mergault Citation1981; Ginsburger-Vogel Citation1989, Citation1991). Nevertheless, other talitrid populations are characterized by a female-biased sex ratio; this is the case for T. saltator at Zouaraa and Korba in Tunisia (Marques et al. Citation2003; Bouslama et al. Citation2007), B. brito at Zouaraa (Gonçalves et al. Citation2003), D. deshayesii (Ayari-Akkari et al. Citation2014 in press), O. mediterranea (Elkaïm et al. Citation1985), O. gammarellus (Jones & Wigham Citation1993; Aksissou & Elkaïm Citation1996; Persson Citation1999; Dias & Sprung Citation2003), and Deshayesorchestia capensis (Van Senus Citation1988).
The fecundity of O. montagui (a mean of nine eggs) in Tunisia was remarkably lower than that of O. gammarellus from Morocco (15 eggs) (Aksissou & Elkaïm Citation1996) and England (19 eggs) (Wildish Citation1979). This result can be explained by the higher survival rate of O. montagui. Moreover, no correlation was found between fecundity and female length, contrary to T. saltator (Williams Citation1978; Bouslama Citation2009) and Deshayesorchestia capensis (Van Senus Citation1988) in which fecundity was positively correlated with female length.
The results of size frequency distributions of O. montagui populations evidenced the presence of 10 new cohorts during 12 months of monitoring. Studying populations of T. saltator from Korba beach during successive years, Bouslama et al. (Citation2007) revealed the presence of nine and eight new cohorts during the first and second year, respectively. During the same time interval, 12 months, six new cohorts were identified at Zouaraa (Marques et al. Citation2003). On Bizerte beach, Ayari-Akkari et al. (Citation2014 in press) revealed the presence of 11 new cohorts for D. deshayesii during the 18 months of their study. At Collelungo, Italy, and at Lavos, Portugal, eight and five cohorts, respectively, were identified (Marques et al. Citation2003).
The lifespan of O. montagui, which had never been studied in Tunisia, was estimated to last from 6 to 7 months. This is similar to that of D. deshayesii (5–7 months; Ayari-Akkari et al. Citation2014 in press), T. saltator (6–9 months; Marques et al. Citation2003; Bouslama Citation2009), B. brito collected from Zouaraa in Tunisia (5–8 months) and from Quiaios in Portugal (6–9 months) (Gonçalves et al. Citation2003), and O. mediterranea in an estuary of Bou Regreg in Morocco (5–9 months; Elkaïm et al. Citation1985). However, some talitrids, such as O. gammarellus, live as long as about 12 months (Jones & Wigham Citation1993; Dias & Sprung Citation2003). In talitrid species, it seems that there is likely a cline in geographical variation, with lifespan increasing from Mediterranean populations to northern European populations (Marques et al. Citation2003).
Our results on the life history traits of O. montagui, in particular, continuous reproduction, male-biased sex ratio, lower fecundity, short life span and seasonal variation in growth rate, depend upon both species and locality. They can also be explained through a combination of factors, namely (a) genetic determinants of the growth exponent, (b) the ability of the individual to exploit resources, (c) environmental conditions, (d) birth date in seasonal environments, (e) the timing of resource investments into reproduction and (f) the timing of reproduction in iteroparous species.
Acknowledgements
We wish to thank Adam Baldinger, Curatorial Associate at the Museum of Comparative Zoology (Harvard University), for his constructive remarks and for revising the English of the manuscript.
References
- Aksissou M, Elkaïm B. 1996. Cycle reproducteur d’une population d’Orchestia gammarellus (Crustácea, Amphipoda) dans le lac Smir (Maroc). Mediterranea. Serie d’estudios biológicos 15:5–10.
- Aksissou M, Errami M, Ménioui M. 1998. Impact des aménagements d’une zone humide Méditerranéenne (Lac Smir, Maroc) sur Orchestia gammarellus (Crustacea, Amphipoda, Talitridae). Rapport Commission International pour l’exploration scientifique de la Mer Méditerranée 35:510–511.
- Angelini E, Longo N, Zane L, Bisol PM. 1996. Biodiversità in anfipodi Talitridi da un punto di vista ecogenetico. Biology Marine Mediterranean 3:115–119.
- Ayari A, Nasri-Ammar K. 2011. Distribution and biology of amphipods on two geomorphologically different sandy beaches in Tunisia. Crustaceana 84:591–599. doi:doi:10.1163/001121611X566776.
- Ayari A, Nasri-Ammar K. 2012a. Seasonal variation of the endogenous rhythm in two sympatric amphipods: Talitrus saltator and Talorchestia deshayesii from Bizerte beach (northern Tunisia). Biological Rhythm Research 43:515–526. doi:10.1080/09291016.2011.613620.
- Ayari A, Nasri-Ammar K. 2012b. Locomotor rhythm phenology of Talitrus saltator from two geomorphologically different beaches of Tunisia: Bizerte (North of Tunisia) and Gulf of Gabes (South of Tunisia). Biological Rhythm Research 43:113–123. doi:10.1080/09291016.2010.537446.
- Ayari-Akkari A, Jelassi R, Khemaissia H, Nasri-Ammar K. 2014. Life history of the sandy beach amphipod Deshayesorchestia deshayesii (Crustacea: Talitridae) from Bizerta beach (North of Tunisia). Invertebrate Reproduction and Development 58:269–277. doi:10.1080/07924259.2014.920422.
- Bousfield EL. 1984. Recent advances in the systematics and biogeography of landhoppers (Amphipoda: Talitridae) of the Indo-Pacific Region. Bishop Museum Special Publication 72:171–210.
- Bouslama MF. 2009. Etude comparative de la dynamique et de la génétique de populations de quelques espèces de Talitridés en Tunisie (Crustacea, Amphipoda). Thèse de doctorat Université de Tunis, Tunisie. 208 pp.
- Bouslama MF, El Gtari M, Charfi-Cheikhrouha F. 2009. Impact of environmental factors on zonation, abundance, and other biological parameters of two Tunisian populations of Talitrus saltator (Amphipoda, Talitridae). Crustaceana 82:141–157. doi:10.1163/156854008X380255.
- Bouslama MF, Neto JM, Charfi-Cheikhrouha F, Marques JC. 2007. Biology, population dynamics and secondary production of Talitrus saltator (Amphipoda, Talitridae) at Korba beach (east coast of Tunisia). Crustaceana 80:1103–1119. doi:10.1163/156854007782008595.
- Cassie RM. 1954. Some uses of probability paper in the analysis of size–frequency distributions. Australian Journal of Marine and Freshwater Research 3:513–522.
- Cassie RM. 1963. Tests of significance for probability paper analysis. New Zealand Sciences Review 6:474–482.
- Cervelli M, Bortolomei A, Linertini A. 2006. Differenziazione genetica tra popolazioni di Orchestia montagui delle coste sarde. Biology Marine Mediterranean 13:10675–11071.
- Charfi-Cheikhrouha F, El Gtari M, Bouslama MF. 2000. Distribution and reproduction of two sandhoppers, Talitrus saltator and Britorchestia brito from Zouaraa (Tunisia). Polskie Archiwum Hydrobiologii 43:621–629.
- Chevreux E, Fage L. 1925. Faune de France: Amphipodes. Vol. 9. Office Central de Faunistique de la Fédération Française des Sociétés de Sciences Naturelles, editor. Paris: Masson. 488 pp.
- Ciavatti G. 1989. Les Talitrides de la plage de la Guadeloupe (Antilles francaises). Faunistique et étude d’une population de Platorchestia platensis (KROYER). Thèse Université Sciences et Techniques Languedoc (Montpellier-II). 248 pp.
- De Matthaeis E, Cobolli M, Davolos D, Mattoccia M. 1995. Stime di flusso genico tra popolazioni di Orchestia montagui (Amphipoda, Talitridae) delle isole circumsarde. Biogeographia 23:249–260.
- De Matthaeis E, Davolos D, Cobolli M, Ketmaier V. 2000a. Isolation by distance in equilibrium and nonequilibrium populations of four talitrid species in the Mediterranean Sea. Evolution 54:1606–1613. doi:10.1111/j.0014-3820.2000.tb00705.x.
- De Matthaeis E, Ketmaier V, Davolos D, Schembri PJ. 2000b. Patterns of genetic diversity in Mediterranean supralittoral amphipods (Crustacea, Amphipoda). Polskie Archiwum Hydrobiologii 47:473–487.
- Dias N, Sprung M. 2003. Population dynamics and production of the amphipod Orchestia gammarellus (Talitridae) in a Ria Formosa saltmarsh (southern Portugal). Crustaceana 76:1123–1141. doi:10.1163/156854003322753448.
- Elkaïm B, Irlinger JP, Pichard S. 1985. Dynamique de la population d’Orchestia mediterranea L. (Crustacé, Amphipode) dans l’estuaire du Bou Regreg (Maroc). Canadian Journal of Zoology 63:2800–2809. doi:10.1139/z85-419.
- Ginsburger-Vogel T. 1989. Determinisme des anomalies de sex ratio á hérédité paternelle chez le crustacé amphipode Orchestia gammarellus Pallas. Invertebrate Reproduction and Development 16:183–194. doi:10.1080/07924259.1989.9672076.
- Ginsburger-Vogel T. 1991. Intersexuality in Orchestia mediterranea Costa, 1853, and Orchestia aestuarensis Wildish, 1987 (Amphipoda): A Consequence of Hybridization or Parasitic Infestation? Journal of Crustacean Biology 11:530–539. doi:10.2307/1548522.
- Ginsburger-Vogel T, Magniette-Mergault F. 1981. The effects of temperature on sexual differentiation in the temperature sensitive thelygenic—intersexual offspring Oforchestia gammarellus (Pallas) (Amphipoda; Crustacea). International Journal of Invertebrate Reproduction 4:39–50. doi:10.1080/01651269.1981.10553417.
- Gonçalves SC, Marques JC, Pardal MA, Bouslama MF, El Gtari M, Charfi-Cheikhrouha F. 2003. Comparison of the biology, dynamics, and secondary production of Talorchestia brito (Amphipoda, Talitridae) in Atlantic (Portugal) and Mediterranean (Tunisia) populations. Estuarine Coastal and Shelf Science 58:901–916. doi:10.1016/j.ecss.2003.07.005.
- Hamilton WD. 1967. Extraordinary sex ratios. Science 156:477–488. doi:10.1126/science.156.3774.477.
- Harding JP. 1949. The use of probability paper for the graphical analysis of polymodal frequency distributions. Journal of the Marine Biological Association of the United Kingdom 28:141–153. doi:10.1017/S0025315400055259.
- Jelassi R, Ayari-Akkari A, Bohli-Abderrazak D, Nasri-Ammar K. 2013. Endogenous locomotor activity rhythm of two sympatric species of Talitrids (Crustacea, Amphipoda) from the supralittoral zone of Bizerte lagoon (Northern Tunisia). Biological Rhythm Research 44:265–275. doi:10.1080/09291016.2012.681845.
- Jelassi R, Khemaissia H, Nasri-Ammar K. 2012. Intra-annual variation of the spatiotemporal distribution and abundance of Talitridae and Oniscidea (Crustacea, Peracarida) at Bizerte Lagoon (northern Tunisia). African Journal of Ecology 50:381–392. doi:10.1111/j.1365-2028.2012.01326.x.
- Jelassi R, Nasri-Ammar K. 2012. Temporal variation in the shore amphipod community in the supralittoral zone of Bizerte lagoon (Northern of Tunisia). Crustaceana 85:433–446. doi:10.1163/156854012X636706.
- Jelassi R, Nasri-Ammar K. 2013. Seasonal variation of locomotor activity rhythm of Orchestia montagui in the supralittoral zone of Bizerte lagoon (North of Tunisia). Biological Rhythm Research 44:718–729. doi:10.1080/09291016.2012.739929.
- Jones MB, Wigham GD. 1993. Reproductive biology of Orchestia gammarellus (Crustacea: Amphipoda) living in a sewage treatment works. Journal of the Marine Biological Association of the United Kingdom 73:405–416. doi:10.1017/S0025315400032951.
- Louis M. 1977. Etude des populations de Talitridae des etangs littoraux mediterraneens I. variations des effectifs au sein des différentes phases et interpretation. Bulletin d’Ecologie 8:63–74.
- Lowry JK, Fanini L. 2013. Substrate dependent talitrid amphipods from fragmented beaches on the north coast of Crete (Crustacea, Amphipoda, Talitridae), including a redefinition of the genus Orchestia and descriptions of Orchestia xylino sp. nov. and Cryptorchestia gen. nov. Zootaxa 3709:201–229. doi:10.11646/zootaxa.3709.3.1.
- Marques JC, Gonçalves SC, Pardal MA, Chelazzi L, Colombini I, Fallaci M, Bouslama MF, El Gtari M, Charfi-Cheikhrouha F, Scapini F. 2003. Comparison of Talitrus saltator (Amphipoda, Talitridae) biology, dynamics, and secondary production in Atlantic (Portugal) and Mediterranean (Italy and Tunisia) populations. Estuarine Coastal and Shelf Science 58:127–148. doi:10.1016/S0272-7714(03)00042-8.
- Marsden ID. 1991. Kelp-sandhopper interactions on a sand beach in New Zealand. II. Population dynamics of Talorchestia quoyana (Milne-Edwards). Journal of Experimental Marine Biology and Ecology 152:75–90. doi:10.1016/0022-0981(91)90136-K.
- Morritt D, Stevenson TDI. 1993. Factors influencing breeding initiation in the beachflea Orchestia gammarellus (Pallas) (Crustacea: Amphipoda). Journal of Experimental Marine Biology and Ecology 165:191–208. doi:10.1016/0022-0981(93)90105-W.
- Nasri-Ammar K, Morgan E. 2005. Variation saisonnière du rythme de l’activité locomotrice de Talitrus saltator issu de la plage de Korba (Cap Bon, Tunisie). Bulletin de la Société zoologique de France 130:19–29.
- Nasri-Ammar K, Morgan E. 2006. Seasonality of the endogenous activity rhythm in Talitrus saltator (Montagu) from a sandy beach in north-eastern Tunisia. Biological Rhythm Research 37:479–488. doi:10.1080/09291010600739765.
- Nogueira A. 1992. ANAMOD—Extracção dos componentes modais de distribuições de frequências de variáveis biométricas. Trabalho de Síntese. Provas de Aptidão Pegagógica e de Capacidade Científica. Portugal: Universidade de Coimbra. 67 pp.
- Pardal MA, Marques JC, Metelo I, Lillebø AI, Flindt MR. 2000. Impact of eutrophication on the life cycle, population dynamics and production of Ampithoe valida (Amphipoda) along an estuarine spatial gradient (Mondego estuary, Portugal). Marine Ecology Progress Series 196:207–219. doi:10.3354/meps196207.
- Pavesi L, De Matthaeis E. 2009. Life history of the talitrid amphipod Macarorchestia remyi (Schellenberg, 1950) on a Tyrrhenian sandy beach, Italy. Hydrobiologia 635:171–180. doi:10.1007/s10750-009-9909-3.
- Pavesi L, Deidun A, De Matthaeis E, Tiedemann R, Ketmaier V. 2012. Mitochondrial DNA and microsatellites reveal significant divergence in the beachflea Orchestia montagui (Talitridae: Amphipoda). Aquatic Sciences 74:587–596. doi:10.1007/s00027-012-0250-y.
- Pavesi L, Iannilli V, Zarattini P, De Matthaeis E. 2007. Temporal and spatial distribution of three supralittoral amphipod species on a sandy beach of central Italy. Marine Biology 151:1585–1595. doi:10.1007/s00227-006-0604-x.
- Pavesi L, Tiedemann R, De Matthaeis E, Ketmaier V. 2013. Genetic connectivity between land and sea: the case of the beachflea Orchestia montagui (Crustacea, Amphipoda, Talitridae) in the Mediterranean Sea. Zoology 10:21.
- Persson L-E. 1999. Growth and reproduction in two brackish water populations of Orchestia gammarellus (Amphipoda: Talitridae) in the Baltic Sea. Journal of Crustacean Biology 19:53–59. doi:10.2307/1549546.
- Prato E, Trono A, Biandolino F. 2009. Life history of Talorchestia deshayesii (Amphipoda, Talitridae) in the Ionian sandy beach (southern Italy). Brazilian Archives of Biology and Technology 52:911–922. doi:10.1590/S1516-89132009000400015.
- Ruffo S. 1993. The Amphipoda of Mediterranean. Part III: Gammaridea (Melphidippidae to Talitridae)-Ingolfiellidae-Caprellidae. Monaco: Mémoire de l’Institut Océanographique Monaco 13. 813 pp.
- Scapini F, Aloia A, Bouslama MF, Chelazzi L, Colombini I, El Gtari M, Fallaci M, Marchetti GM. 2002. Multiple regression analysis of the sources of variation in orientation of two sympatric sandhoppers, Talitrus saltator and Talorchestia brito, from an exposed Mediterranean beach. Behavioral Ecology and Sociobiology 51:403–414. doi:10.1007/s00265-002-0451-9.
- Van Senus P. 1988. Reproduction of the Sandhopper, Deshayesorchestia capensis (Dana) (Amphipoda, Talitridae). Crustaceana 55:93–103. doi:10.1163/156854088X00276.
- Wenner AM. 1972. Sex ratio as a function of size in marine Crustacea. The American Naturalist 106:321–350. doi:10.1086/282774.
- Wildish DJ. 1979. Reproductive consequences of the terrestrial habit in Orchestia (Crustacea: Amphipoda). International Journal of Invertebrate Reproduction 1:9–20. doi:10.1080/01651269.1979.10553295.
- Williams JA. 1978. The annual pattern of reproduction of Talitrus saltator (Crustacea: Amphipoda: Talitridae). Journal of Zoology 184:231–244. doi:10.1111/j.1469-7998.1978.tb03278.x.
- Williamson DE. 1951. On the mating and breeding of some semi-terrestrial Amphipods. Reports of the Dover Marine Laboratory 12:49–62.