Abstract
Neural migration is regarded as a key step for cortical development and cortical lamination. While classical theory holds that immature neurons migrate to their destinations along radial glia, there is some preliminary evidence showing that vasculature is probably involved in that process as well. In this study, we compared vascular development with that of radial glia and the migration of neurons, so that the relationships among them could be elucidated. We found that the radial glial cells and vasculature were highly similar in their distribution and development. For instance, in the external granular layer (EGL, the putative molecular layer), the processes of radial glial cells were arranged in an orderly radial pattern, whereas in the deep areas of the cerebellum, their arrangements were in relative disorder. On the other hand, the vasculatures in EGL were usually orientated radially, which paralleled the projections of radial glia; however, the distribution of vasculature in the internal granular layer (IGL, the putative granular layer) and white matter was in relative disorder as well, similar to the pattern exhibited by radial glial processes. This high harmonization between vasculature and radial glia suggests their interconnected biological relationships during cerebellar development and neural migration. We also observed that a large number of newborn neurons were migrating along blood vessels, suggesting that the vasculature could serve as a scaffold for cell migration. In conclusion, cerebellar vasculature could guide neural migration not only as a platform for biological interaction with radial glia, but also serve scaffolding functions for neural migration.
Introduction
It has been found that radial glial cells play a pivotal role in establishing the lamination of the cortices and neural migration. Radial glial cells can not only give rise to the principal cells of the cerebellar cortex, the Purkinje and granule cells, but also provide a scaffold for the migration of these cells (Fricker-Gates Citation2006; Zhang et al. Citation2010; Sild & Ruthazer Citation2011). Recently, there is evidence showing that blood vasculature in the nervous system is probably involved in a wide range of neuronal functions, including neurogenesis, neuronal migration, neuronal survival and axonal guidance. Both nerves and vasculature often develop and are distributed in a coordinated fashion. For instance, investigators have found that in embryonic skin, arteries were oriented in close association with peripheral nerves and were aligned into two parallel structures (Mukouyama et al. Citation2002; Carmeliet & Tessier-Lavigne Citation2005; Mackenzie & Ruhrberg Citation2012). A recent study indicates that neuronal precursors might also use blood vessels as a physical substrate for their migration (Saghatelyan Citation2009). The vasculature-guided (vasophilic) migration of neuronal precursors has been observed not only under normal conditions, such as in the olfactory bulb (OB) (Bovetti et al. Citation2007; Kishimoto et al. Citation2011; Bozoyan et al. Citation2012), but also in brains undergoing pathological conditions, such as strokes (Saghatelyan Citation2009). The mechanism of the correlation between nerves and vasculature is not yet clear. It has been reported that endothelial stem cells have features such as axonal growth cones that serve guidance functions. Furthermore, axonal growth cones and endothelial tip cells also respond to the same families of molecules in their pathfinding, such as Slits and Roundabouts, Netrins, Semaphorins, Plexins, Neuropilins and so on (Adams & Eichmann Citation2010).
According to the analyses above, radial glial cells and the vasculature in the brain can work together to carry out some functions, such as cell migration guidance and cortical morphogenesis during development. Unfortunately, the details are not yet fully understood. In this study, vascular development was compared with that of radial glial cells and neural migration, so that the relationships between vasculature and radial glial cells, in particular the detail of vasculature-guided neural migration, could be elucidated in a developing cerebellum. This will undoubtedly provide us with new leads in understanding the common mechanisms of nerve and blood vessel wiring, and especially the interplay of vascular guidance with neural migration and cerebellar lamination.
Materials and methods
Animals
All experiments were carried out in accordance with the institutional guidelines of Henan University for animal welfare. Adult male and female C57BL/6J mice were placed in breeding cages in standard laboratory animal housing with a 12 h:12 h light:dark cycle. Embryonic or postnatal offspring were produced from timed pregnancies (E, day of conception; E0, day of vaginal plug in mated females; P, days postnatal; P0, the first 24 h after birth). Pups were born on E19. A total of 385 embryos and postnatal pups at E8–19 and P0–180 were used in this study. To obtain embryonic mice at specific stages, pregnant dams were anesthetized (sodium pentobarbital, 40 mg/kg, intraperitoneal injection or i.p.) and fetuses were harvested by Caesarean section. From E8 to 13, whole embryos were fixed with 4% paraformaldehyde (PFA) in 0.01 M phosphate buffer (PB) (pH 7.2) for 2–3 days at 4°C. From day E14 onwards, the fetal brains were carefully separated and immersion fixed in 4% PFA for 1–2 days at 4°C. For postnatal mice, pups were anesthetized (sodium pentobarbital, 20 mg/kg, i.p.) and perfused transcardially with 4% PFA. Cerebella were removed, and fixation was continued at 4°C for 1–2 days.
Routine histological staining
Embryos (E8–13), whole brains (E14–19) and cerebella (P0 onwards) were dehydrated in gradient ethanol and embedded in paraffin. After horizontal or sagittal sectioning (5–7 µm), hematoxylin and eosin (HE) and Nissl staining were performed according to a standard protocol.
Immunocytochemistry
Paraffin-embedded embryos (< E14) were sectioned horizontally or sagittally (5–7 µm); from E14 onwards, whole brains or separated cerebella were sectioned coronally or sagittally (50 µm) using a vibratome. Sections were rinsed in 0.01 M PB and preincubated in blocking solution (5% normal goat serum) for 30 min at room temperature before immunofluorescent labeling. Nestin, glial fibrillary acidic protein (GFAP), S100, Ki67, double-cortin (DCX), neuronal nuclear antigen (NeuN), calbindin, β-tubulin, Foxp2 and Collagen IV were respectively used as markers to visualize radial glial cells (Nestin, S100), astrocytes (GFAP), newborn neurons (Ki67, double-cortin), mature neurons (NeuN) and Purkinje cells (calbindin). Collagen IV is a marker protein for vascular basal lamina. Sections were incubated overnight at 4°C with the indicated dilutions of the different primary antibodies: mouse monoclonal anti-Nestin antibody (1:200; Santa Cruz, SC-33677), rabbit polyclonal anti-GFAP (1:200; Beijing Zhong Shan-Gloden Bridge, ZA-0117), rabbit polyclonal anti-DCX (1:500; Cell Signaling, 4604), mouse monoclonal anti-NeuN (1:500; Chemicon, MAB377B) and mouse monoclonal anti-calbindin (1:200; Abcam, AB9481). In some sections, double labeling of radial glial cells was performed using rabbit polyclonal anti-Nestin antibody (1:1000, Abcame, AB5968) in addition to mouse anti-Nestin. After multiple washes in 0.01 M PB, appropriate secondary antibodies were added at the indicated dilutions and incubated at room temperature for 3 h. The secondary antibodies were: Alexa Fluro 488 donkey anti-mouse IgG (Immunoglobulin G) (1:500; Invitrogen, A21202), Alexa Fluro 488 donkey anti-rabbit IgG (1:500; Invitrogen, A21206), Alexa Fluro 568 goat antimouse IgG (1:600; Invitrogen, A11004) and Alexa Fluro 568 goat anti-rabbit IgG (1:600; Invitrogen, A11011). Sections were counterstained with DAPI (4’,6-diamidino-2-phenylindole) (1:60,000; Santa Cruz, SC3598) for 1–2 min after immunolabeling. Sections were coverslipped under 65% glycerol in 0.01 M PB and imaged using an epifluorescence microscope (BX61, Olympus) with rhodamine, fluorescein isothiocyanate (FITC) or ultraviolet filter sets. High-quality sections were photographed using an Olympus laser confocal microscope (FV1000, Olympus, Japan), using separate scans with 568 nm (red) and 488 nm (green) laser lines.
5-Bromo-deoxyuridine (BrdU) labeling
The incorporation of BrdU, a marker of S-phase, was used to investigate neuronal proliferation and newborn neuron migration. BrdU (Sigma, B5002) dissolved at 7.5 mg/500 µL sterile 7 mM sodium hydroxide (NaOH) in physiological saline was injected (i.p.) at a dose of 5 µg/gm into pregnant mice at E8-E17 or into postnatal P0-180 mice. To intensify labeling, a second, identical dose was given 3 h later. Animals were killed 2 days after injection. Embryonic brains were immersion fixed; postnatal pups were transcardially perfused and brains were postfixed in 4% PFA in 0.01 M PB overnight at 4°C. After fixation, 125-µm sagittal cerebellar sections were cut using a vibratome. To visualize nuclear BrdU incorporation, sections were incubated in 4 N hydrochloric acid (HCl) for 10 min to denature the DNA, thoroughly rinsed (5×) in PB, and normal goat serum (0.5% volume/volume) was added to block nonspecific binding. Sections were then incubated overnight at 4°C with mouse monoclonal anti-BrdU IgG (1:500; Beijing Zhong Shan-Gloden Bridge, ZM-0013). After washing, Alexa Fluro 488 donkey anti-mouse IgG (1:300; Invitrogen, A21202) was added and sections were incubated at room temperature for 3 h. Sections were coverslipped under 65% glycerol in 0.01 M phosphate-buffered saline and imaged using the fluorescent microscope.
Vasculature labeling with gelatin-ink perfusion
Postnatal pups at various ages were anesthetized peritoneally using 1% pentobarbital sodium at 20 mg/kg as above. 4% PFA was perfused through the left ventricle for 5 minutes; then 6.5% gelatin-ink at 60°C was perfused throughout until the body turned black. After tightening cardiac vessels, the mice were put on ice for about 2 h for the gelatin condensation. Then, immersion fixation was carried out in 4% PFA for 1–2 days at 4°C. Subsequently, the cerebella were taken out and cut sagittally with a vibratome. Some sections were selected for continuous immunocytochemistry as above. The sections were coverslipped with 65% glycerin in 0.01 M PB. The samples were pictured under a normal microscope, fluorescent microscope or confocal microscope to observe the cerebellar vasculature.
Results
Cerebellar development and cortical lamination
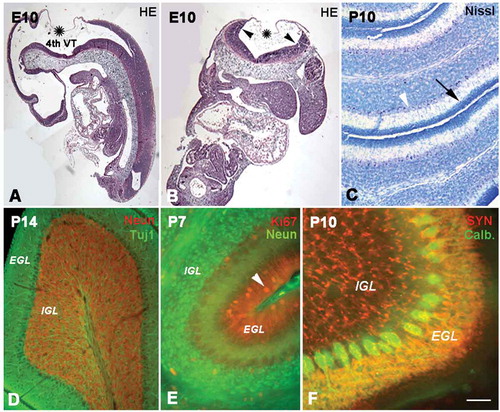
The cerebellum develops from the rhombic lip, a dorsolateral part of the alar plate in the metencephalon. At E10, the rhombic lip, consisting mainly of neuroepithelium, can be clearly discerned in sagittal or horizontal sections. The upper and inner lips were connected with choroid, and the space beneath the rhombic lips and choroid was the 4th ventricle ( and ). The neuroepithelium in rhombic lips differentiated into two proliferative compartments: the subventricular zone (SVZ) and the external granular layer (EGL). Initially, the proliferative zone was located in the SVZ along the axis of the alar plate. Subsequently, neural stem cells from the rostral part of the rhombic lip migrated tangentially into the superficial part of the cerebellum to form the EGL, the presumable molecular layer in the adult (Zhang et al. Citation2010). In view of its proliferative activity, the EGL has been regarded as a secondary proliferative zone in the developing cerebellum, and the newborn postmitotic neuron migrated into the internal granular layer (IGL), which would become the presumable granular layer in adult (). At postnatal day 10 (P10), there were still some proliferative cells in the superficial molecular layer and numerous dense granule cells in the granular layer (). In addition, there was one row of Purkinje cells located between the molecular layer and the granular layer (, and ). The molecular layer now became sparse in cell density after the migration of some cells toward the granular layer. At P14, the cell-free molecular layer was filled with numerous dendrites of Purkinje cells ( and ). The axons of Purkinje cells penetrated through the granular layer and entered into the white matter of the cerebellum (). At P10, numerous functional presynaptic buttons were found on the dendrites of Purkinje cells and granule cells with synaptophysin immunocytochemistry ().
Figure 1. The development of the cerebellum. A and B show the cerebellar anlagen in sagittal (A) and coronal (B) sections at E10 (hematoxylin and eosin staining). Cerebellum was developed from upper and inner rhombic lips (arrow heads) which connected with choroid (stars), and the space surrounded by the rhombic lips and choroid was the 4th ventricle (VT). Photo C shows P10 cerebellum with Nissl staining. Some cells (→) are located in the superficial external granular layer (EGL), while some granule cells have migrated into the internal granular layer (IGL). With cell migration, the cell density in the EGL became sparse, and there are few cells still remaining in the superficiality of the EGL or molecular layer. The area between the EGL and IGL is presumed to be the Purkinje cell layer (arrow head). Photo D shows the cerebellum at P14 (Tublin and NeuN double immunofluorescent labeling). A row of Purkinje cells (green) was seen in the Purkinje cell layer (PCL), and their dendrites extended into the EGL. The IGL contained numerous granule cells (red) underneath the PCL. Photo E shows the cerebellar lamination and cell migration at P7 with Ki67 (red) and NeuN (green) immunofluorescent labeling. At this age, the three-layered structure of cerebellar lamination was formed, and EGL and IGL could be clearly recognized. The Ki67 positive cells (arrow head) in EGL were migrating toward IGL to form the mature granule cells (green). Photo F shows the P10 cerebellum with functional presynaptic buttons (synaptophysin and calbindin double immunolabeling), which were located in EGL and IGL (red). These presynaptic buttons in EGL can be connected with the dendrites of Purkinje cells (green). Scale bars: A and B: 200 µm; C and D: 80 µm; E: 60µm; F: 30 µm.
Development of radial glial cells
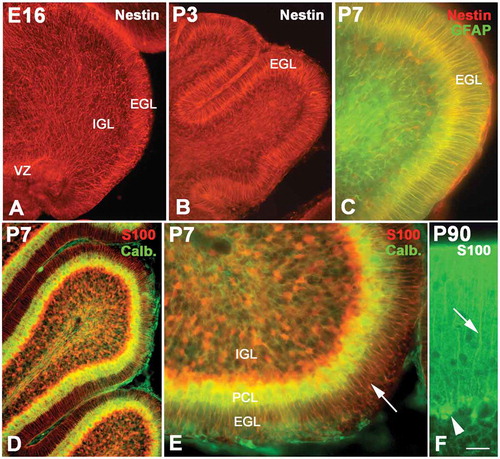
Radial glial cells as early as E16 could be labeled with Nestin immunolabeling (). At this age, the cerebellar surface was smooth, without cortical sulci. The processes of radial glial cells spanned over the entire cerebellar parenchyma from neuroepithelium to the pia. However, the alignment of these processes was different in various areas. In the superficial zone of the cerebellum (putative molecular layer or EGL), they were arranged in an orderly fashion with a radial pattern; however, in the deep areas of the cerebellum, the arrangement of the processes of radial glial cells was in relative disorder. The EGL widened with age increase, with many sulci appearing in the cerebellar cortex. The processes of the glial cells were in a more typical radial distribution in the EGL. On the other hand, the projections of radial glial cells in IGL and white matter were more tangled, with a network-like distribution (). Some chemical alterations also occurred in radial glial cells during development. Before P7, radial glial cells only expressed Nestin; thereafter, however, the radial glial projections could express both Nestin and GFAP (), and after P14 only GFAP was specifically expressed in radial glia (data not show). On the other hand, S100 could be expressed in radial glial cells as well. The S100 positive projections were only sparingly observed in embryonic cerebellum, and dense radial glial cells could not be seen clearly until P7 ( and ). The morphology of radial glial cells changed greatly during cerebellar development as well. Initially the cell bodies of radial glial cells were located in the neuroepithelium, after which the cells moved up and settled down in the Purkinje cell layer (). Therefore, the radial glial cells are called Bergmann cells in the cerebellum (–).
Figure 2. The development of radial glial cells in cerebellum. Photo A shows the radial glial cells (red) at E16 (Nestin immunolabeling), whose processes spanned from the ventricular zone (VZ) to the pia. However, their distribution patterns were quite different in different areas. In the superficial zone of the cerebellum, such as the external granular layer (EGL), the processes were arranged in an orderly radial pattern, but in the internal granular layer (IGL), their arrangement was in relative disorder. With age increase, the EGL became wide with the typical radial pattern for the processes of radial glial cells (B). Photo C shows the P7 cerebellum with Nestin and glial fibrillary acidic protein (GFAP) double immunolabeling. The structure of the cerebellum became more typical at P7. At this age, the radial glial cells expressed both Nestin (red) and GFAP (green). Photo D shows the radial glial cells (red) stained with S100 immunolabeling, and the Purkinje cells (green) were stained with calbindin immunochemistry. Photo E is the high magnification of photo D. At this age, the radial glial cells assumed the shape of typical Bergmann cells. Their cell bodies were located in the Purkinje cell layer (PCL), and the processes extended into the EGL. A high magnification of Bergmann cells (green) with cell bodies (arrow head) and processes (arrow) is also showed in photo F with S100 immunochemistry. Scale bars: A and B:, 100 µm; C: 30 µm; D: 60 µm; E: 25 µm; F: 15 µm.
Cell migration in the cerebellum
There are two different patterns of cell migration in the cerebellum. One is the way from the neuroepithelium to the Purkinje cell layer, and the other is the way from EGL to IGL. – shows the migration and differentiation of Purkinje cells. At E15, there were numerous Foxp2-positive cells distributed between the neuroepithelium and the putative Purkinje cell layer. These cells probably were the neurons migrating toward the Purkinje cell layer, which serves as the precursor cells of Purkinje cells. At E18, the Foxp2-positive cells migrated up further and concentrated near the Purkinje cell layer. At P5, only 2–3 rows of Foxp2-positive cells were located in the Purkinje cell layer and differentiated into Purkinje cells (–). On the other hand, additional evidence also showed cell migration from EGL into IGL. In the prenatal and early postnatal stages, there were numerous neural stem cells and newborn neurons in the EGL, which would to migrate toward the IGL ( and –). At P5, these neural stem cells and newborn neurons occupied the whole EGL, and some newborn neurons in the EGL started to migrate into the IGL and differentiated into granule cells ( and ). At P10, only a few cells were located in the superficial zone of the molecular layer (). Almost all neurons in the molecular layer had migrated into the IGL and differentiated into granule cells (). The laminar alterations of cerebellar cortex supported the hypothesis that the neurons in EGL migrated into IGL along Bergmann cells (Buffo & Rossi Citation2013).
Figure 3. Neural migration in the cerebellum. Photos A–C, neural migration from the ventricular zone (VZ) to the Purkinje cell layer (PCL) (Nestin and Foxp2 double immunolabeling). At E15, numerous Foxp2 positive cells (green) migrated from VZ to PCL along the processes of radial glial cells (red) A, At E18, the migrating Foxp2-positive cells were concentrated near the PCL B, At P5 only one or two rows of Purkinje cells were located in the PCL C, Photos D–F, the migration of newborn neurons from the superficial external granular layer (EGL) to the internal granular layer (IGL). With double-cortin (DCX, red) and NeuN (green) double immunolabeling, photos D and E show that DCX-positive newborn neurons (red) in EGL are ready to migrate toward IGL and differentiate into granule cells (green). Photo F shows the cerebellar cortex at P10 (NeuN and Foxp2 double immunolabeling). There are few post-mitotic neurons in the superficial EGL (green, arrow), since almost all neurons have migrated into the IGL and differentiated into granule cells (green). The Purkinje cells (red and arrow head) are visualized between EGL and IGL. Photos G–H, neural proliferation and neural migration in the cerebellum (5-Bromo-deoxyuridine (BrdU) assay). The proliferative cells and migrating neurons (green) decreased with age increase. The proliferative cells in EGL are marked with arrows. At P14, only a few proliferative cells were located in the superficial zone of EGL (I). Scale bars: A and B: 100 µm; C: 50 µm; D and E: 100 µm; F: 50 µm; G–I: 100 µm.
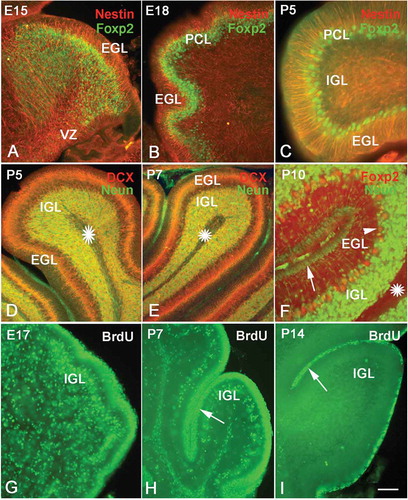
BrdU assay further vividly showed the process of neural migration. Since BrdU can be utilized as a thymine alternative during the DNA synthesis of the S phase, it is usually used to mark the proliferating cells. In the present study, the animals were sacrificed 2 days later after injection; therefore, some BrdU-positive cells could also represent postmitotic neurons, which were the daughters of neural progenitor cells. We found the BrdU-positive cells not only in proliferative zones, such as SVZ and EGL, but also outside of proliferative zones as well. In this way, the BrdU-positive cells outside SVZ and EGL could be regarded as migrating newborn neurons. In our study, at E17, there were many BrdU-positive cells in the EGL, and many of them started to migrate into the IGL as well (). Both neural progenitor cells and migrating newborn neurons decreased with age increase (), since the neural proliferative ability decreased with central nervous system development, as other authors have reported (Schuller et al. Citation2007; Kaslin et al. Citation2013). At P14, only a few BrdU-positive cells remained in the EGL, and almost no migrating neurons were found in the IGL (–).
Cerebellar vasculature, radial glial cells and neural migration
Collagen IV immunolabeling indicated that the vascular network appeared in the cerebellum as early as E15. Interestingly, the vasculature in the presumable EGL was orientated radially and paralleled with the processes of radial glial cells. On the other hand, the vasculature in the area from the neuroepithelium to the PCL was orientated in a disorderly, networked pattern, where the processes of radial glial cells ran in a state of disarray as well (). With age increase, cortical sulci appeared on the surface of the cerebellum, and the EGL developed into the typical wide structure (). Furthermore, the vasculature in the cerebellum became high in density, but the distributions of vessels were still highly identical with the processes of radial glial cells. For instance, at P0 and P3, the vessels in EGL ran in a radial pattern and were paralleled by the orientation of the processes of radial glial cells. In the rest of the cerebellum, white matter and IGL, the vasculature appeared in a networked pattern, similar to the disorder of the processes of radial glial cells ( and ). In adulthood, the cerebellar vasculature became very dense, but its vascular distribution was still similar to that of the early developmental stages – that is, a radial arrangement in the molecular layer and a networked arrangement in the granule layer (). The similarity of the processes of radial glial cells and the cerebellar vasculature suggested that there was close crosstalk between them during development. Ink profusion experiments showed similar results to Collagen IV immunolabeling (–). In addition, we found that the vasculature in the cortex mainly consisted of capillaries, in contrast to the larger vessels in the white matter and pia, suggesting these small arteries and veins collected blood from the cortical capillaries ().
Figure 4. The development of vasculature and radial glial cells in the cerebellum. Photos A–C, the distribution of vessels and radial glial cells at various ages with Collagen IV (green) and Nestin (red) double immunolabeling. The vascular network (green) appeared in the cerebellum as early as E15. The vasculature in the presumable superficial external granular layer (EGL) was orientated radially and paralleled with the processes of radial glia (red) in same area. However, the vasculature in the internal granular layer (IGL) appeared disorderly; so did the processes of the radial glia in the same area. At P0 and P3, the vasculature became dense, and their distributions were still highly identical with the arrangement of processes of radial glia. Photo D shows the vascular network at P90 with ink perfusion (dark). The vasculature in white matter is marked by a star, and the relatively large vessels in the pia and white matter are marked by an arrowhead and arrow respectively, showing that the cortical capillary network converges into the vessels in the pia and white matter. Photos E–F, cerebellar vasculature and the development of radial glial cells by ink perfusion and Nestin or glial fibrillary acidic protein (GFAP) immunochemistry. The orientation of vessels (dark) was highly harmonized with the distribution of the processes of radial glia (green) as described above, again suggesting the close interaction between radial glial cells and blood vasculature in the cerebellum. Photo G, cerebellar vessels and neural migration by ink perfusion, S100 immunochemistry and 5-Bromo-deoxyuridine (BrdU) assay triple labeling. The proliferative BrdU-positive cells were mainly concentrated in the subventricular zone (SVZ) and EGL. Since BrdU injections were carried out 2 days before animal sacrifice, some BrdU-positive neural progenitors had differentiated into post-mitotic neurons and migrated elsewhere. In the photo, many BrdU-positive migrating cells were observed along the cerebellar vessels (arrowhead), suggesting that the cerebellar vasculature served as a migration scaffold similar to the function of radial glial cells. The arrow shows the proliferative area in EGL, which are the source of migrating neurons into IGL. Photo H, the neural migration under vascular guidance with high magnification using triple labeling (ink, S100 and BrdU). Many BrdU-positive cells were migrating along the vascular scaffold as described above (arrow head). Many BrdU-positive cells (green) expressed S100 (red) with a merged yellow color, suggesting that proliferative stem cells were transiting toward newborn post-mitotic neurons. Scale bars: A–C: 200 µm; D–F: 100 µm; G: 60 µm; H: 40 µm.
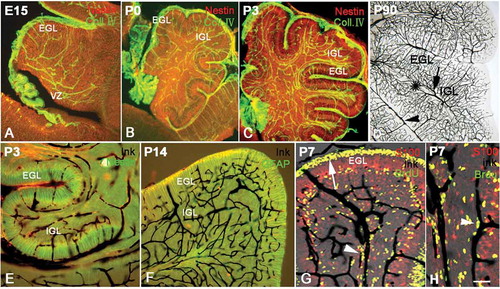
Triple labeling was carried out in order to further observe the interplay among vasculature, radial glial processes and neural migration. The migrating newborn neurons were labeled with BrdU assay, Bergmann cells were labeled with S100 immunochemistry and cerebellar vasculature was visualized with ink perfusion. In this study, the vasculature could distribute in pia, EGL, PCL, IGL and white matter as well. Meanwhile, an interesting phenomenon appeared under the confocal microscope. A large part of migrating newborn neurons was often located along the vessels ( and ), suggesting that blood vessels served as migration scaffolds to guide the cell migration, as radial glial cells did. In the meantime, many BrdU-positive cells (green) expressed S100 (red) with a merged yellow color. This was probably evidence that newborn post-mitotic neurons were transited from BrdU-positive stem cells.
Discussion
During brain development, some post-mitotic newborn neurons use the processes of radial glia cells as a scaffold to migrate and reach their final destination. New evidence has shown that brain blood vessels are probably involved in neurogenesis and neural migration as well, because circulatory and nervous systems have close interactions with each other in brain development. At first, the embryonic “vascular niche” functions as a microenvironment for neurogenesis, neuronal migration and neurite extension (Javaherian & Kriegstein Citation2009; Paez-Gonzalez et al. Citation2011; Decimo et al. Citation2012). Stubbs et al. (Citation2009) also demonstrated that proliferative cells were found in close proximity to the vasculature rather than just appearing in random distribution; furthermore, neurites extended toward and along labeled blood vessels as well, suggesting close vascular-neuronal interactions during development. Secondly, neural migration in the olfactory bulb is also dependent upon the guidance of vasculature, since radial glial cells have almost disappeared in adults (Peretto et al. Citation2005; Yang et al. Citation2005). Finally, the distributions of blood vessels could also affect cortical lamination. For instance, in the reeler mutant mice, which have defects in neuronal migration produced by the lack of the extracellular matrix protein Reelin, relatively layer-specific characteristics of the vascular pattern were found to be changed in the hippocampus and dentate gyrus. The vascular network in the dentate gyrus was remodeled by the dispersed granule layer, contributing to decreased vessel bifurcations in the reeler dentate gyrus (Lindhorst et al. Citation2012). All of this evidence suggests that causality should exist between vascular development and cortical formation, and the secrets behind this vascular–neuronal interplay are waiting to be revealed in further detail.
Crosstalk between radial glial cells and angiogenesis
Cerebellar development and the establishment of the lamination in the cerebellar cortex are subservient to the complex processes of neural proliferation, neuronal differentiation and cell migration (Lossi et al. Citation1995). Radial glial cells not only serve as neural progenitor cells, but are also thought to provide a scaffold for cell migration (Malatesta et al. Citation2003; Anthony et al. Citation2004; Zhang et al. Citation2010). The cerebellum is proposed as a good model to study subjects such as vascularization, radial glial cells, neural migration and cortical lamination. In the present study, the evidence initially showed that there was a close association between vascularization and radial glial cells. The vasculature in the molecular layer was orientated radially and paralleled with the processes of radial glial cells. However, the vasculature in the PCL area and the granule layer was in disorder, where the processes of radial glial cells ran in a state of disarray as well, suggesting there were associations between them. No doubt this association is linked by some molecular crosstalk between radial glial cells and vasculature. Some axon guidance molecules, such as Netrins, Slits, Semaphorins, Ephrins and their receptors, have also been implicated in this crosstalk as well (Yoshida Citation2001; Gerhardt et al. Citation2004; Wang et al. Citation2008). Recently, vascular endothelial growth factor (VEGF) has also been proposed to play a critical role in mechanisms that cause the crosstalk between vasculature and radial glial cells. VEGF protein is mainly expressed in endothelial cells (Yu et al. Citation2012) and nerve tissues, such as nervous cells, neuroblasts and radial glial cells, or even in some non-neuronal cells. In nervous tissues, VEGF can regulate angiogenesis and responds to the high metabolic demands of the developing neocortex (Virgintino et al. Citation2003). VEGF’s high-affinity receptor VEGFR-2 can be expressed in radial glia, suggesting VEGF can act on the radial glial cells (Sentilhes et al. Citation2010). VEGF can guide the polarity and orientation of neurons and glia (Mackenzie & Ruhrberg Citation2012). In our hypothesis, the molecules above, especially VEGF and its receptor, can probably determine the parallel orientations of radial glial cells and vasculature (Zheng et al. Citation2011). In spite of their parallel orientations, the density of radial glial cells and vasculature were quite different. This phenomenon ruled out the possibility of a one-to-one inductive influence during the genesis of radial glial cells and vasculature, which have their own specific ways for growth and development. After establishing the relationship between radial glial cells and vasculature, the patterns of neural migration and cortical lamination in the cerebellum will be determined accordingly.
Vasculature-guided neural migration
In this study, we had observed that the source of migrating neurons was the neuroepithelium of rhombic lips, and the neuroepithelium could evolve further into two proliferative compartments: SVZ and EGL. The Purkinje cells migrated from SVZ, while the granule cells migrated from EGL, the presumed molecular layer (Zhang et al. Citation2010). Neural migrations toward IGL and PCL were dependent upon the functional performance of radial glial cells and cerebellar vasculature. The processes of radial glial cells spanned the entire cerebellar parenchyma from the neuroepithelium to the pia. The arrangement of these cellular projections was different in various areas. In this study, we compared the relationships among the cerebellar vessels, radial glial cells and the neural migration together. The highly identical distribution patterns of vasculature and radial glial cells suggests that the close crosstalk between cerebellar vasculature and the processes of radial glia, as well as the corresponding changes in the biological characteristics occurring in cerebellum, were vital factors in neural migration during development. In order to understand the role that blood vessels play in cell migration directly, triple labeling for BrdU-positive cells, Bergmann cells and cerebellar vasculature was carried out. BrdU assay is usually used to label the proliferating cells at the S stage. As we know, there are two patterns of cell division in neural progenitor cells: symmetric division and asymmetric division. Through symmetric division, more and more neural progenitors can be replicated. However, through asymmetric division, some post-mitotic newborn neurons can be produced by cell differentiation (Pontious et al. Citation2008), and these postmitotic newborn neurons have strong migration ability. In the present study, since BrdU was injected 2 days before animal sacrifice, many BrdU-positive cells were observed in the path of neural migration in the cerebellum, and these cells were assumed to be the post-mitotic newborn neurons that migrated from SVZ and EGL. Other parts of BrdU-positive cells concentrated in the proliferative areas, such as VZ, SVZ and EGL, still kept their proliferative ability of neural progenitor cells. Interestingly, we found that a large part of the BrdU-positive cells were located along the vessels, suggesting that these cells migrated to their destination through a vascular scaffold instead of through radial glial cells. Our study confirmed the findings of Peretto et al. (Citation2005) and Yang et al. (Citation2005), and the neural migration along the blood vessels is probably intermediated by VEGF as well (Rosenstein et al. Citation2010). VEGF has participated in cell migration and the formation of migration-promoting vasculature scaffold as well, which occurs in migrating neurons in rostral migratory stream (RMS) (Kishimoto et al. Citation2011; Bozoyan et al. Citation2012). However, to better understand neural migration under vascular guidance, time lapse images will be helpful, and it is necessary to observe neural migration through a VEGF deletion assay.
In summary, by investigating cerebellar development and neural migration, and especially by comparing the orientation of blood vessels with the distribution of radial glial cells, we found that radial glial cells and vasculature were highly similar in their distribution and development, suggesting that vasculature could affect neural migration by altering the biological characteristics of radial glial cells. Furthermore, the evidence showed that post-mitotic neurons could migrate along blood vessels directly and that VEGF is probably an important molecule in radial glia–vasculature interaction and vasculature-guided migration.
Conflict of interests
The authors declare no conflicts of interests.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Grant No. 30771140, 31070952, U1204311).
References
- Adams RH, Eichmann A. 2010. Axon guidance molecules in vascular patterning. Cold Spring Harbor Perspectives in Biology 2:a001875. doi:10.1101/cshperspect.a001875.
- Anthony TE, Klein C, Fishell G, Heintz, N. 2004. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron 41:881–890. doi:10.1016/S0896-6273(04)00140-0.
- Bovetti S, Hsieh Y-C, Bovolin P, Perroteau I, Kazunori T, Puche AC. 2007. Blood vessels form a scaffold for neuroblast migration in the adult olfactory bulb. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 27:5976–5980. doi:10.1523/JNEUROSCI.0678-07.2007.
- Bozoyan L, Khlghatyan J, Saghatelyan A. 2012. Astrocytes control the development of the migration-promoting vasculature scaffold in the postnatal brain via VEGF signaling. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 32:1687–1704. doi:10.1523/JNEUROSCI.5531-11.2012.
- Buffo A, Rossi F. 2013. Origin, lineage and function of cerebellar glia. Progress in Neurobiology 109:42–63. doi:10.1016/j.pneurobio.2013.08.001.
- Carmeliet P, Tessier-Lavigne M. 2005. Common mechanisms of nerve and blood vessel wiring. Nature 436:193–200. doi:10.1038/nature03875.
- Decimo I, Bifari F, Krampera M, Fumagalli G. 2012. Neural stem cell niches in health and diseases. Current Pharmaceutical Design 18:1755–1783. doi:10.2174/138161212799859611.
- Fricker-Gates RA. 2006. Radial glia: A changing role in the central nervous system. NeuroReport 17:1081–1084. doi:10.1097/01.wnr.0000230505.32726.65.
- Gerhardt H, Ruhrberg C, Abramsson A, Fujisawa H, Shima D, Betsholtz C. 2004. Neuropilin-1 is required for endothelial tip cell guidance in the developing central nervous system. Developmental Dynamics: An Official Publication of the American Association of Anatomists 231:503–509. doi:10.1002/dvdy.20148.
- Javaherian A, Kriegstein A. 2009. A stem cell niche for intermediate progenitor cells of the embryonic cortex. Cerebral Cortex 19 Suppl 1:70–77. doi:10.1093/cercor/bhp029.
- Kaslin J, Kroehne V, Benato F, Argenton F, Brand M. 2013. Development and specification of cerebellar stem and progenitor cells in zebrafish: From embryo to adult. Neural Development 8:9–25. doi:10.1186/1749-8104-8-9.
- Kishimoto N, Alfaro-Cervello C, Shimizu K, Asakawa K, Urasaki A, Nonaka S, Kawakami K, Garcia-Verdugo JM, Sawamoto K. 2011. Migration of neuronal precursors from the telencephalic ventricular zone into the olfactory bulb in adult zebrafish. The Journal of Comparative Neurology 519:3549–3565. doi:10.1002/cne.22722.
- Lindhorst T, Kurz H, Sibbe M, Meseke M, Förster E. 2012. Congruence of vascular network remodeling and neuronal dispersion in the hippocampus of reelin-deficient mice. Histochemistry and Cell Biology 137:629–639. doi:10.1007/s00418-012-0912-9.
- Lossi L, Ghidella S, Marroni P. 1995. The neurochemical maturation of the rabbit cerebellum. Journal of Anatomy 187:709–722.
- Mackenzie F, Ruhrberg C. 2012. Diverse roles for VEGF-A in the nervous system. Development. 139:1371–1380. doi:10.1242/dev.072348.
- Malatesta P, Hack MA, Hartfuss E, Kettenmann H, Klinkert W, Kirchhoff F, Götz M. 2003. Neuronal or glial progeny regional differences in radial glia fate. Neuron 37:751–764. doi:10.1016/S0896-6273(03)00116-8.
- Mukouyama Y-S, Shin D, Britsch S, Taniguchi M, Anderson DJ. 2002. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell 109:693–705. doi:10.1016/S0092-8674(02)00757-2.
- Paez-Gonzalez P, Abdi K, Luciano D, Liu Y, Soriano-Navarro M, Rawlins E, Bennett V, Garcia-Verdugo JM, Kuo CT. 2011. Ank3-dependent SVZ niche assembly is required for the continued production of new neurons. Neuron 71:61–75. doi:10.1016/j.neuron.2011.05.029.
- Peretto P, Giachino C, Aimar P, Fasolo A, Bonfanti L. 2005. Chain formation and glial tube assembly in the shift from neonatal to adult subventricular zone of the rodent forebrain. The Journal of Comparative Neurology 487:407–427. doi:10.1002/cne.20576.
- Pontious A, Kowalczyk T, Englund C, Hevner, RF. 2008. Role of intermediate progenitor cells in cerebral cortex development. Developmental Neuroscience 30:24–32. doi:10.1159/000109848.
- Rosenstein JM, Krum JM, Ruhrberg C. 2010. VEGF in the nervous system. Organogenesis 6:107–114. doi:10.4161/org.6.2.11687.
- Saghatelyan A. 2009. Role of blood vessels in the neuronal migration. Seminars in Cell & Developmental Biology 20:744–750. doi:10.1016/j.semcdb.2009.04.006.
- Schuller U, Zhao Q, Godinho SA, Heine VM, Medema RH, Pellman D, Rowitch DH. 2007. Forkhead transcription factor FoxM1 regulates mitotic entry and prevents spindle defects in cerebellar granule neuron precursors. Molecular and Cellular Biology 27:8259–8270. doi:10.1128/MCB.00707-07.
- Sentilhes L, Michel C, Lecourtois M, Catteau J, Bourgeois P, Laudenbach V, Marret SAL. 2010. Vascular endothelial growth factor and its high-affinity receptor (VEGFR-2) are highly expressed in the human forebrain and cerebellum during development. Journal of Neuropathology and Experimental Neurology 69:111–128. doi:10.1097/NEN.0b013e3181ccc9a9.
- Sild M, Ruthazer ES. 2011. Radial glia: Progenitor, pathway, and partner. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry 17:288–302. doi:10.1177/1073858410385870.
- Stubbs D, DeProto J, Nie K, Englund C, Mahmud I, Hevner R, Molnar Z. 2009. Neurovascular congruence during cerebral cortical development. Cerebral Cortex 19 Suppl 1:i32–i41. doi:10.1093/cercor/bhp040.
- Virgintino D, Errede M, Robertson D, Girolamo F, Masciandaro A, Bertossi M. 2003. VEGF expression is developmentally regulated during human brain angiogenesis. Histochemistry and Cell Biology 119:227–232.
- Wang LJ, Zhao Y, Han B, Ma YG, Zhang J, Yang DM, et al. 2008. Targeting Slit-Roundabout signaling inhibits tumor angiogenesis in chemical-induced squamous cell carcinogenesis. Cancer Science 99:510–517. doi:10.1111/j.1349-7006.2007.00721.x.
- Yang P, Baker KA, Hagg T. 2005. A disintegrin and metalloprotease 21 (ADAM21) is associated with neurogenesis and axonal growth in developing and adult rodent CNS. The Journal of Comparative Neurology 490:163–179. doi:10.1002/cne.20659.
- Yoshida M. 2001. Glial-defined boundaries in Xenopus CNS. Developmental Neuroscience 23:299–306. doi:10.1159/000048713.
- Yu CH, Yhee JY, Kim JH, Im KS, Kim NH, Kwon SY, Hur TY, Sur JH. 2012. Increased expression of vascular endothelial growth factor in neo-vascularized canine brain tissue. Canadian Journal of Veterinary Research = Revue canadienne de recherche vétérinaire 76:62–68.
- Zhang Y, Niu B, Yu D, Cheng X, Liu B, Deng J. 2010. Radial glial cells and the lamination of the cerebellar cortex. Brain Structure & Function 215:115–122. doi:10.1007/s00429-010-0278-5.
- Zheng Y, Henderson PW, Choi NW, Bonassar LJ, Spector JA, Stroock AD. 2011. Microstructured templates for directed growth and vascularization of soft tissue in vivo. Biomaterials 32:5391–5401. doi:10.1016/j.biomaterials.2011.04.001.
