Abstract
The hydroid fauna of the Mediterranean Sea is considered one of the best known in the world, but the hydrozoans of the Aegean Sea remain poorly studied, hindering efforts to identify alien and invasive species in the region. The spatial and seasonal composition of the shallow-water (0–20 m depth) benthic hydrozoan assemblage from Gökçeada Island was investigated in summer 2012 and winter 2013. Overall, 48 hydrozoan taxa were identified, and their presence and ecological features are discussed herein. Twelve species are recorded for the first time in the Aegean Sea, and the same number for the Turkish coasts. Differences in species composition were detected between the northern and southern coasts of Gökçeada by cluster, multidimensional scaling (MDS) and permutational multivariate analysis of variance (PERMANOVA) analysis, whereas seasonal and vertical distribution patterns were not statistically significant. Differences in species richness and composition between the northern and southern coasts may be explained by the distinct geomorphological aspects of the shores, providing a spatial heterogeneity in the availability of substrates for the hydroid colonies. Observed differences are attributable to the occurrence and/or abundance of common species such as Sertularella ellisii, Aglaophenia tubiformis, Clytia hemisphaerica, Clytia linearis, Eudendrium racemosum, Plumularia obliqua, Eudendrium capillare, Turritopsis dorhnii and Dynamena disticha, rather than to the presence of rare, exclusive species.
Introduction
The Mediterranean Sea, a biodiversity hotspot with approximately 17,000 marine species so far described, is renowned as “extremely well” studied (Coll et al. Citation2010), and its Hydrozoa fauna (siphonophores excluded) can be considered one of the best known in the world, with a total of 400 recorded species (Gravili et al. Citation2013). Because of their high reproductive potential (Boero et al. Citation2002), hydroids represent a key component of shallow-water communities, especially on hard bottoms. Nonetheless, knowledge of these organisms is not uniform throughout the entire Mediterranean Sea, because marine research efforts have historically been concentrated in the proximity of main oceanographic stations along the Western basin and the Adriatic Sea. Therefore, several areas of the Mediterranean, such as the African coasts and the eastern basin, still remain relatively undersampled (Boero et al. Citation1997). Recent studies have aimed to reduce the existing gap of information (e. g. Morri et al. Citation2009; Soto-Àngel & Peña-Cantero Citation2013), but much remains to be done in the Aegean Sea and, more generally, in the Levantine basin (Morri & Bianchi Citation1999).
The available information on the hydroids of the Aegean Sea and neighboring regions is summarized in the papers of Yamada (Citation1965), Marinopoulos (Citation1979), and Morri and Bianchi (Citation1999). Other records of hydrozoans in the basin come from the works of Morri et al. (Citation1999), Cocito et al. (Citation2000) and Bianchi et al. (Citation2011) in the hydrothermal vents of the Greek island of Milos, and of Rayyan et al. (Citation2002) and Rayyan et al. (Citation2004) in the northern (mainly Greek) part of the Aegean. The Turkish section of this sea, on the other hand, is relatively unexplored, and its hydrozoan fauna virtually unknown. Ninety hydrozoan species have been reported from the Turkish coasts so far, 63 of them from the Aegean Sea (Kocataş Citation1976; Marinopoulos Citation1979; Ünsal Citation1981; Marques et al. Citation2000; Gülşahin et al. Citation2013), and 42 from the Sea of Marmara (Demir Citation1954; Ünsal Citation1981; Marques et al. Citation2000; Isinibilir et al. Citation2010), with only 10 species of hydroids reported from Gökçeada Island (Ünsal Citation1981). Furthermore, an alien hydropolyp species (Macrorhynchia philippina) was recently recorded from the Levantine coasts of Turkey (Çinar et al. Citation2006).
The large Turkish island of Gökçeada is located at the core of the above-mentioned undersampled region of the northern Aegean Sea. The complex geomorphology of the island (Koral et al. Citation2009) is encircled by a coastal surface circulation driven by the anticyclone gyre, associated with the Dardanelles low-salinity water input (Olson et al. Citation2007). Gökçeada has a strategic position within the Aegean archipelago, mirroring the role of the Aegean Sea as a biogeographical crossroads between the Marmara Sea (and eventually the Black Sea) and the greater Mediterranean basin, as observed for a number of marine and terrestrial taxa (Ergüven et al. Citation1988; Topaloğlu Citation2001; Isinibilir & Tarkan Citation2002; Ateş et al. Citation2006; Karakulak et al. Citation2006; Aslan-Cihangir Citation2012). Given the above, the present study represents the first inventory of benthic hydrozoans (sub-class Hydroidolina, sensu Marques & Collins Citation2004), from shallow waters (0–20 m depth) at Gökçeada Island, with an assessment of spatial and seasonal ranges of distribution.
Material and methods
Study area
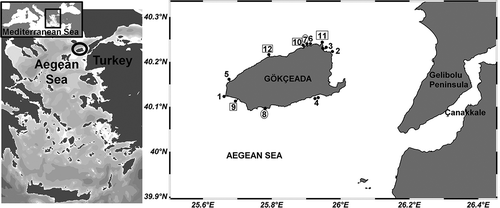
The island of Gökçeada, with a coastline of 92 km and a surface area of 279 km2, is located about 20 km off the Gelibolu Peninsula, in the Aegean Sea, between 40° 05’ 12’’–40° 14’ 18’’ N and 25°40’06’’–26°02’05’’ E. On the northern coast of the island, the continental slope is steep and the Saroz Pit, a tectonic trench, is located immediately off the coast, creating a narrow continental shelf that rapidly gains depth and that is characterized mainly by a rocky substratum. The southern coast, on the other hand, has wide sandy beaches and a wider continental shelf with a slowly deepening, soft substratum. Particularly in summer, the wind circulation around Gökçeada is dominated by northeasterly and southwesterly winds (Kocataş & Bilecik Citation1992). Furthermore, due to the presence of numerous close islands, the water movements in the northern Aegean Sea in the vicinities of Gökçeada vary greatly; the Black Sea waters from the Dardanelles Strait, for example, have an orientation towards the north in winter and towards the south in summer (Kocataş & Bilecik Citation1992). The area is enriched by nutrients of the Meriç River and has a variety of pelagic fish feeding on the abundant phytoplankton (Ulutürk Citation1987). The water mass surrounding the island is affected by cooler and less salty water coming from the Sea of Marmara (Kocataş & Bilecik Citation1992).
Twelve sampling stations were selected around Gökçeada Island; eight (2-Kanyer [KY], 3-Kuzulimanı [KZ], 5-Şeytankale [ŞE], 6-Mavikoy [MA], 7-Yıldızkoy [YI], 10-Kaleköy [KL], 11-Kaşkaval [KŞ] and 12-Pirinç (Kuş Burnu) [KU]) were located on the northern side while four (1-Avlaka [AV], 4-Aydıncık [AY], 8-Laz koyu [LA] and 9-Uğurlu [UG]) were positioned on the southern side of the island (). Stations 1, 11 and 12 are pristine sites with no access from land. Stations 6 and 7 are located within a Marine Protected Area, while 7 hosts thousands of tourists that reside elsewhere on the island but come to Yıldızkoy for clean beaches in the summer. Station 8 suffers similar tourism pressure while stations 2, 3, 4 and 9 are influenced by a somewhat lower (but still consistent) number of tourists in the same season. Additionally, station 7 suffers from sand dumping for recreational purposes by the local municipality before the summer season, and the sand is withdrawn into the sea in autumn, causing temporary turbidity and covering sessile fauna. Station 3 is located in the main harbor of the island and includes samples from both inside and outside the pier. Station 9 is located at a pier with low use by local fishermen. Station 10 is heavily influenced by the nearby human settlement of Kaleköy, and is the only location on the island where sewage discharge occurs.
Sampling and analysis
Sampling took place at the 12 selected stations in either summer (July 2012) or winter (February 2013), or both seasons, depending upon the prevailing climatic conditions (). All sampling stations were characterized by a substrate composed of a mixture of rocks, sand and Posidonia meadows. At each station, benthic hydroid colonies from the bottom and fragments of diverse substrates supporting or susceptible to support hydrozoan colonies (e.g. algae, sea-grasses, invertebrates, etc.) were collected by SCUBA diving in each of four depth zones (0–5 m, 5–10 m, 10–15 m and 15–20 m) along a vertical transect. For the purposes of a faunistic inventory, a visually oriented collection was carried out (Piraino et al. Citation2013). Trained divers selectively picked up only hydroid colonies or their potential substrates from a single, larger homogeneous bottom belt (30 cm height × 1000 cm length = 3000 cm2) at one intermediate depth within each depth zone, whenever available substrates offered a sampling opportunity.
Specimens were sorted in the laboratory and anesthetized using 7% magnesium chloride solution in seawater before being observed alive under a binocular stereomicroscope for primary identification. Hydroids were fixed in 4% formaldehyde a few hours after sampling in order to preserve them for ultimate confirmation of their taxonomic identity under both low- and high-power microscopes. When possible, subsamples were fixed in 90% ethanol to keep a tissue bank for future genetic studies. Nematocysts were examined either on live specimens or from formalin-preserved material, with the aid of a light microscope. Biological samples were deposited at the Hydrozoa Collection of the University of Istanbul (HCUI). Due to the benthic target and adopted sampling, representatives of orders Actinulida, Limnomedusae, Narcomedusae and Trachymedusae (collectively grouped also in the subclass Trachylinae) were not included in our investigation.
Morphological description, updates on synonymy and distribution at the Mediterranean scale are not dealt with in detail since these issues have been recently covered elsewhere for all of the hydroid species found in this study (e. g. Peña-Cantero & García-Carrascosa Citation2002; Bouillon et al. Citation2004; Schuchert Citation2004, Citation2006, Citation2007, Citation2008a, Citation2008b, Citation2010).
There is still a lack of consensus concerning the higher classification of the class Hydrozoa and alternative names are still in use for suprafamily taxa, especially at subclass and order levels (see Bouillon et al. Citation2006; Cartwright & Collins Citation2007; Schuchert Citation2014a). The classification given in Bouillon et al. (Citation2006) is mainly adopted here, although corresponding nomenclature is often provided as from the online World Hydrozoa Database (Schuchert Citation2014b).
Cluster and ordination analysis were carried out using the presence/absence data of taxa in order to identify spatiotemporal patterns of species composition in the study area. The selected similarity index was that of Sørensen (Citation1948). The generated similarity matrix was used to obtain a cluster diagram through the hierarchical grouping method and a multidimensional scaling (MDS) representation in order to identify the existing relationships among sampling stations based on biological information. The SIMPROF (similarity profile) test was used in association with the cluster analysis (Clarke et al. Citation2008) in order to test the null hypothesis of no meaningful structure within groups of samples (average of 1000 permutations and 999 simulations, α = 0.05).
Differences in the hydrozoan assemblages according to the sampled region of the island (south vs. north), season (summer vs. winter) and depth of collection (0–5 m, 5–10 m, 10–15 m and 15–20 m) were tested using a permutational multivariate analysis of variance (PERMANOVA, Anderson Citation2001) in which each term was tested using 9999 random permutations, while a similarity percentage analysis (SIMPER) was performed to determine the contribution of each taxon to the dissimilarities between groups obtained from the cluster and MDS. Our experimental design included three factors: (1) “exposure” (fixed, two levels, north and south); (2) “season” (fixed, two levels, summer and winter); (3) “depth” (fixed, four levels, 0–5 m, 5–10 m, 10–15 m and 15–20 m). All of the above analyses were carried out with the Primer v. 6 software (Clarke & Gorley Citation2006).
Results and discussion
Forty-eight hydrozoan (Hydroidolina) taxa () were found along the coasts of Gökçeada, out of which 12 represented first records for the Aegean Sea, and 13 for the Turkish coastal area. Overall, we collected 19 representatives of the Athecata/Anthomedusae and 29 of the Thecata/Leptomedusae (also synonymized as orders Anthoathecata and Leptothecata, respectively, by Cornelius Citation1992). Twenty-seven species were collected from both the northern and southern coasts, whereas 21 species were recorded only from one of the two coasts, 15 from the northern shores and six from the southern shores of Gökçeada.
Table I. List of Hydrozoa taxa recorded in the present study on Gökçeada Island. Species marked with “ + ” are first records for the Aegean Sea, and those marked with “#” are first records for the Turkish coasts. Previous records of the species in the Turkish Aegean coasts, Greek Aegean Sea, Marmara Sea and the Levantine Basin are indicated with: TA, A, MS and L, respectively (references for these records are Demir Citation1954; Kocataş Citation1976; Marinopoulos Citation1979; Ünsal Citation1981; Svoboda & Cornelius Citation1991; Morri & Bianchi Citation1999; Marques et al. Citation2000; Morri et al. Citation2009). Acronyms of biogeographic affinity are as follows: C, cosmopolitan; ME, Mediterranean endemic; B, boreal; I, Indo-Pacific; CT, circumtropical; AM, Atlanto-Mediterranean; TA, tropical Atlantic. Acronyms of seasonality and reproductive status: R, fertile; J, June; F, February.
The high number of new records in this study is mainly due to the remarkable scarcity of studies in the region. Two species (Bougainvillia muscus and Filellum cf. serpens) were previously recorded in the Marmara Sea, but there were no further records from the Turkish Aegean coasts. In addition, Clytia linearis is a lessepsian species that was recorded for the first time in Turkey in the present work. Thus, the present record of 48 species, all previously known in the western Mediterranean, constitutes a significant increase in the number of hydroid species recorded in the Aegean Sea. Previously, Yamada (Citation1965) had recorded 12 hydrozoan species from the vicinity of Athens, while Kocataş (Citation1976) found 10 species in the Gulf of İzmir, Morri and Bianchi (Citation1999) observed 31 in two southern Aegean islands (Kos and Milos), and Ünsal (Citation1981) recorded another 23 in the Turkish Aegean coasts. Thus, this study provides the basis for the increase of the number of hydroid species known from the Aegean Sea to a total of 88.
The hydroid fauna of Gökçeada Island is dominated by circumtropical species (15 species, 33%). This proportion is similar to that reported from the Levantine Sea (Morri et al. Citation2009) and the Maltese islands (Soto-Àngel & Peña-Cantero Citation2013), the general warm-water affinity of the Gökçeada hydrozoan fauna being evident. Overall, the present collection showed a relatively low proportion of Atlantic–Mediterranean elements; these data, however, must be taken with caution, since the total number of Atlantic–Mediterranean species may increase, provided that more studies are conducted in the region.
As for substrate preference, most species are epibionts, being found mainly on hard surfaces such as algal bioconcretions, bryozoans and polychaete tubes. Although some species (e.g. Aglaophenia elongata, Kirchenpaueria halecioides) were found to be only epilithic in our samples (eight species), they have been observed growing on other substrate types elsewhere (see for example Boero Citation1981; Svoboda & Cornelius Citation1991). Twenty-one species in our samples were both epilithic and epibionts; three other species were exclusively found on other hydroids (Lafoeina tenuis, Anthohebella parasitica and Clytia cf. paulensis), and five species could utilize both hydroids and other substrates. Clytia cf. paulensis, however, was observed only once, so the information on its preferred substrate is by no means conclusive. Only four species were found on P. oceanica meadows, and two of them, namely Aglaophenia harpago and Sertularia perpusilla, are obligate and specialized epibionts restricted to this sea grass species. Posidonia oceanica meadows exhibit a complex biotic community and have a very high productivity and diversity of invertebrates (Pergent et al. Citation1994; Borg et al. Citation2006). Hydrozoans are one of the most remarkable taxa inhabiting these meadows, as pointed out in several studies (e. g. Boero Citation1981, Citation1987). Hydrozoan diversity may, however, be underestimated in the Gökçeada Posidonia meadows, because of our sampling approach which was not focused on the leaves of this species. Notwithstanding this, the present collection of species suggests that substrate diversity at Gökçeada Island directly influences hydrozoan diversity in the region.
In general terms, a pattern of variation between the samples collected on the northern coasts of Gökçeada and those obtained from the southern shoreline was identified by the classification analysis () and the PERMANOVA (), and to a lesser extent also by the MDS ().
Figure 2. Similarity dendrogram (Sørensen index) of the samples based on the hydroid species composition of 12 stations along the coasts of Gökçeada (Aegean Sea). The codes identifying the samples are as follows: number of the station; station acronym; “su” for summer or “wi” for winter; depth of the sample. Thick lines connect samples which differ significantly (similarity profile, SIMPROF P < 0.05).
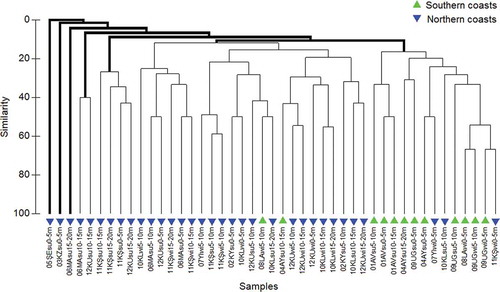
Figure 3. Tridimensional representation of the multidimensional scaling (MDS) of the samples based on the hydroid species composition of 12 stations along the coasts of Gökçeada (Aegean Sea). Acronyms identifying the samples are omitted for clarity, except for the most distinctive Stations 3 and 5 (see text).
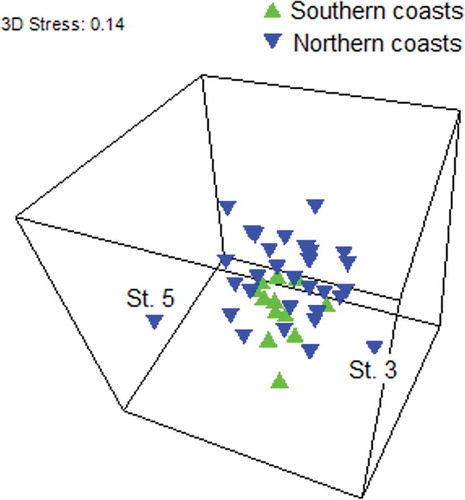
Table II. Permutational multivariate analysis of variance (PERMANOVA) of hydrozoan assemblages (48 taxa) of Gökçeada Island, (Aegean Sea). Statistically significant results are highlighted within a rectangle. Sources of variation are as follow: Se = season; Ex = exposure (north or south); De = depth; df = degrees of freedom; SS = sum of squares; MS = mean square; Pseudo-F = Pseudo-F statistic; P (perm) = probability after the permutations.
The PERMANOVA comparisons revealed that no significant difference can be attributed to the season or the depth of sampling, whereas the (southern or northern) exposure of the collection sites was significant in determining the observed differences between samples ( and ). The main difference between the northern and the southern coasts of the island is related to their geomorphology. The southern coast has a much more gradual bottom slope and soft substrates are much more abundant than in the northern area, where it is common to find vertical or sub-vertical rocky cliffs. This difference has therefore influenced the number of sampling stations in the northern area (eight), double the number of hydroid samplings available in the southern coast (four).
Soft bottoms indeed offer a limited selection of natural substrates and a reduced diversity of benthic species which may represent potentially available substrates for epibiotic hydrozoans. The nature of the substrate significantly influences species composition, diversity, abundance and distribution of benthic organisms, including hydrozoans, and hydroid assemblages are generally more diverse and abundant on hard substrates (Calder Citation1991). It is worth noting that the differences observed between the northern and southern samples seem to be related to the presence of common, locally abundant species (Sertularella ellisii, Aglaophenia tubiformis, Clytia hemisphaerica, Clytia linearis, Eudendrium racemosum, Plumularia obliqua, Eudendrium capillare, Turritopsis dorhnii and Dynamena disticha) rather than rare, exclusive species as shown in the SIMPER analysis (). Common, dominant species have often been held responsible for richness and diversity patterns in hydrozoan assemblages elsewhere in Mediterranean habitats, and, in fact, several spatial patterns in richness reported in the literature appear to be caused by relatively few, common species (Megina et al. Citation2013). Besides C. linearis, no new alien species were detected, providing evidence that Gökçeada may represent a still-unaffected hotspot of Eastern Mediterranean hydrozoan biodiversity.
Table III. Similarity percentage analysis (SIMPER) analysis of hydrozoan contributions to dissimilarities between northern and southern samples from Gökçeada Island, Northern Aegean Sea. δ = Average dissimilarity between northern and southern samples; SD = standard deviation of δ; Contrib.% = percentage contribution of each species to the overall dissimilarity; Cum.% = cumulative percentage of contribution; only main contributors (up to a cumulative 40%) are shown. Species in bold contributed the most to the similarity of southern stations. Species with * contributed the most to the similarity of northern stations.
The cluster diagram () and MDS representation () showed that stations 3 (Kuzulimanı) and 5 (Şeytankale) were highly different from the other sampling stations, mainly due to their low number of species and unique species composition. Both stations present very particular conditions, which most probably are responsible for their singularity in terms of the hydrozoan fauna. Station 3 (Kuzulimanı) is the main harbor of the island, being subjected to a high anthropogenic pressure that seems to condition the presence of a reduced assemblage of hydrozoans. Only some widespread and opportunistic species (e.g. the well-known Clytia hemisphaerica, Clytia linearis and Aglaophenia picardi) are able to establish permanent populations in this disturbed environment. These results are consistent with the findings of Megina et al. (Citation2013), who observed harbor hydroid assemblages to be significantly different from natural ones mainly due to their qualitative composition.
An interesting finding related also to the fauna of the harbor and its vicinity in Gökceada is that of Ectopleura larynx, which is considered a species found very often in or around harbors in temperate and cold waters around the world, and that may sometimes cause problems to aquaculture and port operations (Guenther et al. Citation2009, Citation2010). We found this species exclusively in station 2 (Kanyar), very close to the harbor of Kuzulimanı (station 3). This vicinity suggests that the species may actually be present in the harbor but was missed in the only sampling conducted at this station. The abundance of E. larynx in our samples was not very high, however, and more studies are needed to establish the extent of its populations in the study area.
Species inventories and distribution records represent key tools to biodiversity research. The diversity of benthic hydrozoans recorded in the last decade in the Mediterranean Sea is restricted to 156 species (Gravili et al. Citation2013) and, out of them, 48 taxa were collected by this study on the Gökçeada coast. In spite of the limited temporal samplings (1 week in summer; 1 week in winter), and the depth limitation (0–20 m) due to diving operations, nearly one third of the contemporary (i.e. recently collected) Mediterranean species of hydroids were detected in Gökçeada. Therefore, the northern Aegean Sea appears to be a representative, high-diversity area of the Eastern basin, probably acting as a biogeographic crossroads across the different ecological and hydrological features of the Black Sea, the Aegean Sea and the Ionian Sea. The near absence of hydrozoan aliens in the present faunistic inventory qualifies Gökçeada as a hotspot for future biodiversity studies and for early monitoring of the impact of climate change in the Eastern Mediterranean.
Acknowledgements
The authors are grateful to the scientists and technicians of Gökçeada Marine Research Center for their help during samplings and analyses. This study was supported by the Istanbul University (project number IRP-20587).
References
- Anderson MJ. 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecology 26:32–46.
- Aslan-Cihangir H. 2012. The Echinoderm fauna of Gokceada island (NE Aegean Sea). Journal of Animal and Veterinary Advances 11:26–29. doi:10.3923/javaa.2012.26.29.
- Ateş A, Katagan T, Kocatas A, Sezgin M. 2006. Decapod crustaceans on the Gökçeada (Imbros) island continental shelf (north-eastern Aegean Sea). Mediterranean Marine Science 7:55–60. doi:10.12681/mms.169.
- Bianchi CN, Dando PR, Morri C. 2011. Increased diversity of sessile epibenthos at subtidal hydrothermal vents: Seven hypotheses based on observations at Milos Island, Aegean Sea. Advances in Oceanography and Limnology 2:1–31. doi:10.1080/19475721.2011.565804.
- Boero F. 1981. Systematics and ecology of the hydroid population of two Posidonia oceanica meadows. Marine Ecology 2:181–197. doi:10.1111/j.1439-0485.1981.tb00093.x.
- Boero F. 1987. Evolutionary implications of habitat selection in the hydroids of Posidonia oceanica meadows. In: Bouillon J, Boero F, Cicogna F, Cornelius PFS, editors. Modern trends in the systematics, ecology, and evolution of hydroids and hydromedusae. Oxford: Clarendon Press. pp. 251–256.
- Boero F, Bouillon J, Piraino S, Schmid V. 2002. Asexual reproduction in the Hydrozoa. In: Hughes RN, editor. Reproductive biology of invertebrates - progress in asexual reproduction. New Delhi and Oxford: IBH Publishing. pp.141–158.
- Boero F, Gravili C, Denitto F, Miglietta MP, Bouillon J. 1997. The rediscovery of Codonorchis octaedrus (Hydroidomedusae, Anthomedusae, Pandeidae), with an update of the Mediterranean hydroidomedusan biodiversity. Italian Journal of Zoology 64:359–365. doi:10.1080/11250009709356223.
- Borg JA, Rowden AA, Attrill MJ, Schembri PJ, Jones MB. 2006. Wanted dead or alive: High diversity of macroinvertebrates associated with living and ‘dead’ Posidonia oceanica matte. Marine Biology 149:667–677. doi:10.1007/s00227-006-0250-3.
- Bouillon J, Gravili C, Pagès F, Gili JM, Boero F. 2006. An introduction to Hydrozoa. Paris: Publications Scientifiques du Muséum.
- Bouillon J, Medel MD, Pagès F, Gili JM, Boero F, Gravili C. 2004. Fauna of the Mediterranean Hydrozoa. Scientia Marina 68:5–438. doi:10.3989/scimar.2004.68s25.
- Calder DR. 1991. Associations between hydroid species assemblages and substrate types in the mangal at Twin Cays, Belize. Canadian Journal of Zoology 69:2067–2074. doi:10.1139/z91-288.
- Cartwright P, Collins A. 2007. Fossils and phylogenies: Integrating multiple lines of evidence to investigate the origin of early major metazoan lineages. Integrative and Comparative Biology 47:744–751. doi:10.1093/icb/icm071.
- Çinar ME, Bilecenoğlu M, Öztürk B, Can A. 2006. New records of alien species on the Levantine coast of Turkey. Aquatic Invasions 1:84–90. doi:10.3391/ai.2006.1.2.6.
- Clarke KR, Gorley RN. 2006. Primer v6: User Manual/Tutorial. 1st ed. Plymouth, UK: Plymouth Marine Laboratory.
- Clarke KR, Somerfield PJ, Gorley RN. 2008. Testing of null hypotheses in exploratory community analyses: Similarity profiles and biota-environment linkage. Journal of Experimental Marine Biology and Ecology 366:56–69. doi:10.1016/j.jembe.2008.07.009.
- Cocito S, Bianchi CN, Morri C, Peirano A. 2000. First survey of sessile communities on subtidal rocks in an area with hydrothermal vents: Milos Island, Aegean Sea. Hydrobiologia 426:113–121. doi:10.1023/A:1003991117108.
- Coll M, Piroddi C, Steenbeek J, Kaschner K, Ben Rais LF et al. 2010. The biodiversity of the Mediterranean Sea: Estimates, patterns, and threats. PLoS ONE 5:e11842.
- Cornelius PFS. 1992. Medusa loss in leptolid Hydrozoa (Cnidaria), hydroid rafting, and abbreviated life-cycles among their remote-island faunae: An interim review. Scientia Marina 56:245–261.
- Demir M. 1954. Boğaz ve Adalar sahillerinin omurgasız dip hayvanları. İst.Üniv. Fen Fak. Hidrobiyoloji Araşt.Enst.Yay.no:3. (in Turkish).
- Ergüven H, Ulutürk T, Oztürk B. 1988. Gökceada’nin Porifera (sünger) faunasi ve üretim imkanlari. Journal of Aquatic Products 2:173–189. (in Turkish).
- Gravili C, Di Camillo CG, Piraino S, Boero F. 2013. Hydrozoan species richness in the Mediterranean Sea: Past and present. Marine Ecology 34:41–62. doi:10.1111/maec.12023.
- Guenther J, Carl C, Sunde LM. 2009. The effects of colour and copper on the settlement of the hydroid Ectopleura larynx on aquaculture nets in Norway. Aquaculture 292:252–255. doi:10.1016/j.aquaculture.2009.04.018.
- Guenther J, Misimi E, Sunde LM. 2010. The development of biofouling, particularly the hydroid Ectopleura larynx, on commercial salmon cage nets in Mid-Norway. Aquaculture 300:120–127. doi:10.1016/j.aquaculture.2010.01.005.
- Gülşahin N, Tarkan AN, Bilge G. 2013. The hydrozoan Geryonia proboscidalis (Forskål, 1775), new for Turkey (Hydrozoa). Zoology in the Middle East 59:93–94. doi:10.1080/09397140.2013.795077.
- Isinibilir M, Tarkan AN. 2002. Distribution of the invasive ctenophore Mnemiopsis leidyi (Agassiz, 1865) in the northeastern Aegean Sea in August 1998. Turkish Journal of Fisheries and Aquatic Sciences 2:129–132.
- Isinibilir M, Yilmaz IN, Piraino S. 2010. New contributions to the jellyfish fauna of the Marmara Sea. Italian Journal of Zoology 77:179–185. doi:10.1080/11250000902895766.
- Karakulak FS, Erk H, Bilgin B. 2006. Length–weight relationships for 47 coastal fish species from the northern Aegean Sea, Turkey. Journal of Applied Ichthyology 22:274–278. doi:10.1111/j.1439-0426.2006.00736.x.
- Kocataş A. 1976. Note sur le Peuplement a Cytoseira crinita Bory Dans le Golfe d’İzmir (Turquie). Tethys 2-3:241–248.
- Kocataş A, Bilecik N. 1992. Ege Denizi ve Kaynakları. T.C. Tarım ve Köyişleri Bakanlığı Su Ürünleri Araştırma Enstitüsü. Seri A, Yayın no 7.
- Koral H, Öztürk H, Hanilçi N. 2009. Tectonically induced coastal uplift mechanism of Gökçeada Island, Northern Aegean Sea, Turkey. Quaternary International 197:43–54. doi:10.1016/j.quaint.2008.04.001.
- Marinopoulos J. 1979. Biological survey of the eastern Mediterranean Sea: Hydroids (preliminary study). Rapports et Procès Verbaux des Réunions, Commission Internationale pour l’Exploration Scientifique de la Mer Méditerranée: 119–120.
- Marques AC, Collins AG. 2004. Cladistic analysis of Medusozoa and cnidarian evolution. Invertebrate Biology 123:23–42. doi:10.1111/j.1744-7410.2004.tb00139.x.
- Marques AC, Peña Cantero AL, Vervoort W. 2000. Mediterranean species of Eudendrium Ehrenberg, 1834 (Hydrozoa, Anthomedusae, Eudendriidae) with the description of a new species. Journal of Zoology 252:197–213. doi:10.1111/j.1469-7998.2000.tb00615.x.
- Megina C, González-Duarte MM, López-González PJ, Piraino S. 2013. Harbours as marine habitats: Hydroid assemblages on sea-walls compared with natural habitats. Marine Biology 160:371–381. doi:10.1007/s00227-012-2094-3.
- Morri C, Bianchi CN. 1999. Hydroids (Cnidaria: Hydrozoa) from the Aegean Sea, mostly epiphytic on algae. Cahiers de Biologie Marine 40:283–291.
- Morri C, Bianchi CN, Cocito S, Peirano A, De Biase AM, Aliani S, Pansini M, Boyer M, Ferdeghini F, Pestarino M, Dando P. 1999. Biodiversity of marine sessile epifauna at an Aegean island subject to hydrothermal activity: Milos, Eastern Mediterranean Sea. Marine Biology 135:729–739. doi:10.1007/s002270050674.
- Morri C, Puce S, Bianchi CN, Bitar G, Zibrowius H, Bavestrello G. 2009. Hydroids (Cnidaria: Hydrozoa) from the Levant Sea (mainly Lebanon), with emphasis on alien species. Journal of the Marine Biological Association of the United Kingdom 89:49–62. doi:10.1017/S0025315408002749.
- Olson DB, Kourafalou VH, Johns WE, Samuels G, Veneziani M. 2007. Aegean surface circulation from a satellite-tracked drifter array. Journal of Physical Oceanography 37:1898–1917. doi:10.1175/JPO3028.1.
- Peña-Cantero AL, Garcia Carrascosa AM. 2002. The benthic hydroid fauna of the Chafarinas Islands (Alboran Sea, western Mediterranean). Zoologische Verhandelingen 337:1–180.
- Pergent G, Romero J, Pergent-Martini C, Mateo M-A, Boudouresque C-F. 1994. Primary production, stocks and fluxes in the Mediterranean seagrass Posidonia oceanica. Marine Ecology-Progress Series 106:139–146. doi:10.3354/meps106139.
- Piraino S, De Vito D, Brodbeck E, Di Camillo CG, Fanelli G, Boero F. 2013. Destructive standard squares or low-impact visually driven collection? A comparison of methods for quantitative samplings of benthic hydrozoans. Italian Journal of Zoology 80:424–436. doi:10.1080/11250003.2013.787461.
- Rayyan A, Christidis J, Chintiroglou CC. 2002. First record of the bivalve-inhabiting hydroid Eugymnanthea inquilina in the eastern Mediterranean Sea (Gulf of Thessaloniki, north Aegean Sea, Greece). Journal of the Marine Biological Association of the UK 82:851–853. doi:10.1017/S0025315402006239.
- Rayyan A, Photis G, Chintiroglou CC. 2004. Metazoan parasite species in cultured mussel Mytilus galloprovincialis in the Thermaikos Gulf (North Aegean Sea, Greece). Diseases of Aquatic Organisms 58:55–62. doi:10.3354/dao058055.
- Schuchert P. 2004. Revision of the European athecate hydroids and their medusae (Hydrozoa, Cnidaria): Families Oceanidae and Pachycordylidae. Revue Suisse de Zoologie 111:315–369.
- Schuchert P. 2006. The European athecate hydroids and their medusae (Hydrozoa, Cnidaria): Capitata Part 1. Revue Suisse de Zoologie 113:325–410.
- Schuchert P. 2007. The European athecate hydroids and their medusae (Hydrozoa, Cnidaria): Filifera Part 2. Revue Suisse de Zoologie 114:195–396.
- Schuchert P. 2008a. The European athecate hydroids and their medusae (Hydrozoa, Cnidaria): Filifera Part 3. Revue Suisse de Zoologie 115(2):221–302.
- Schuchert P. 2008b. The European athecate hydroids and their medusae (Hydrozoa, Cnidaria): Filifera Part 4. Revue Suisse de Zoologie 115:677–757.
- Schuchert P. 2010. The European athecate hydroids and their medusae (Hydrozoa, Cnidaria): Capitata part 2. Revue suisse de Zoologie 117:337–555.
- Schuchert P. 2014a. High genetic diversity in the hydroid Plumularia setacea: A multitude of cryptic species or extensive population subdivision? Molecular Phylogenetics and Evolution 76:1–9. doi:10.1016/j.ympev.2014.02.020.
- Schuchert P. 2014b. World Hydrozoa database. Available: http://www.marinespecies.org/hydrozoa on 20140721. Accessed May 2014 7.
- Sørensen TA. 1948. A method of establishing groups of equal amplitude in plant sociology based on similarity of species content, and its application to analyses of the vegetation on Danish commons. Kongelige Danske Videnskabernes Selskabs Biologiske Skrifter 5:1–34.
- Soto-Àngel JJ, Peña-Cantero Á L. 2013. Shallow-water benthic hydroids from the Maltese Islands (Central Mediterranean). Marine Ecology 34:177–196. doi:10.1111/maec.12035.
- Svoboda A, Cornelius PFS. 1991. The European and Mediterranean species of Aglaophenia (Cnidaria: Hydrozoa). Zoologisches Verhandelingen 274:1–72.
- Topaloğlu B. 2001. A preliminary study on sponge fauna of the north shore of Gökceada Island. Gökceada: Ulus. Ege Adal.Toplant. (in Turkish).
- Ulutürk T. 1987. Gökçeada Çevresinin Balık Faunası ve Çevre Fon Radyoaktivitesi. İ.Ü. Su Ürünleri Dergisi, Cilt 1, Sayı 1.
- Ünsal İ. 1981. Ege ve Marmara denizlerinin hidroid polipleri. (Doçentlik Tezi). İst. Üniv. Fen Fak.Biyo1oji Bolümü. 70 pp. (in Turkish).
- Yamada M. 1965. Marine hydroids from Greece. Publications of the Seto Marine Biological Laboratory 12:359–362.