Abstract
This paper reports the first finding of the sea pen Protoptilum carpenteri Kölliker, 1872 in the Mediterranean Sea. A total of three colonies were collected in 2010 with an epibenthic sledge, and one colony in 2013 with a bottom-trawl net, in the Santa Maria di Leuca (SML) coral province in the Ionian Sea. The main anatomical features and taxonomic characteristics are reported and discussed in order to update the knowledge of this species. A description of morphologies and dimensions of the sclerites, taken from different parts of the colony, is reported. A comparison with Atlantic records is given and discussed.
Introduction
Protoptilum carpenteri Kölliker, 1872 is a sea pen belonging to the family Protoptilidae, which currently includes six accepted species of the genus Protoptilum and one of the genus Distichoptilum. This family is characterized by long slender colonies, with bilateral symmetry and with a prominent axis, rounded in cross-section or sometimes slightly flattened and present throughout the length of the colony (Kükenthal Citation1915; Williams Citation1990). The rachis presents solitary polyps that arise laterally and sometimes ventrally on the colony. Autozooids are arranged in one to three more or less distinct longitudinal series, which can simultaneously form diagonally ascending rows (Kükenthal Citation1915). Polyps are retractile into spiculate calyces, which appear asymmetrically developed, and adhere to the rachis along their inner proximal portions and face upward. Between the autozooids there are several to numerous siphonozooids. The sclerites are numerous, three-flanged and mostly spindles (Williams Citation1995). Protoptilum carpenteri is characterized by a narrow, thin rachis, ca. 1–2 mm wide, with a narrow stripe bare of zooids on the dorsal side and throughout the length of the rachis (Jungersen Citation1904; Kükenthal Citation1915). The colony is supported by a calcareous axis, cylindrical in cross section, about 0.4 mm in diameter, and located in the whitish coenenchyme throughout the whole colony. Autozooids of P. carpenteri are arranged in two longitudinal rows along opposite sides of the rachis: a ventral and a dorsal row on either side (Jungersen Citation1904; Kükenthal Citation1915). Autozooid calyces are formed like a horn or straight cornucopia, without evident teeth, and are 2–3 mm long and 1–1.1 mm wide (Jungersen Citation1904; Kükenthal Citation1915; Grasshoff Citation1981). Polyps are reinforced by sclerites arranged obliquely and only located along the aboral side of the tentacle-stem (Jungersen Citation1904; Kükenthal Citation1915). Siphonozooids have a calyx of similar shape and appearance to that of the autozooids, but much smaller. Along the colony of P. carpenteri, there are more than 2–3 siphonozooids per autozooid, often numerous and congested around the autozooids. The siphonozooids are arranged in 2–3 rows along each side of the dorsal midline. However, on the ventral side, there are fewer siphonozooids, approximately half the number of the dorsal ones (Jungersen Citation1904; Kükenthal Citation1915).
The sclerites of the polyps, calyces, rachis and peduncle are three-flanged spindles, needles or rods, completely smooth on their surface (Jungersen Citation1904; Kükenthal Citation1915). The sclerites of calyces and rachis are bright red in color and give this color to the rachis too, with the exception of the bare streak on the dorsal side which is yellowish-white. The sclerites of calyces and rachis are about 0.240–0.480 mm long and 0.016–0.032 mm wide (Jungersen Citation1904; Kükenthal Citation1915), whereas polyp sclerites are smaller, measuring about 0.064 mm in length and up to 0.0112 mm in breadth (Jungersen Citation1904). These sclerites form a red streak on the tentacle-stem, which is clearly visible due to the contrast with the whitish polyps.
The sclerites on the peduncle are yellowish to white, as is the whole peduncle. Their size is not reported in the literature.
The presence of a terminal bulb at the base of the colony is not a specific character of the species (Jungersen Citation1904).
This paper reports the first record of Protoptilum carpenteri in the Mediterranean Sea. Furthermore, a detailed description of the collected colonies is provided in order to increase the knowledge of this species.
Distribution of Protoptilum carpenteri
Protoptilum carpenteri shows a North Atlantic distribution and it is considered a deep-sea species (Kükenthal Citation1915). It has been found in several areas of the North Atlantic Ocean, from Canada and Northern Europe to West Africa. In particular, P. carpenteri has been found from Massachusetts to North Carolina, in West Africa and Northern Europe, between 1334 and 2194 m in depth (samples in the US National Museum of Natural History collection, Ocean Biogeographic Information System); south of New York by the Challenger Expedition (indicated as P. aberrans), at depths of 2268, 2469 and 3109 m (Kölliker Citation1880); in the North Atlantic Ocean by the Porcupine Expedition, at 1262 and 1326 m depth (Marshall & Fowler Citation1888); south of Iceland by the Ingolf Expedition, at 1545 and 2150 m in depth (Jungersen Citation1904); from the North Atlantic to the Azores, between depths of 650 and 4270 m (Grasshoff Citation1981); from Baffin Bay to Newfoundland (Canada), between depths of about 600 and 1000 m (Gilkinson & Edinger Citation2009); in Newfoundland, off the Grand Banks, between about 400 and 2300 m in depth (Baker et al. Citation2012). Therefore, this species can live in a wide bathymetric range, since it is certainly present between depths of 400 and 4270 m in the Atlantic Ocean.
Although Protoptilum carpenteri is considered to be present in the North Atlantic and European waters (Williams & van der Land Citation2001), there are no previous documented records for the Mediterranean Sea.
Material and methods
Sampling was carried out in April 2010, in the Santa Maria di Leuca (SML) coral province (Ionian Sea; Central Mediterranean) () (Tursi et al. Citation2004; Taviani et al. Citation2005; Corselli Citation2010), during a research cruise on board the R/V (Research Vessel) Minerva I, within the EU 7FP CoralFISH project. The first findings (Stations BT47 and BT64; ) were carried out by means of an epibenthic sledge with a fixed iron opening of 3.8 m by 0.46 m and a mesh size of 0.5 cm. Sampling was carried out between 412 and 451 m in depth on soft bottom habitats (SB sensu Vertino et al. Citation2010) characterized by a gently sloping sea floor and a smooth surface, without mounds or any other seafloor irregularity (Mastrototaro et al. Citation2013). Each tow was carried out for about 30 min at a speed of about 3 knots.
Figure 1. Study area: (a) location within the Mediterranean Sea; (b) bathymetric map of the area with detail of the Santa Maria di Leuca (SML) coral province.
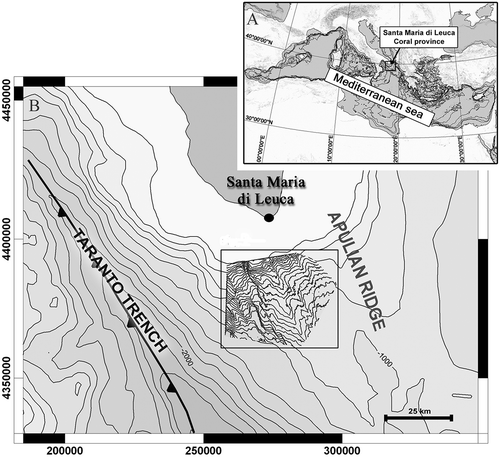
Table I. Number of colonies of Protoptilum carpenteri collected at each sampling station, relative period, depth range, geographic coordinates and sampling tool.
A second benthic survey took place in June 2013, in the same area, during an experimental bottom trawl fishing sampling within the MEDITS (International bottom trawl survey in the Mediterranean) project. This second sampling event was carried out using an experimental trawl net on a muddy bottom area (Station ST69; ), close to the SML coral province, in a depth range of 240–244 m. The trawl net was equipped with a SCANMAR (sonar system for monitoring fishing gears) net sensor to monitor the net opening and a MINILOG submersible temperature logger.
The sampled colonies of Protoptilum carpenteri were anaesthetized with a saturated solution of menthol in seawater for 2–4 hours according to the size of the colonies and the contractions of the polyps. After this procedure, the colonies were preserved in 5% seawater–formalin solution.
All the collected colonies were measured. The total length of colonies and the lengths of rachis and peduncle were measured, as well as the width of the colonies (measured at the middle area of each colony, without including autozooid or siphonozooid calyces). The colonies were identified considering the main taxonomic characters of the species, such as the distribution of polyps on the rachis, the morphology of autozooids and siphonozooids, and the shape and the distribution of carbonatic sclerites in both the rachis and peduncle. The length and width of calyces of autozooids and siphonozooids and the distance between autozooid calyces along the axis for the whole length of rachis were also measured.
Microscope slides of sclerites were prepared by collecting small portions of tissue from the peduncle and rachis. From the rachis in particular, separated samples were taken from the polyps, autozooid calyces, siphonozooid calyces and body portions without zooids, in order to isolate and distinguish sclerites from different parts of the colony. Each portion of tissue was digested in sodium hypochlorite; then the sclerites were washed in distilled water and dehydrated with a sequence of alcohol solutions (70%, 80%, 95% and absolute ethanol). A portion of the sclerites was transferred to slides, observed and measured using an optical microscope. The length and width of a total of 750 sclerites were measured: 150 for each portion of the colony (peduncle, polyps, autozooids calyces, siphonozooid calyces and rachis without zooids). The Student’s t-test was also applied for samples with similar variance to compare the size of sclerites from the autozooid calyces, siphonozooid calyces and body parts without any calyces.
Results
During the sampling that occurred in April 2010 with the epibenthic sledge, a total of three colonies of Protoptilum carpenteri were collected: one colony between 412 and 446 m in depth and two colonies between 429 and 451 m in depth. One of these two colonies was incomplete so it was not possible to take biometric measurements. Another colony of P. carpenteri was collected during the bottom trawl fish sampling in June 2013, between 240 and 244 m in depth (). The bottom temperature registered was 14.00°C. Two complete colonies of P. carpenteri collected in April 2010 in the SML coral province are now part of the collection of the Zoological Museum of the University of Bari (Code MUZAC-6277 for the BT47 colony and MUZAC-6278 for the BT64 one).
Morphology and biometry of the colonies
The colonies are long and thin, with a red–orange rachis and a yellowish-white peduncle. The colonies are supported by a central calcareous axis, whitish and rounded in cross section, of about 0.5 mm in diameter. The axis extends for the whole length of the colony within the coenenchyme. There is no evident terminal bulb in any of the colonies sampled (a).
Figure 2. Protoptilum carpenteri Kölliker, 1872 collected in the Santa Maria di Leuca (SML) coral province (Mediterranean Sea): A, whole colony 451 mm long; B, rachis with autozooids (Aut) and siphonozooids (Siph); C, detail of the middle zone of rachis with autozooids and siphonozooids; D, polyp of autozooids; E, sclerites of autozooid polyp; F, autozooid calyx with sclerites; G, detail of polyp tentacle. Scale bars: A, 20 mm; B, C, 2 mm; D, E, 1 mm; F, G, 0.40 mm.
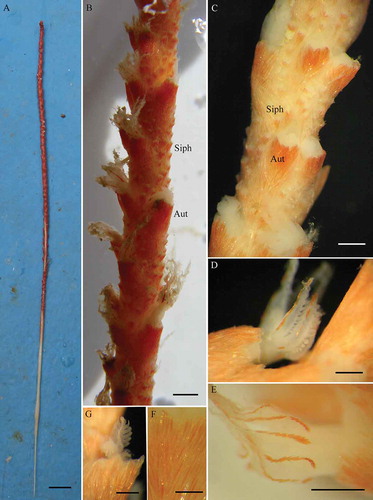
Along the entire length of the rachis there are solitary autozooids and siphonozooids, with the exception of a thin white dorsal streak devoid of zooids. This stripe becomes wider towards the peduncle.
On the ventral side of the neck area of the colony (between the rachis and peduncle) where the bare streak widens, there is a bare white area which, going down towards the peduncle, joins with the opposite dorsal bare area. The two bare areas, the dorsal one and the ventral one, widen to meet each other and to open up the peduncle. However, going up towards the rachis, the ventral bare area soon disappears while the polyps on the two lateral sides join, and the only bare area that remains is the dorsal streak.
The solitary autozooids arise laterally and ventrally on the rachis, arranged in one to three more or less distinct longitudinal series along opposite sides (b and c). The calyces are spiculated, horn-like in shape, adherent to the rachis along their ventral side and facing upward, without evident teeth (f). The polyps are retractile into the calyces and have obliquely located sclerites along the aboral side of the tentacles (d, e and g). Several siphonozooids are present along the whole rachis, between and around autozooids, sometimes in groups and sometimes forming longitudinal series.
The three complete colonies collected measure between 350 and 451 mm in length and between 2.0 and 2.5 mm in width, with a rachis between 275 and 331 mm long that represent 73–81% of the total length of the colony (). The peduncles are between 74 and 120 mm long.
Table II. Minimum, maximum, mean and standard deviation of the biometric measurements (mm) and the % of the rachis of Protoptilum carpenteri in relation to the total length of the colony in the four colonies collected close to the Santa Maria di Leuca (SML) coral province.
The autozooid calyces have a mean length of 2.44 ± 0.92 mm, with a mean width of 1.05 ± 0.33 mm. The mean length of the siphonozooid calyces is 0.54 ± 0.12 mm, and their mean width is 0.20 ± 0.07 mm. Moreover, the mean distance between autozooid calyces along the same line of the colonies is 4.96 ± 2.38 mm ().
Description and biometry of sclerites
Sclerites of rachis are fusiform macrosclerites, up to 1.05 mm long and 0.06 mm wide, with a three-flanged spindle shape and with an evident orange colour that also persists in the preserved colonies (both in 5% seawater–formalin solution and in 99% ethanol solution). The fusiform shape of the mascrosclerites can be more or less convex in the central part or, sometimes, in the distal part of the sclerite (a). Fusiform macrosclerites are distributed throughout the rachis, covering it completely.
Figure 3. Different types of sclerites of Protoptilum carpenteri: from A, rachis, B, polyps, C, peduncle.
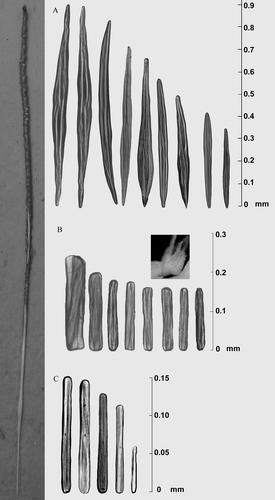
In contrast, the peduncle is covered by elongate round-ended microsclerites, up to 0.17 mm long and 0.02 mm wide, with a three-flanged rod shape and a pale yellowish color. Some of these microsclerite rods can be more or less clavate (c).
The sclerites of the polyps are three-flanged rods, like the peduncle sclerites but more robust and bright orange colored (b), up to 0.56 mm long and 0.05 mm wide. However, it is also possible to find some clavate rod-like sclerites and some fusiform spindle-like ones. The measurements of sclerites from the different parts of the colony are reported in , expressed as the minimum, maximum and mean with standard deviation.
Table III. Sclerite size (mm) of one colony of Protoptilum carpenteri expressed as minimum, maximum, mean and standard deviation (SD).
The difference in length and width between the sclerites of the autozooids and those of the siphonozooids is statistically significant (α = 0.01; p = 0.00001688 for length; p = 0.00165768 for width) as is the difference between the sclerites of siphonozooids and the ones taken from the parts of the body without polyps (α = 0.01; p = 0.000000077 for length; p = 0.00007549 for width), while the difference in size between the autozooid and body sclerites is not statistically significant (α = 0.01; p = 0.0633 for length; p = 0.1814 for width).
Discussion
The finding of Protoptilum carpenteri in the SML coral province represents the only documented record of the species in the Mediterranean Sea and increases the knowledge of the biodiversity of this area (Carlier et al. Citation2009; Mastrototaro et al. Citation2010; Vertino et al. Citation2010).
The three colonies sampled in April 2010 were found within the facies of Kophobelemnon stelliferum described in the same area by Mastrototaro et al. (Citation2013) and wrongly reported as Distichoptilum sp.
The depth of the first finding, 412–451 m, is in agreement with the lower limit of distribution of the species reported in the literature for the Atlantic Ocean (Baker et al. Citation2012). By contrast, the depth of the second finding, about 242 m, lowers the minimum bathymetrical limit known for this species.
The water temperature of 14°C, recorded during the second finding (June 2013), widens the temperature range of the species previously recorded in Atlantic waters at 3.3°C (2150 m depth in South of Iceland) (Jungersen Citation1904).
The presence of P. carpenteri in the bathyal mud near the SML coral province is most probably due to the protection from the trawl fishing which occurs around this area offered by hard substrates and coral mounds. Indeed, hard substrates interspersed with coral mounds and muddy bottoms are less accessible to trawl fishing activities, and therefore they can provide a natural refuge not only for mobile fauna, as reported by D’Onghia et al. (Citation2011), but also for sessile species such as P. carpenteri and other sea pens (Mastrototaro et al. Citation2013). It is well known that the Mediterranean bathyal mud was covered by octocoral facies, such as the gorgonian Isidella elongata on compact mud or the sea pen Funiculina quadrangularis on viscous mud (Pérès & Picard Citation1964). These facies constitute an essential habitat for some commercial crustaceans, such as Aristeus antennatus and Aristeomorpha foliacea in the first case, and Parapenaeus longirostris and Nephrops norvegicus in the second one. Both I. elongata and F. quadrangularis facies have almost completely disappeared due to trawl fishing activity in many Mediterranean areas (D’Onghia et al. Citation2003; Sardà et al. Citation2004). This could also be true for sea pens such as P. carpenteri, although the actual distribution and abundance of this species in the Mediterranean is not known. Indeed, the low number of colonies found with both the epibenthic sledge and the trawl net could also be related to the highly patchy distribution of the species, which usually characterizes sea pens and other sessile organisms in deep-sea habitats (Carpine & Grasshoff Citation1975; Marshall Citation1979; Gage & Tyler Citation1991; Williams Citation1992; Morris Citation2011; Mastrototaro et al. Citation2013).
Concerning the biometry of P. carpenteri, the colonies described here (between 350 and 451 mm in length) are much longer than the ones found by Jungersen (Citation1904) (from 75 to 190 mm in length). Moreover, in the colonies described by Jungersen (Citation1904), the rachis represents a lower percentage of the colony (about 56%) than the values found in the present study (about 78%). On the contrary, the width of the colonies here measured, from 2 to 2.5 mm, is in accordance to the values reported in the literature (Jungersen Citation1904; Kükenthal Citation1915). The recorded autozooid calyx sizes expand the known interval from 2–3 mm long and 1–1.1 mm wide (Jungersen Citation1904; Kükenthal Citation1915; Grasshoff Citation1981) to 1.1–3.9 mm long and 0.5–1.6 mm wide.
The length and width of the siphonozooid calyces, as well as the distance between the autozooid calyces, can be informative of the biometry of the species, even though never previously recorded.
The analysis of sclerite size and morphology also increases knowledge about this species. The range of known rachis sclerite dimensions has been expanded to 0.10–1.05 mm in length and to 0.007–0.063 mm in width, amplifying the previously known range of about 0.240–0.480 mm in length and 0.016–0.032 mm in width (Jungersen Citation1904; Kükenthal Citation1915). In addition, it has been possible to distinguish between the dimensions of rachis sclerites from autozooids and those from siphonozooids (). In fact, autozooid calyx sclerites seem to be about 20% longer and 10% wider than those from siphonozooid calyces (p << 0.01), while the difference between siphonozooid sclerites and those taken from the parts of the body without polyps is not statistically significant (p > 0.01).
The size of polyp sclerites has been reconsidered too, varying from 0.05 to 0.56 mm in length and from 0.003 to 0.063 mm in width.
The present work also provides peduncle sclerite sizes that seem to be about 25% shorter and about 30% narrower than those of the rachis.
Considering the small number of findings, it is difficult to hypothesize whether there are true biometric differences between the Atlantic and Mediterranean populations of Protoptilum carpenteri or if these biometric differences are due to the intrinsic variability of the species.
Acknowledgements
This work was supported by the EU 7FP CoralFISH project (www.eu-fp7-coralfish.net) and MEDITS program conducted within the Data Collection Framework (DCF) with financial support from the European Commission (DG MARE) and Member States.
References
- Baker KD, Wareham VE, Snelgrove PVR, Haedrich RL, Fifield DA, Edinger EN, Gilkinson KD. 2012. Distributional patterns of deep-sea coral assemblages in three submarine canyons off Newfoundland, Canada. Marine Ecology Progress Series 445:235–249. doi:10.3354/meps09448.
- Carlier A, Le Guilloux E, Olu K, Sarrazin J, Mastrototaro F, Taviani M, Clavier J. 2009. Trophic relationships in a deep Mediterranean cold-water coral bank (Santa Maria di Leuca, Ionian Sea). Marine Ecology Progress Series 397:125–137. doi:10.3354/meps08361.
- Carpine C, Grasshoff M. 1975. Les gorgonaires de la Méditerranée. Bulletin de l’Institut Océanographique de Monaco 71:1–140.
- Corselli C. 2010. Introduction: Cold-water coral communities in the Mediterranean Sea. Deep Sea Research Part II: Topical Studies in Oceanography 57:323–325. doi:10.1016/j.dsr2.2009.08.018.
- D’Onghia G, Indennidate A, Giove A, Savini A, Capezzuto F, Sion L, Vertino A, Maiorano P. 2011. Distribution and behaviour of deep-sea benthopelagic fauna observed using towed cameras in the Santa Maria di Leuca cold-water coral province. Marine Ecology Progress Series 443:95–110. doi:10.3354/meps09432.
- D’Onghia G, Mastrototaro F, Matarrese A, Politou CY, Mytilineou C. 2003. Biodiversity of the upper slope demersal community in the eastern Mediterranean: Preliminary comparison between two areas with and without trawl fishing. Journal of Northwest Atlantic Fishery Science 31:263–273.
- Gage JD, Tyler PA. 1991. Deep-sea biology: A natural history of organisms at the deep-sea floor. Cambridge: Cambridge University Press.
- Gilkinson K, Edinger EN. 2009. The ecology of deep-sea corals of Newfoundland and Labrador waters: Biogeography, life history, biogeochemistry and relation to fishes. Canadian Technical Report of Fisheries and Aquatic Sciences 2830, VI. 136 pp.
- Grasshoff M. 1981. Die Gorgonaria, Pennatularia und Antipatharia des Tiefwassers der Biskaya (Cnidaria, Anthozoa). Bulletin du Musée National d’Histoire Naturelle, 4ème Série 3:731–766.
- Jungersen HFE. 1904. Pennatulida. Danish Ingolf-Expedition 5:1–95.
- Kükenthal WG. 1915. Pennatularia. Das Tierreich 43:i–xv. Berlin: R. Friedländer und Sohn. 132 pp.
- Marshall NB. 1979. Developments in deep-sea biology. Poole, Dorset: Blandford Press.
- Marshall AM, Fowler GH. 1888. Report on the Pennatulida dredged by H. M. S. Porcupine. Transactions of the Royal Society of Edinburgh 33:453–464. doi:10.1017/S0080456800028039.
- Mastrototaro F, D’Onghia G, Corriero G, Matarrese A, Maiorano P, Panetta P, Gherardi M, Longo C, Rosso A, Sciuto F, Sanfilippo R, Gravili C, Boero F, Taviani M, Tursi A. 2010. Biodiversity of the white coral bank off Cape Santa Maria di Leuca (Mediterranean Sea): An update. Deep Sea Research Part II: Topical Studies in Oceanography 57:412–430. doi:10.1016/j.dsr2.2009.08.021.
- Mastrototaro F, Maiorano P, Vertino A, Battista D, Indennidate A, Savini A, Tursi A, D’Onghia G. 2013. A facies of Kophobelemnon (Cnidaria, Octocorallia) from Santa Maria di Leuca coral province (Mediterranean Sea). Marine Ecology 34:313–320. doi:10.1111/maec.12017.
- Morris KJ. 2011. North Atlantic octocorals: Distribution, Ecology and Phylogenetics. PhD thesis. Faculty of Natural and Environmental Science School of Ocean and Earth Sciences. University of Southampton. 281 pp.
- Pérès JM, Picard J. 1964. Nouveau manuel de bionomie benthique de la Méditerranée. Recueil des Travaux de la Station Marine d’Endoume 31:1–137.
- Sardà F, Calafat A, Flexas MM, Tselepides A, Canals M, Espino M, Tursi A. 2004. An introduction to Mediterranean deep-sea biology. Scientia Marina 68:7–38.
- Taviani M, Remia A, Corselli C, Freiwald A, Malinverno E, Mastrototaro F, Savini A, Tursi A. 2005. First geo-marine survey of living cold-water Lophelia reefs in the Ionian Sea (Mediterranean basin). Facies 50:409–417. doi:10.1007/s10347-004-0039-0.
- Tursi A, Mastrototaro F, Matarrese A, Maiorano P, D’Onghia G. 2004. Biodiversity of the white coral reefs in the Ionian Sea (Central Mediterranean). Chemistry and Ecology 20:S107–S116. doi:10.1080/02757540310001629170.
- Vertino A, Savini A, Rosso A, Di Geronimo I, Mastrototaro F, Sanfilippo R, Gay G, Etiope G. 2010. Benthic habitat characterization and distribution from two representative sites of the deep-water SML Coral Province (Mediterranean). Deep Sea Research Part II: Topical Studies in Oceanography 57:380–396. doi:10.1016/j.dsr2.2009.08.023.
- Von Kölliker RA. 1880. Report on the Pennatulida dredged by HMS Challenger during the years 1873–1876. Report on the Scientific Results of the Voyage of the HMS Challenger during the years 1873–76. Zoology 1:1–41.
- Williams GC. 1990. The Pennatulacea of southern Africa (Coelenterata, Anthozoa). Annals of the South African Museum 99:31–119.
- Williams GC. 1992. Biogeography of the octocorallian coelenterate fauna of southern Africa. Biological Journal of the Linnean Society 46:351–401. doi:10.1111/j.1095-8312.1992.tb00869.x.
- Williams GC. 1995. Living genera of sea pens (Coelenterata: Octocorallia: Pennatulacea): Illustrated key and synopses. Zoological Journal of the Linnean Society 113:93–140. doi:10.1111/j.1096-3642.1995.tb00929.x.
- Williams GC, van der Land J. 2001. Octocorallia-Pennatulacea. In: Costello MJ, Emblow C, White RJ, editors. European Register of Marine Species: A check-list of the marine species in Europe and a bibliography of guides to their identification. Collection Patrimoines Naturels 50. Paris: Pubblication Scientifiques du MNHN. pp. 105–106.
