Abstract
Rana latastei and Rana dalmatina are two explosive breeder amphibians whose mating seasons last less than 1 month. The two species have very different repertoires of vocalisations, as Rana dalmatina uses only one advertisement call while Rana latastei uses two different vocalisations with opposite structural features. In our research, we continually recorded the calling activity of the two species during a whole breeding season in a pond where they breed in syntopy in order to assess the possible functions of their vocalisations. Males of both species increased their activity in coincidence with the peak of activity of females, but Rana latastei males reached the peak 2–3 days before the deposition peak. By contrast, males of Rana dalmatina reached the peak at the same time as the deposition peak, and called at high intensity long after the deposition peak. These different acoustic patterns led us to infer different prevailing functions for the vocalisations of these species. Male vocalisations of Rana latastei are addressed only to males in order to gain and confirm their position within male hierarchy. By contrast, Rana dalmatina males might vocalise also in order to attract females, rather than to deter rival males. Thus, vocalisation in this second species might also play an intersexual function. The two call types of Rana latastei were used since the beginning of the breeding season. No difference was found also in daily activity, and both vocalisations were used preferentially during night time.
Introduction
The Italian agile frog (Rana latastei Boulenger, Citation1879) and common agile frog (Rana dalmatina Fitzinger in Bonaparte, 1838) are two amphibian species protected by the European Union under the Habitats Directive 92/43/CEE within the Natura 2000 network. Both species are medium sized with males attaining 50 mm in Rana latastei and 60–65 mm in Rana dalmatina (Lanza et al. Citation2007). In Northern Italy, R. latastei and R. dalmatina frequently mate synchronously in the same ponds (Vercesi et al. Citation2000; Barbieri & Bernini Citation2004; Bernini et al. Citation2004) even if their general distribution is partially allopatric, and interspecific interactions between the two frog species have been proposed (Hettyey & Pearman Citation2003; Ficetola & De Bernardi Citation2005; Sindaco et al. Citation2006). Males of R. dalmatina show territoriality (Lodé & Lesbarrères Citation2004; Lodé et al. Citation2008) while little is known about territoriality in R. latastei, except that males seem to aggregate together when they are present in the pond at medium densities (Seglie et al. Citation2008). Both species are explosive breeders whose mating seasons last less than 1 month (Lanza Citation1983; Lanza et al. Citation2007). Mating starts at the end of winter, in early February, with water temperatures of 4–5°C, and continues until the end of March or mid-April (Dolce et al. Citation1982, Citation1985; Andreone Citation1992; Barbieri & Bernini Citation2004). The highest activity of the breeding period occurs with water temperatures of about 7°C (Dolce et al. Citation1982, Citation1985; Barbieri & Bernini Citation2004; Lanza et al. Citation2007), and females of both species lay a single clutch for every reproductive season (Lanza Citation1983; Lanza et al. Citation2007). Typically, males are spaced a few decimetres apart, and call underwater during the whole breeding period. However, the two species have very different repertoires of vocalisations. R. dalmatina’s repertoire includes only one vocalisation type, that consists of a train of pulse groups repeated several times; each pulse group is composed of five subunits increasing in volume (Schneider et al. Citation1988; Lesbarrères et al. Citation2008). The mean duration ranges from 2 to 8 ms, while the fundamental frequency ranges from 542 to 778 Hz (Lesbarrères et al. Citation2008). By contrast, little is known about vocalisations of R. latastei, as previous descriptions by Boulenger (Citation1879), Pozzi (Citation1980), Lanza (Citation1983), Nöllert and Nöllert (Citation1992) were based just on mating calls heard out of water. Using a microphone protected by a thin waterproof layer, Farronato et al. (Citation2000) documented for the first time the two different vocalisations of this species, which are uttered underwater only (Farronato et al. Citation2000; Seglie et al. Citation2006). The first type, called “mew”, is a shrill, very short sound, while the second type, called “brum”, is a grave, guttural sound, constituted by the repetition of a single note. The dominant frequency of mews varies from 411 to 1752 Hz and the duration varies from 287 to 930 ms, whereas the single note of brums has a frequency ranging between 277 and 444 Hz and a duration between 700 and 2110 ms (Farronato et al. Citation2000).
In our study, we recorded the male call activity of R. latastei and R. dalmatina during a whole breeding season in a pond where both species breed in syntopy. At the same time, we recorded egg laying by females. The specific aims of this study were to assess (i) if mew and brum calls were used differently during the day or over the season by R. latastei males, and (ii) if the call activity peaks of males correlate with the egg laying peaks of females in both species.
Materials and methods
Recording device and sound analysis
The research was carried out in the natural reserve “Bosco Giuseppe Negri”, southwest of the city of Pavia (Northern Italy, 45°10’20”N, 9°8’27”E). The recordings were made in a small (9 × 3 m) pond with a depth ranging from 10 to 35 cm. We used a continuum recording system consisting of an omnidirectional underwater hydrophone (DolphinEAR Arretec, piezo transducer, frequency response 7–22000 Hz) connected by a 20-m shielded wire to a PC that recorded sounds non-stop within the pond. The hydrophone was fixed to a float and settled approximately on the centre of the pond in a way that it was hanging at half of the pond depth, and could record calls emitted all over the pond since sound attenuation in those conditions does not exceed 3–10dB/doubling distance (Fine & Lenhardt Citation1983; Forrest Citation1994). Recordings were carried out using the Sound Forge 5.0 Software in the 0–22 kHz frequency range, 16-bit with a sampling rate of 44,100 sample/s. We started the recording at midday on 23 February 2005, and stopped it at midday on 6 April 2005, when the calling activity of frogs ended.
Every day we saved the recording and stored it on an external hard disk, thus obtaining 42 files of nearly 24 hours, one for each day.
Recordings were analysed using the software Cool Edit Pro v. 2.1 (Syntrillium). The best resolution was achieved analysing vocalisations in the 0–22 kHz frequency range (bandwidth of 28 Hz, frequency resolution 56 Hz and time resolution 46 ms), and we identified and counted the calls emitted by frogs directly on the screen. In all sound files, we were able to count the total number of mew calls emitted by Rana latastei and the total number of calls emitted by Rana dalmatina every 30 seconds. We were also able to identify the maximum number of calling individuals, under the assumption that frogs can be reliably individually recognised on the bases of the acoustic features of their calls, such as fundamental and dominant frequencies, intensity and call rate (Davis Citation1987; Bee et al. Citation2001; Lesbarrères & Lodé Citation2002). A similar approach is considered a standard method to provide continuous estimates of population size and breeding activity for target frog species (Rand & Drewry Citation1994) and was recently adopted to estimate the population size of tree frogs in Switzerland (Pellet et al. Citation2007). The song rate per hour for these two vocalisations was therefore expressed by the total number of calls emitted during 1 hour divided by the maximum number of singers. This procedure was not used in the case of the brum calls of the R. latastei, since the brums calls are long, constant, continuous and overlapping when several frogs called simultaneously. In these cases, we were unable to count calls or distinguish different individuals. Consequently, we assigned a rank of song rate to each 30-second interval within an hour, according to the scale reported in . The song rate of brum for an hour was therefore expressed by the mean rank over the 30-second intervals composing that hour; this procedure was similar to the one proposed by Bridges and Dorcas (Citation2000) and by Weir and Mossman (Citation2005).
Table I. Rank of song rate used to score singing activity of Italian agile frog brums.
Given the unfeasibility to analyse all 42 daily recordings in their entirety, we sampled the first 2.5 minutes of a given hour to estimate song rates during that hour (Møller Citation1987; Saino & Møller Citation1995). The consistency between the 2.5-minute sampling intervals and the corresponding hour was estimated in a sample of 7 hours randomly selected from as many different frogs, which were fully analysed. The song rate variability within an hour was significantly lower than that among hours [one way-analysis of variance (ANOVA) including the song rate as dependent variable and the hour as grouping factor: mew call: F6,7 = 5.07, P = 0.02; brum call: F6,7 = 25.88, P < 0.001; R. dalmatina call: F6,7 = 4.61, P = 0.03]. The R2 of linear regression models between song rates computed in the full hour and in the sampling intervals exceeded 0.84 (mew call: 0.90, F1,5 = 17.49, P = 0.004; brum call: 0.84, F1,5 = 27.52, P = 0.003; R. dalmatina call: 0.85, F1,5 = 28.79, P = 0.003) confirming the high correlation between the estimate of song rate within 2.5 minutes and that in the corresponding hour.
Egg-laying monitoring and measurement of water temperature
During the whole study, we monitored the pond daily in order to count the egg-masses laid the previous night. Clutches of each species can be distinguished by a trained researcher (Bernini et al. Citation2000; Lanza et al. Citation2007). The surveys were made visually by walking along the edge of the pond and reporting the position of each egg-mass (usually attached to predisposed submerged branches) on a map to avoid multiple counts. Finally, we recorded (at noon) the temperature of the water (°C) at the surface and bottom of the pond daily using a Greisinger GTH-175/MO digital thermometer.
Statistical data analyses
Series data were log transformed and analysed using the packages TSA and tseries in R (v. 2.11.1, R Development Core Team Citation2010). Autocorrelations in the three calling activity series were evaluated using sample autocorrelation functions (ACF). First and diurnal (lag = 24) differencing were used to highlight the general trend and cycling component in the calling activity.
The daily trend in calling activity was initially modelled using a 24-level factor, giving the expected average activity for each of the 24 hours of the day (Cryer & Chan Citation2008). Since this approach involved a high number of parameters, we modelled the diurnal trend economically with cosine curves that incorporate the smooth change expected from one hour to the next while still preserving the diurnal cycle (Cryer & Chan Citation2008). In these analyses, we considered cosine models up to and including the fifth harmonic frequency. The better model was selected using the Akaike’s Information Criterion (AIC) model selection (Burnham & Anderson Citation2002; Mazerolle Citation2006). Days with no calling activity were removed from the series, so analyses were done on the sub-series 8 March–1 April for R. latastei, and on the sub-series 11 to 29 March for R. dalmatina.
General seasonal trends in calling activity were investigated using polynomial regressions on the residuals of the best models obtained from daily trend analyses. Residuals were used to remove the confounding effects of daily fluctuations from the series (Cryer & Chan Citation2008). For each call type we fitted all the polynomial models to the fifth degree, and the best model was selected using AIC model selection.
Results
Calling activity
The breeding season in Rana latastei () started on 23 February, with the melting of the ice layer on the pond surface, but the activity lasted for only 5 days, as the temperature then dropped, leading the pond surface to ice over again. The real beginning of the calling activity of both species coincided with the first strong rising of water temperature, especially in the case of R. latastei (). In effect, the calling activity of Rana dalmatina only began 3 days following the temperature increase (). R. latastei called from 8 March to 1 April, and both mew and brum calls followed the same pattern ( and ). R. dalmatina called for a shorter time period, from 11 to 29 March ().
Figure 1. Calling activity of Rana latastei and Rana dalmatina recorded from 23 February and 6 April. Solid and dotted lines represent bottom and surface temperature, while the bold straight segments report the presence of ice on the pond surface.
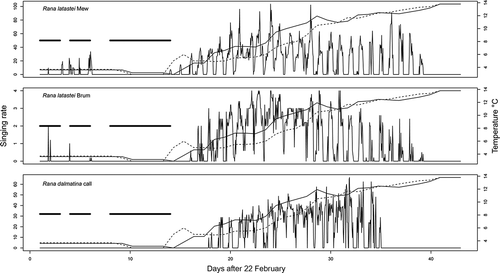
The mean numbers of individuals recorded per sampling interval were 5.7 (range 1–10, n = 395 intervals) and 3.1 (range 1–8, n = 303 intervals) for R. latastei and R. dalmatina, respectively. Male R. latastei emitted on average 22.8 ± 0.9 mews per hour (range: 4.8–103.5), the mean rank of brums per hour was 2.1 ± 0.7, whereas R. dalmatina emitted 30.4 ± 0.7 calls per hour (range: 4.8–66.4).
Daily trends
ACFs revealed a prominent daily autocorrelation in the song rate of all three vocalisations, with a strong correlation at lags 24, 48, 96 and so on (). Daily differencing removed this general cycling trend from the series, but the ACFs revealed now a positive correlation at lags 1 to 6–7, suggesting the presence of another substantial correlation that needed to be modelled. Taking both first (lag = 1) difference and daily difference (lag = 24), very little autocorrelation remains in the series, suggesting that both daily and general trend components characterise calling activity in both species. All daily trend models made for mew call activity were highly significant (all P-values < 0.01), and the fourth harmonic frequency model turned out to be the best one (). The mew calling rate showed marked day–night fluctuations: it reached its maximum during night time (between 22:00 and 5:00 h), sharply decreased in the early morning (after 5:00 h), which represents the minimum of the diurnal cycle (i.e. between 7:00 and 9:00 h), and slowly increased starting from midday up to dusk (19:00 h), when it rapidly rose to the night maximum (). The brum calling rate followed a similar pattern: all generated models were highly significant (all P-values < 0.01), and showed a marked day–night fluctuation pattern similar to that just described for mew calls. The best model in this case was the third harmonic frequency (), which pointed the maximum brum calling activity during night-time, between 20:00 and 5:00 h, and the minimum in the morning, between 7:00 and 9:00 h (). Similarly to mew call, brum calling activity increased from midday and reached the maximum at the beginning of the night, but without a sharp increase at dusk (). Indeed, mew and brum calling activity were positively correlated (Spearman rank correlation: r = 0.80).
Figure 2. Simple, diurnal (lag = 24) and first differencing autocorrelation function (ACF) for the calling activity of Rana latastei and Rana dalmatina; the dashed lines marks significant correlations.
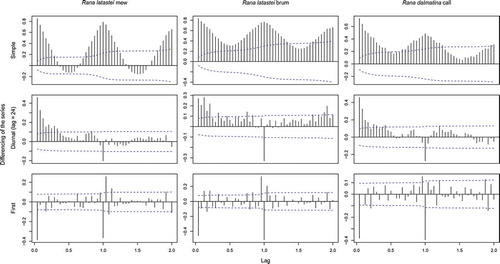
Figure 3. Daily trend in calling activity of Rana latastei and Rana dalmatina: a, mew call; b, brum call of Rana latastei; c and d, advertisement call of Rana dalmatina using first and second harmonic frequency models, respectively. The dashed lines represent 95% confidence intervals.
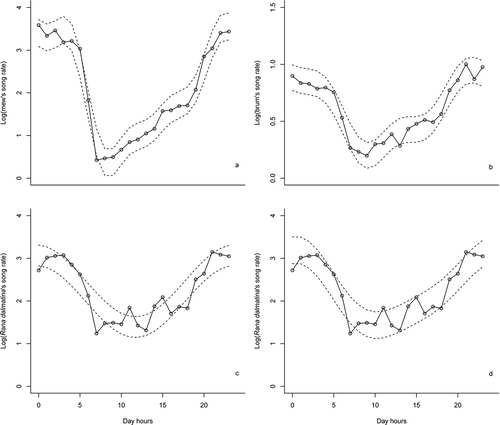
Table II. Ranking of candidate models for the daily trend in singing activity of the vocalisation of common and Italian agile frogs according to the corrected Akaike’s Information criterion (AICc) model selection. K represents the number of parameters, and models are listed in order of best AICc. Delta_AICc is the difference with respect to the best model, AICcWt is the AICc weight, Cum.Wt is the cumulative weight, LogLik is the log likehood of the model.
Rana dalmatina calling activity followed a pattern similar to that of Rana latastei (). Indeed, all models were highly significant (all P-values < 0.01) and revealed marked day–night fluctuations. The best model (i.e. the first harmonic frequency, ) showed that activity reached the maximum during night-time (between 21:00 and 4:00), and sharply decreased during the morning. However, activity did not drop completely at night as with R. latastei, and R. dalmatina males continued to call during daytime at lower intensity. At twilight (i.e. after 18:00–19:00 h), R. dalmatina males increased calling activity until the night maximum (). Eight data points fell out of the 95% confidence intervals of the model (), indicating that the model slightly overestimated the calling rate during the morning (6:00–7:00 h), while it underestimated it during the evening (10:00 h, and particularly during 14:00 and 15:00 h). These deviations suggested that the calling activity trend may not be strictly symmetrical before and after noon, as predicted by the model. The nearest model, i.e. the second harmonic frequency, better followed the mean activity trend (), suggesting that frogs sharply decreased their calling activities in the morning, without ceasing altogether, then, from 10:00, gradually increased their activity until the night maximum.
Seasonal trends
The general seasonal trends for both species were better described by the fifth polynomial model (). Mew and brum calling activity by male Rana latastei rapidly increased and reached their respective peaks of the season in 5–6 days after beginning. Peaks occurred on day 22 (16 March) and 23 (17 March) for mew and brum calls, respectively. After the peak, males gradually decreased calling, and stopped by the tenth day ().
Figure 4. Seasonal trend in calling activity of Rana latastei and Rana dalmatina in relation to the number of egg masses laid (histograms); solid lines represent the trend predicted by the best model and dotted lines represent the confidence and prediction intervals for the fitted model.
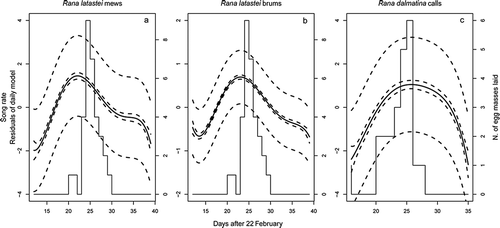
Table III. Ranking of candidate models for the seasonal trend in singing activity of the vocalisation of common and Italian agile frogs according to the AICc model selection. K represents the numberof parameters and models are listed in order of best AICc.
Rana dalmatina males showed a slightly different trend, as they reached a peak of calling activity some days later (i.e. days 24–25) and, contrary to the other species, males maintained high activity rates for a week (). After that, calling activity rapidly decreased and stopped a few days later ().
Egg laying
Rana latastei females started egg laying on day 21 (15 March) and stopped 9 days later, on day 30 (24 March). We counted 32 egg masses overall. The highest deposition rates occurred during day 25 (19 March), with nine egg masses (28%, ). Therefore, in this species, the calling activity peaked within the egg laying period, but a few days before the deposition peak (3 and 2 days for mew and brum, respectively; ).
Egg laying by Rana dalmatina largely overlapped with that of the other species, and occurred between days 16 and 28 (10 and 22 March). We found 23 egg masses overall, and the deposition peak (n = 6. 26%) occurred during day 26 (20 March, ). In this species, the deposition peak occurred coincidentally with the calling activity peak of males, and calls at the highest intensity also occurred when most females ended egg laying (); or, as an alternative hypotheses, it could be the presence of females that animated males to call more.
Discussion
In this paper, we provide a detailed description of the calling activity of Rana latastei and Rana dalmatina. We showed that both species call preferentially by night and underwater following a daily activity similar to that described for other Paleartic frogs like Rana graeca Boulenger, 1891, Rana italica Dubois, 1987, Rana iberica Boulenger, Citation1879 and, probably, also Rana pyrenaica (Nöllert & Nöllert Citation1992; García-París et al. Citation2004; Schneider Citation2005; Razzetti et al. Citation2006).
The main results of this study rely on the different relationships between calling activity and egg laying of R. latastei and R. dalmatina. Indeed, males of both species increased their activity in coincidence with the peak of activity of females, but males of R. latastei reached their peak 2–3 days before the deposition peak. By contrast, males of R. dalmatina reached their peak at the same time as the deposition peak, and called at the highest intensity significantly after the deposition peak. A second relevant difference between the species regards the distribution of activity over the season. R. latastei spread its calling activities over a much longer period: they started from day 1, and the bulk of activity lasted for 27 days (from day 13 to 39). After reaching the peak, males maintained high activity levels for only 2–3 days, and, when females reached the pond in large numbers (the deposition peak), calling activity was just declining. On the contrary, R. dalmatina started calling later, and concentrated its activity in fewer days than the syntopic species (i.e. 19 days, from day 17 to 35). In addition, R. dalmatina males increased activity in close correlation with increasing female density (as estimated by egg masses), leading to a coincidence of the peak activity of the two sexes. This different acoustic behaviour led us to consider different prevailing functions of vocalisations between species. Both the beginning and peaking of calling activity clearly predate the maximum of egg deposition, suggesting that R. latastei might vocalise primarily against conspecific males. Rana latastei is a classical explosive breeder, which reaches the pond as soon as the environmental conditions allow it. The male priority is to gain the better sites, and individuals wintering inside ponds have been reported (Bernini et al. Citation2004; F. Bernini & E. Razzetti, pers. obs.). Male density within ponds might be high, causing a very intense intrasexual competition, especially at the beginning of the breeding season (Ficetola et al. Citation2010). Females of R. latastei reach the breeding pond later than the males (Lanza et al. Citation2007), when dominance hierarchies among males are probably already well established.
R. dalmatina is also classified as an explosive breeder, but its calling behaviour is better conformed to the female’s egg laying trend than that of R. latastei, as the activity calling peak matches the deposition peak, and lasts for some days after. This correlation suggests that males might vocalise in order to attract females, rather than to deter rival males. Indeed, males in this species reach the pond before females and the operational sex ratio is usually male biased (Lodé et al. Citation2008). Therefore, males probably do not have detailed information on how many females are arriving at the pond. The observed pattern suggests that the increase in calling activity might be a direct response to the increase in the number of females reaching the pond to lay eggs. Also, the prolonged calling activity after the female deposition is consistent with a female-attractive function of male calls, since males might maintain calling activity at the highest intensity, increasing chances for new mating opportunities.
A second relevant aim of this study was to investigate different functions of mew and brum calls of R. latastei. A previous study (Seglie et al. Citation2008) hypothesised that mew and brum are used as different male strategies, depending on male density. At low density, males should principally use the mew call, which is adapted for long-distance communication, while at high density, males should use the brum call, which is more suited for short-range communication. This leads to the prediction that mews should be used more frequently at the beginning of the breeding season, while the brum rate should increase progressively, as new males reach the pond. Contrary to this prediction, we were not able to detect any substantial difference in the use of mew and brum calls by R. latastei over the breeding season. The two call types were both used from the beginning of the breeding season and both reached a maximum before the egg deposition peak. At the deposition peak, the calling rates of both vocalisations were just declining. These findings suggest that mew and brum cannot be related to the density of males. We agree with the hypothesis by Seglie et al. (Citation2008) that the two song types reflect long- vs short-range communication, but for reasons that are probably related to efficiency in signal transmission. As stated before, male–male competition is high in this species, and the effectiveness of acoustic signal transmission is crucial in both deterring rivals and achieving (and maintaining) the highest rank in dominance hierarchies.
Acknowledgements
We would like to thank the Municipality of Pavia and Stefania Ratano of Lega Italiana Protezione Uccelli (LIPU) for authorisation and assistance during field research in the natural reserve “Bosco Giuseppe Negri”. We are also grateful to Carlo Violani who revised the English of this manuscript, and to Francesco Ficetola and two other anonymous referees for their useful suggestions. Research was authorised by the Ministero dell’Ambiente e della Tutela del Territorio e del Mare [DPN/2D/2006/16275]. We also thank four anonymous referees for their useful suggestions that sensibly improved previous versions of the manuscript.
References
- Andreone F. 1992. Valutazione e categorizzazione dello status della batracofauna (Amphibia) in Piemonte e Valle d’Aosta. Quaderni della Civica Stazione di Idrobiologia di Milano 19:27–40.
- Barbieri F, Bernini F. 2004. Distribution and status of Rana latastei in Italy (Amphibia, Ranidae). Italian Journal of Zoology 71(sup1):91–94. doi:10.1080/11250003.2004.9525542.
- Bee M, Kozich CE, Blackwell KJ, Gerhardt HC. 2001. Individual variation in advertisement calls of territorial male green frogs, Rana clamitans: Implications for individual discrimination. Ethology 107:65–84. doi:10.1046/j.1439-0310.2001.00640.x.
- Bernini F, Barbieri F, Vercesi A. 2000. Nuove metodologie di cattura e di marcatura negli Anuri: prima esperienze su Rana latastei e Rana dalmatina. In: Giacoma C, editor. Atti I Congr. Naz. S.H.I. Torino: Museo Regionale di Scienze Naturali. pp. 269–276.
- Bernini F, Gentilli A, Scali S. 2004. Rana di lataste. In: Bernini F, Bonini L, Ferri V, Gentilli A, Razzetti E, Scali S, editors. Atlante degli Anfibi e dei Rettili della Lombardia, Monografie di Pianura n. 5. Provincia di Cremona. pp. 108–110.
- Boulenger GA. 1879. Études sur les grenouilles rousses Ranae temporariae et description d’espèces nouvelles ou méconnues. Bullétin de la Societé Zoologique de France 4:158–193.
- Bridges AS, Dorcas ME. 2000. Temporal variation in anuran calling behavior: Implications for surveys and monitoring programs. Copeia 2000:587–592. doi:10.1643/0045-8511(2000)000[0587:TVIACB]2.0.CO;2.
- Burnham KP, Anderson DR. 2002. Model selection and multimodel inference a practical information-theoretic approach. New York: Springer.
- Cryer JD, Chan K-S. 2008. Time series analysis with applications in R. New York: Springer.
- Davis MS. 1987. Acoustically mediated neighbor recognition in the North American bullfrog, Rana catesbeiana. Behavioral Ecology and Sociobiology 21:185–190. doi:10.1007/BF00303209.
- Dolce S, Lapini L, Stergulc F. 1982. Contributo preliminare allo studio dell’erpetofauna della bassa pianura friulana. Note eco-etologiche sugli Anfibi e Rettili del Bosco Baredi e Selva di Arvonchi (Muzzana del Turgnano, Udine). Quaderni sulla “Struttura delle Zoocenosi Terrestri”, Collana del Programma Finalizzato “Promozione della Qualità dell’Ambiente”. Roma: CNR ed., AQ/1/181–186.
- Dolce S, Lapini L, Stoch F. 1985. Indagini ecologiche su Rana latastei Boul. (Amphibia, Anura) nei boschi della Bassa Pianura friulana (Italia nord-orientale). Gortania - Atti Museo Friulano Storia Naturale Udine 6:227–238.
- Farronato I, Pesente M, Fracasso G, Carlotto L. 2000. Osservazioni sulle manifestazioni sonore di Rana latastei Boulenger, 1879. In: Bon M, Scarton F, editors. Atti 3° Convegno Faunisti Veneti. Venezia: Museo Civico di Storia Naturale di Venezia. pp. 32–36.
- Ficetola GF, De Bernardi F. 2005. Interspecific social interactions and breeding success of the frog Rana latastei: A field study. Ethology 111:764–774. doi:10.1111/j.1439-0310.2005.01089.x.
- Ficetola GF, Padoa-Schioppa E, Wang J, Garner TWJ. 2010. Polygyny, census and effective population size in the threatened frog, Rana latastei. Animal Conservation 13(Suppl. 1):82–89. doi:10.1111/j.1469-1795.2009.00306.x.
- Fine ML, Lenhardt ML. 1983. Shallow-water propagation of the toadfish mating call. Comparative Biochemistry and Physiology Part A: Physiology 76:225–231. doi:10.1016/0300-9629(83)90319-5.
- Forrest TG. 1994. From sender to receiver propagation and environmental effects on acoustic signals. American Zoologist 34:644–654.
- García-París M, Montori A, Herrero P. 2004. Fauna Iberica – Vol. 24. Amphibia Lissamphibia. Madrid: Museo Nacional de Ciencias, Consejo Superior de Investigaciones Científicas.
- Hettyey A, Pearman PB. 2003. Social environment and reproductive interference affect reproductive success in the frog Rana latastei. Behavioral Ecology 14:294–300. doi:10.1093/beheco/14.2.294.
- Lanza B. 1983. Guide per il riconoscimento delle specie animali delle acque interne italiane 27 - Anfibi e Rettili. Roma: CNR.
- Lanza B, Andreone F, Bologna MA, Corti C, Razzetti E. 2007. Fauna d’Italia, Amphibia. Bologna: Calderini.
- Lesbarrères D, Lodé T. 2002. Variations in male calls and responses to an unfamiliar advertisement call in a territorial breeding anuran, Rana dalmatina: Evidence for a “dear enemy” effect. Ethology Ecology and Evolution 14:287–295. doi:10.1080/08927014.2002.9522731.
- Lesbarrères D, Merilä J, Lodé T. 2008. Male breeding success is predicted by call frequency in a territorial species, the agile frog (Rana dalmatina). Canadian Journal of Zoology 86:1273–1279. doi:10.1139/Z08-121.
- Lodé T, Holveck M-J., Lesbarrères D. 2008. Asynchronous arrival pattern, operational sex ratio and occurrence of multiple paternities in a territorial breeding anuran, Rana dalmatina. Biological Journal of the Linnean Society 86:191–200. doi:10.1111/j.1095-8312.2005.00521.x.
- Lodé T, Lesbarrères D. 2004. Multiple paternity in Rana dalmatina, a monogamous territorial breeding anuran. Naturwissenschaften 91:44–47. doi:10.1007/s00114-003-0491-7.
- Mazerolle MJ. 2006. Improving data analysis in herpetology: Using Akaike’s Information Criterion (AIC) to assess the strength of biological hypotheses. Amphibia-Reptilia 27:169–180. doi:10.1163/156853806777239922.
- Møller AP. 1987. Mate guarding in the swallow Hirundo rustica. Behavioral Ecology and Sociobiology 21:119–123. doi:10.1007/BF02395439.
- Nöllert A, Nöllert C. 1992. Die Amphibien Europas. Stuttgard: Frank-Kosmos Verglas-GmbH & Co.
- Pellet J, Helfer V, Yannic G. 2007. Estimating population size in the European tree frog (Hyla arborea) using individual recognition and chorus counts. Amphibia-Reptilia 28:287–294. doi:10.1163/156853807780202530.
- Pozzi A. 1980. Ecologia di Rana latastei. Bollettino Atti Società Italiana di Scienze Naturali, Museo Civico di Storia Naturale, Milano 121:221–274.
- R Development Core Team. 2010. R A language and environment for statistical computing R Vienna, Foundation for Statistical Computing. URL: http//wwwR-projectorg/
- Rand AS, Drewry GE. 1994. Acoustic monitoring at fixed sites. In: Heyer WR, Donnelly MA, McDiarmid RW, Hayek LC, Foster MS, editors. Measuring and monitoring biological diversity: Standard methods for amphibians. Washington: Smithsonian Institution Press. pp. 150–152.
- Razzetti E, Sacchi R, Platz JE. 2006. First description of the acoustic repertoire of Rana italica (Anura, Ranidae). Herpetological Journal 16:229–231.
- Saino N, Møller AP. 1995. Testosterone correlates of mate guarding, singing and aggressive behaviour in male barn swallows, Hirundo rustica. Animal Behaviour 49:465–472. doi:10.1006/anbe.1995.0060.
- Schneider H. 2005. Bioakustik der Froschlurche Einheimische und verwandte Arten. Zeits. Feldherpetoliske 6:1–135.
- Schneider H, Sofianidou TS, Kyriakopoulou-Sklavounou P. 1988. Calling behavior and calls of Rana dalmatina (Anura, Ranidae) in Greece. Zoologische Jahrbuecher Systematik 92:231–243.
- Seglie D, Giacoma C, Marzona E, Paschetto D. 2006. Advertisement calls of Rana latastei (Boulenger, 1879). In: Zuffi MAL, editor. Atti del V Congresso Nazionale della Societas Herpetologica Italica. Firenze: Firenze University Press. pp. 217–224.
- Seglie D, Marzona E, Ficetola GF, Giacoma C. 2008. Comportamento riproduttivo e vocale della rana di Lataste, Rana latastei (Amphibia Anura). In: Corti C, editor. Herpetologia Sardiniae. Latina: Belvedere. pp. 439–443.
- Sindaco R, Doria G, Razzetti E, Bernini F. 2006. Atlante degli Anfibi e dei Rettili d’Italia/Atlas of Italian Amphibians and Reptiles. Societas Herpetologica Italica. Firenze: Polistampa.
- Vercesi A, Bernini F, Barbieri F. 2000. La sintopia di Rana dalmatina e Rana latastei nei boschi planiziali del fiume Ticino aspetti della biologia riproduttiva. In: Giacoma C, editor. Atti del 1° Congresso Nazionale Societas Herpetologica Italica. Torino: Museo Regionale di Scienze Naturali. pp. 269–276.
- Weir LA, Mossman MJ. 2005. North American Amphibian Monitoring Program (NAAMP). In: Lanoo MJ, editor. Amphibian declines: Conservation status of united states species. Berkeley: University of California Press. pp. 307–313.
