Abstract
Chalinula nematifera (Porifera: Haplosclerida) is an encrusting sponge able to overgrow live corals. It was described from the Western-Central Pacific Ocean and recently has been reported along the Mexican Pacific coast, where it is considered introduced and a potential threat for corals. However, there is a lack of accurate data about the distribution and abundance of Chalinula nematifera. Here we report a first wide assessment of its effect on different coral species and its growth rate. This is a first knowledge baseline for C. nematifera, based on visual and photo surveys, along the eastern coast of the Sulawesi Island (Indonesia).
Introduction
The key role of sponges in coral reefs is well known (Bell Citation2008). In some areas, sponges can exceed scleractinian corals in terms of both biomass and species diversity (Diaz & Rützler Citation2001). Sponges play a fundamental role in structuring coral reefs, taking part not only in the bioconstruction and bioerosion processes (Diaz & Rützler Citation2001; Wulff Citation2006), but also leading to a complex web of functional interactions (Cerrano et al. Citation2006). Sponges play an essential role in the flows of energy and organic matter on coral reefs by retaining large amounts of POM (particulate organic matter) and DOC (dissolved organic carbon) (Scheffers et al. Citation2010) and promoting a DOM (dissolved organic matter)-sponges-detritus loop (de Goeij et al. Citation2013).
Sponges and corals interact strictly, sometimes to the extent that sponges overgrow corals and outcompete them completely. Sponges are considered strong competitors of scleractinian corals in the colonisation of areas affected by impacts of both anthropogenic and natural origin (Plucer-Rosario Citation1987; Rützler & Muzik Citation1993), but only a few sponges are able to outcompete reef-building corals (Calcinai et al. Citation2007; Coles & Bolick Citation2007; Benzoni et al. Citation2008; Wang et al. Citation2012).
Chalinula nematifera (De Laubenfels) belongs to the order Haplosclerida, family Chalinidae. It is characterised by a mauve colouration and by white, wavy filaments, produced by symbiotic fungi (Weerdt De et al. Citation2002). This species was originally described by De Laubenfels (Citation1954, as Nara nematifera) in association with corals of the Western-Central Pacific Ocean (Great Barrier Reef, Marshall Islands, Micronesia). Information about the distribution and abundance of Chalinula nematifera is scanty. This sponge occurs throughout the tropical Indo-West Pacific (Weerdt De et al. Citation2002; Ávila & Carballo Citation2009) and has also been reported from the tropical Eastern Pacific ocean where it is considered a recent introduction (probably by fouling), and a potential threat to the Pacific Mexican coral reefs, since it overgrows or outcompetes live corals (Ávila & Carballo Citation2009). Its presence in Indonesian reefs, particularly in Borneo, has been documented (see Ávila & Carballo Citation2009), but there is no published information about its abundance and distribution in this area.
The present report provides a baseline of knowledge on the distribution and habitats colonised by C. nematifera on Sulawesi Island, Indonesia. We also provide an estimation of the growth rate of the species in this region.
Materials and methods
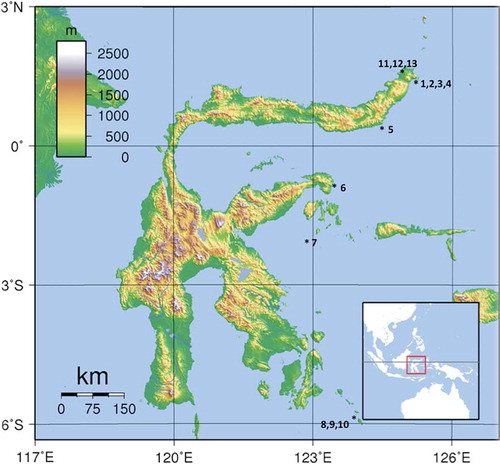
Visual surveys were conducted by SCUBA diving, from 15 to 25 of April 2012, along the eastern side of the Island of Sulawesi (Indonesia) from Bitung (Lembeh Strait) to the Wakatobi Marine Park (), for a total length of 830 nautical miles. In total, we examined 13 sites in the course of 1-hour dives ().
Table I. Areas and dive sites where Chalinula nematifera was recorded associated with hard corals.
Chalinula nematifera is easily recognisable by its characteristic brilliant mauve-coloured coat and white, wavy filaments covering coral substrate. Standard procedures, according to Rützler et al. (Citation1978), were followed to confirm the taxonomic identification. Spicule complement is characterised by thin, straight oxeas (90–105 × 2–2.5 µm) creating a delicate skeleton with triangular and polygonal meshes two spicules long.
Every time the presence of C. nematifera was detected, the coral covered by the sponge was identified (mainly at the genus level; ). Data about coral morphology (massive, branching, encrusting, etc.) and observations about coral and sponge interactions were also recorded (). The category or type of interactions was assessed following Aerts and van Soest (Citation1997): overgrowth of living coral, peripheral contact of sponge for more than 3 cm, tissue contact considered accidental, and non contact.
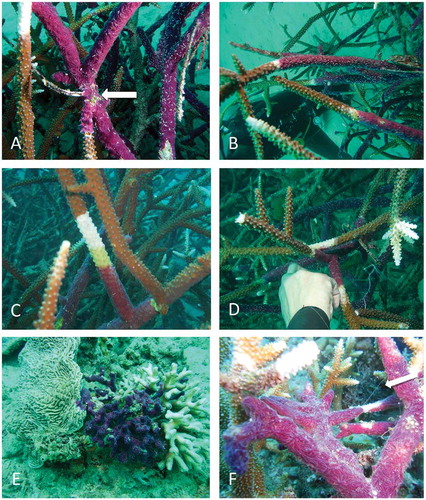
Besides the visual survey, the growth rate of C. nematifera was evaluated on two coral species. Two branches of Stylophora sp. and 13 branches of Acropora sp. partially covered by the sponge were monitored at 12 m depth at Bangka Island North Sulawesi, Indonesia (Pearl Farm station 01°48.626ʹ N, 125°06.762ʹ E). For each infested branch, a plastic wire was tied up at the boundary between sponge and coral to measure sponge growth on 31 July 2013; after 31 days (31 August 2013) and 67 days (6 October 2013) the spread of the sponge over the coral () was measured with a calliper (modified from Chaves-Fonnegra & Zea Citation2011). The growth rate was calculated in centimetres of growth per day.
Figure 2. Chalinula nematifera from Bangka Island: A–C, Acropora necrosis (white band) in the area in contact with the sponge; arrow in (A) points the starting point where the plastic wire was tied to measure sponge growth; D, white area of dead Acropora on a branch in contact with a covered colony; E, sponge invading the encrusting coral Pachyseris sp. and Seriatopora sp.; F, filaments of mucus (arrow) produced by the sponge.
Results
Chalinula nematifera was widely distributed throughout the entire monitored range of the eastern coast of Sulawesi (). Its presence was observed at each explored site (), where numerous coral colonies were infested.
The sponge was recorded between 5 to 32 m depth, growing on massive-encrusting corals such as Mycedium elephantothus, members of the Faviidae family (Platygyra daedalea, Echinopora sp., Favia sp. and Goniastrea sp.), leafy or laminar corals such as Pectinia sp. and branching corals such as Pocillopora sp. ().
Figure 3. Chalinula nematifera overgrowing hard corals: A, unidentified species; B, Pectinia sp.; C, Pocillopora sp.; D, Pectinia sp.; E, Mycedium elephantothus; F, Platygyra daedalea; G, Echinopora sp.; H, Favia sp.; I, Goniastrea sp.; J, Faviidae. Note particularly in (H–J) the white areas of necrosis, and the sponge spreading out over living portions of coral in (C–E).

According to the scheme of Aerts and van Soest (Citation1997), the most common pattern of infestation was by peripheral contact (, F, G), but sometimes an evident overgrowth was documented ().
Chalinula nematifera was observed spreading both over dead portions of corals () and over living coral colonies (, –E, H). In most cases, the sponge grew on parts of coral colonies already dead, covered by algae or sediment (). In the early phase of growth on living corals, coral necrosis (evident as a white band) was observed in the area in direct contact with the sponge; it looked like an evident thin boundary rim between the growing sponge and the living coral (, –J).
On branching corals, owing to the disappearance of coral tissues, a white rim between the spreading sponge and the living coral was always very evident (–C). In few cases, similar white areas of dead coral were observed on branches of colonies that came in contact with other colonies infested by Chalinula ().
At Bangka Island, the sponge was observed () growing mainly on Acropora sp. (–D, F), Stylophora sp. (overgrowth of living coral sensu Aerts & van Soest Citation1997), Seriatopora sp. and Pachyseris sp. ().
After 31 days (31 August 2013), the sponge growth on Acropora sp. branches was measured. It ranged between 1.2 and 9.2 cm with a growth rate of 0.03–0.29 cm/day, on average 0.17 (± 0.08 standard deviation, SD) cm/day. For the two specimens of Stylophora sp., the growth rate was 0.03 cm/day. On 6 October 2013, after 67 days, it was possible to measure the growth of C. nematifera only on seven branches of Acropora sp., as the other specimens of Acropora sp. and Stylophora sp. were totally covered, broken or lost. Considering only these seven branches and the entire monitored period (67 days), the growth rate was on average 0.22 (± 0.04 SD) cm/day, confirming the values recorded during the first measurements.
Discussion
The distribution and presence of C. nematifera was documented by visual observations of a qualitative nature; in each dive, we detected the sponge on various coral colonies, suggesting a general abundance at most sites. C. nematifera was observed associated to corals with several growth forms: laminar, encrusting, branching and massive (, ). Ávila and Carballo (Citation2009), on the contrary, reported the sponge almost exclusively on branching corals such as Pocillopora spp. (mainly P. verrucosa), very rarely on rocks and never overgrowing massive corals, suggesting a high specificity towards branched corals. This specificity is not present in C. nematifera overgrowing corals in Indonesia, as our observations didn’t highlight any preference for a particular coral growth form.
According to the different patterns of infestations (, ), we hypothesise that this sponge kills corals as reported by other studies (Plucer-Rosario Citation1987; Coles & Bolick Citation2007; Benzoni et al. Citation2008). We documented dead areas of coral along the boundary with the sponge (–D, G–J), strongly suggesting the production of allelochemicals harmful to the coral tissue, as demonstrated in other studies (Porter & Targett Citation1988). The chemical compounds could come in contact with coral tissues by the release of a mucous secretion (as documented for other sponges), frequently observed in C. nematifera when manipulated (Ávila & Carballo Citation2009). We also observed filaments of mucus in the sponge covering colonies of Acropora sp. ().
In a few instances (, B, F), we observed the sponge surrounded by wide areas of dead coral. These could be explained considering that the sponge, after spreading over the coral, could in turn die, leaving the dead coral skeleton available for the colonisation of other organisms, as reported by Benzoni et al. (Citation2008). This behaviour was never observed on colonies of Acropora sp. during this study. Further investigations are needed to clarify if C. nematifera can settle on and overgrow both dead and living corals.
Between the sites of Lembeh and Wakatobi, the damages caused by bomb fishing were evident both in the limited presence of the species targeted by this practice and in the direct impacts on the reefs. If C. nematifera had a preference for dead corals, these unfavourable conditions could increase the substratum available for sponge spreading and growth. However, at Isla Isabel National Park (Mexico), where a considerable coral reef deterioration was recorded after the El Niño event of 1982 (Nava & Carballo Citation2013), this sponge occurred in low percentages on rock, and was not found on coral rubble or mollusc shells (Ávila & Carballo Citation2009).
This study showed that the C. nematifera growth rate was about 50 mm/month, which is comparatively higher than the growth rate of other sponges such as Terpios hoshinota (Rützler & Muzik Citation1993, 23 mm/month) or Clathria (Microciona) sp. (Benzoni et al. Citation2008, 10 mm/month).
According to this study, C. nematifera appears to be able to rapidly spread and to eventually become dominant over corals, confirming it to be a potential threat for corals and coral reefs (Ávila & Carballo Citation2009). For this reason, monitoring actions should be conducted in the near future to better understand its growth dynamic, recruitment and distribution in the Indonesian coral reef ecosystems.
References
- Aerts LAM, van Soest RWM. 1997. Quantification of sponge/coral interactions in a physically stressed reef community, NE Colombia. Marine Ecology Progress Series 148:125–134. doi:10.3354/meps148125.
- Ávila E, Carballo JL. 2009. A preliminary assessment of the invasiveness of the Indo-Pacific sponge Chalinula nematifera on coral communities from the tropical Eastern Pacific. Biological Invasions 11:257–264. doi:10.1007/s10530-008-9230-5.
- Bell JJ. 2008. The functional roles of marine sponges. Estuarine, Coastal and Shelf Science 79:341–353. doi:10.1016/j.ecss.2008.05.002.
- Benzoni F, Calcinai B, Eisinger M, Klaus R. 2008. Coral disease mimic: Sponge attacks Porites lutea in Yemen. Coral Reefs 27:695.
- Calcinai B, Azzini F, Bavestrello G, Gaggero L, Cerrano C. 2007. Excavating rates and boring pattern of Cliona albimarginata (Porifera: Clionaidae) in different substrata. In: Custódio MR, Lôbo-Hajdu G, Hajdu E, Muricy G, editors. Porifera Research: Biodiversity, Innovation and Sustainability. Proceeding of 7th International Sponge Symposium, pp. 203–210.
- Cerrano C, Calcinai B, Pinca S, Bavestrello G. 2006. Reef sponges as hosts of biodiversity: cases from North Sulawesi. In: Suzuki Y, Nakamori T, Hidaka M, Kayanne H, Casareto BE, Nadao K, Yamano H, Tsuchiya M, editors. Proceedings Xth Coral Reef Symposium, June 28–July 2, 2004. Okinawa: Japanese Coral Reef Society, Tokyo, Japan, pp. 208–213.
- Chaves-Fonnegra A, Zea S. 2011. Coral colonization by the encrusting excavating Caribbean sponge Cliona delitrix. Marine Ecology 32:162–173. doi:10.1111/j.1439-0485.2010.00416.x.
- Coles S, Bolick H. 2007. Invasive introduced sponge Mycale grandis overgrows reef corals in Kane’ohe Bay, O’ahu, Hawai’i. Coral Reefs 26:911. doi:10.1007/s00338-007-0295-x.
- de Goeij JM, van Oevelen D, Vermeij MJA, Osinga R, Middelburg JJ, de Goeij AFPM, Admiraal W. 2013. Surviving in a marine desert: The sponge loop retains resources within coral reefs. Science (Wash.) 342:108–110. doi:10.1126/science.1241981.
- Diaz MC, Rützler K. 2001. Sponges: an essential component of Caribbean coral reefs. Bulletin of Marine Science 69:535–546.
- Laubenfels De MW. 1954. The sponges of the west-central Pacific. Oregon state college Corvallis, Oregon. Printed at the college press. Studies in Zoology 7:1–306.
- Nava H, Carballo JL. 2013. Environmental factors shaping boring sponge assemblages at Mexican Pacific coral reefs. Marine Ecology 34:269–279. doi:10.1111/maec.12012.
- Plucer-Rosario G. 1987. The effect of substratum on the growth of Terpios, an encrusting sponge which kills corals. Coral Reefs 5:197–200. doi:10.1007/BF00300963.
- Porter JW, Targett NM. 1988. Allelochemical interactions between sponges and corals. Biological Bulletin 175:230–239. doi:10.2307/1541563.
- Rützler K. 1978. Sponges in coral reefs. In: Stoddart DR, Johannes RE, editors. Coral reefs: Research methods, monographs on oceanographic methodology. Paris: UNESCO, pp. 299–313.
- Rützler K, Muzik K. 1993. Terpios hoshinota, a new cyanobactiosponge threatening Pacific reefs. Scientia Marina 4:395–403.
- Scheffers SR, van Soest RWM, Nieuwland G, Bak RPM. 2010. Coral reef framework cavities: is functional similarity reflected in composition of the cryptic macrofaunal community? Atoll Research Bulletin 583:1–24. doi:10.5479/si.00775630.583.1.
- Wang JT, Hirose E, Hsu CH, Chen YY, Meng PJ, Chaolun AC. 2012. A coral-killing sponge, Terpios hoshinota, releases larvae harboring cyanobacterial symbionts: An implication of dispersal. Zoological Studies 51:314–320.
- Weerdt De WH. 2002. Family Chalinidae Gray, 1867. In: Hooper JNA, Van Soest RWM, editors. Systema Porifera. A guide to the classification of sponges 1. New York, Boston, Dordrecht, London, Moscow: Kluwer Academic/Plenum Publishers, pp. 852–873.
- Wulff JL. 2006. Ecological interactions of marine sponges. Canadian Journal of Zoology 84:146–166. doi:10.1139/z06-019.