Abstract
Andalusian brown trout populations represent the southwestern limit of this species in Europe, show a high genetic diversity, are subjected to extreme habitat conditions and environmental alterations, and are very sensitive to extinction. However, there is a lack of scientific studies on them. A necessary first step to preserve them is to describe their spatial distribution. We detected the species in eastern Andalusia along almost 710 km of rivers, finding 38 populations inhabiting streams and reservoirs in the upper reaches of three basins (Guadalquivir, Segura and South) in an altitude range between 200 and 2200 m above sea level. Populations are constrained by natural causes in their upper limits, and by anthropogenic causes in their lower limits (mainly related to water management). Currently, the populations are very isolated in protected areas (62% of their distribution) or downstream of those areas (32%), and a range displacement towards higher altitudes compared with their distribution in the nineteenth century is observed.
Introduction
Brown trout (Salmo trutta Linnaeus, 1758) is a species native to Europe, showing a high genetic, ecological and morphological variability throughout its wide distribution area (Jonsson & Jonsson Citation2011). In the Iberian Peninsula, it is naturally located at the headwaters of almost all rivers, except some rivers in the east and south of Spain, and the Guadiana basin (Doadrio Citation2001).
South Iberian populations together with those of North Africa represent the southwestern limit in the natural distribution of the species. In the southernmost region of the Iberian Peninsula, Andalusia, this species is the only endemic salmonid. Andalusian brown trout populations include two of the six evolutionarily significant units (ESUs) present in the Iberian Peninsula (Machordom et al. Citation2000), with five new worldwide haplotypes recently discovered (Almodóvar et al. Citation2010). Thus, preserving the integrity of these populations is crucial for the conservation of the entire genetic diversity of the species (Almodóvar et al. Citation2006).
Deleterious effects of overfishing (Almodóvar & Nicola Citation2004; Johnston et al. Citation2012), problems associated with habitat loss, alteration and fragmentation (e.g. Gosset et al. Citation2006; Maceda-Veiga & De Sostoa Citation2011), introduction of exotic species (e.g. Elvira & Almodóvar Citation2001; Leunda Citation2010; De Silva Citation2012) and introgression of foreign genes (e.g. Laikre Citation1999; Madeira et al. Citation2005; Almodóvar et al. Citation2006) on the brown trout populations are problems that have been intensively studied by many authors in all the wide distribution range of this species. Although overfishing has affected its current distribution and conservation status in some Spanish rivers (García de Jalón & Schmidt Citation1995), since 2005, brown trout in Andalusia may be fished only in the modality of catch and release. Hence, this problem does not affect trout populations in the study area.
Moreover, it is noteworthy that human civilizations have shown an intense and historic predilection for settling in areas of the Mediterranean basin, so that the rivers flowing in Mediterranean climate regions have suffered the largest anthropogenic interventions on Earth (Blondel et al. Citation2010). This high impact has resulted mainly in pollution, water abstraction and droughts, restricted distribution ranges of species or limited dispersal of taxa. Thus, in the Mediterranean rivers, 36% of freshwater fish species can be included as threatened (critically endangered, endangered or vulnerable), seven species are extinct and one is extinct in the wild (Tierno de Figueroa et al. Citation2013). This situation is even more worrying when considering only Mediterranean endemic species, with 56% of them threatened (Smith & Darwall Citation2006). In Andalusia, 19 of the 22 native freshwater fishes (more than half being Iberian endemisms) are under some degree of threat. Thus, while brown trout in Europe is catalogued as “least concern, LC” (Freyhof Citation2011) and in Spain as “vulnerable, VU” (criterion 1cde; Doadrio Citation2001), in Andalusia it is catalogued as “endangered, EN” (criterion A1e; Franco Ruíz & Rodríguez de los Santos Citation2001).
All of these previous data show the importance of conservation of Andalusian brown trout populations. However, only two studies on historical distribution in the nineteenth century (Menor & Prenda Citation2006; Sáez Gomez Citation2010), and another on genetic analysis (Almodóvar et al. Citation2010), have been published regarding brown trout conservation in the study area.
Thus, the aims of the present study are (1) to identify external factors responsible for the current distribution of brown trout in the study area, which is crucial for the future conservation of the species and its habitats; and (2) to determine the number and distribution of brown trout populations that inhabit the fresh waters of Andalusia, establishing their limits of distribution and identifying the factors that set those limits.
Materials and methods
Study area
Andalusia is the southwesternmost region of the European continent (), and is the second largest region of Spain (17.3% of the surface of the country). Noteworthy is the fact that it has the largest network of protected natural areas in Europe, which constitute 30% of the entire protected areas in Spain.
Andalusia has 46,415 km of water courses within four basins: Guadalquivir, South Mediterranean (hereafter South), Guadiana and Segura (; ).
Table I. Overview of the four Andalusian basins and results obtained for brown trout in them.
Methods
Historical information was obtained from: (1) the distribution of brown trout in Andalusia in the nineteenth century (Menor & Prenda Citation2006; Sáez Gomez Citation2010); (2) a Spanish inventory of terrestrial and freshwater species (Spanish law, Real Decreto 556/2011); and (3) interviews with people related to the aquatic environment (environmental agents, local fishermen, shepherds, etc.). This information allowed us to identify the rivers where the presence of the species was known in the past, and rivers where their presence could be likely, being next to reaches of inhabited rivers, tributaries with permanent water or isolated reaches of difficult access.
To set the current limits of the distribution range, field sampling was conducted by means of electrofishing qualitative surveys (presence/absence) during the summers of 2008 and 2009. To minimize fish mortality, electric current intensity was regulated depending on water conductivity (Johnson et al. Citation2007) at every sampling site.
Single-pass electrofishing surveys were conducted at each sampling site (maximum length 400 m). If the species was detected, the following sampling station was located 1 km upstream or downstream, depending on whether we were delimiting the upper or lower limit in that river, respectively. This procedure was repeated until no brown trout was detected at the following sampling station. In such cases, one more sampling was performed 500 m away from the former site to corroborate the limit.
The geographical distribution of the brown trout populations was defined by the limits of the presence of brown trout in a continuous river network (Berryman Citation1999). Each population was characterized by the geographical coordinates and the altitude of its limits. Subsequently, the type of factors (natural or anthropogenic) and the causes that prevented the expansion of the species were identified. Natural factors (NA) were classified into four categories (causes): (1) water temperature (Te); (2) the limit is the source of the stream (Rs); (3) impassable waterfall (Iw); and (4) naturally dry in summer (Sd). Six main sources of perturbation on biodiversity and ecology of freshwater rivers pointed out by Clavero et al. (Citation2010) were considered as anthropogenic factors (AN): (1) reservoirs and channel construction (Da); (2) agriculture (leading to agricultural water pollution, Ag); (3) water abstraction (Wa); (4) invasive species (Is); (5) overfishing (Of); and (6) pollution (Po). Moreover, an additional category was considered, multiple (Mu), when more than one cause acted.
Furthermore, other factors that may be relevant to the current distribution of the species in this area were considered: (1) the distribution of mountain systems in Andalusia and (2) the location of its protected natural areas.
To obtain inhabited total distances in the study area and identify how much of them were located within protected areas, the limits of trout populations were digitalized using ArcGIS 9.3.1. and the boundaries of protected natural areas were obtained from the Environmental Information Network of Andalusia (REDIAM, http://www.juntadeandalucia.es/medioambiente/site/web/rediam).
Results
Eighty-one water courses, including main rivers and tributaries, were sampled. It should be noted that a population may have one or more upper limits (as many as there are tributaries), but only one lower limit after all the inhabited tributaries converge. Thereby, 38 isolated populations, 64 upper limits and 37 lower limits have been identified.
Brown trout distribution is currently concentrated in the east of the Andalusian region (). Brown trout has been detected along almost 710 km of rivers, streams and reservoirs in the upper reaches of the Guadalquivir, Segura and South basins ( and ; see also supplemental online data): 70.1, 20.3 and 9.6%, respectively.
Figure 1. Location of Andalusia (in gray shading). The Iberian Peninsula basins are delimited in the figure. Four of them form part of Andalusia: (1) Guadiana, (2) Segura, (3) Guadalquivir and (4) South.
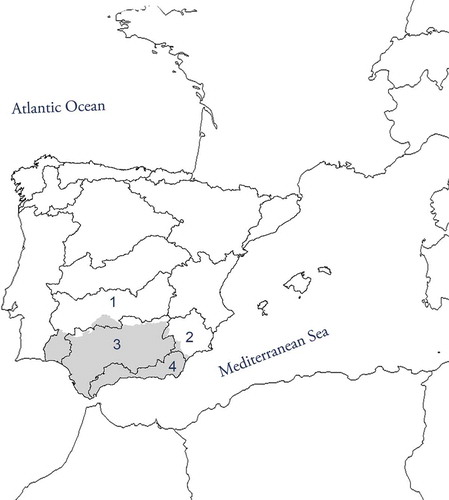
Figure 2. (A) Altimetry of the study region (meters above sea level, m a.s.l.); (B) distribution of the brown trout in Andalusia: thick lines delimit Andalusia, shaded lines demarcate the basins (see ) and the boxes show the areas where brown trout populations are present; (C, D) detailed distributions. The light gray surfaces are natural parks and the dark gray surfaces are national parks. These natural reserves are named, and the inhabiting rivers are numbered, according to the codes in the supplemental online data.
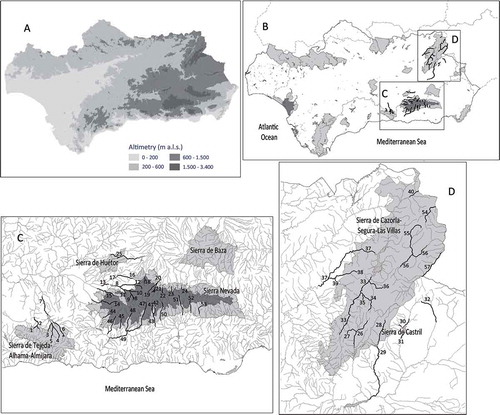
Figure 3. Altitudinal ranges (meters above sea level, m a.s.l.) inhabited by each population (reaches codes in brackets are as listed in the supplemental data), causes of their upper and lower limits (natural indicated with empty symbols, and anthropogenic with solid symbols) and natural reserves containing all, or part, of the reaches inhabited by the population. Causes of the limits: ![]()
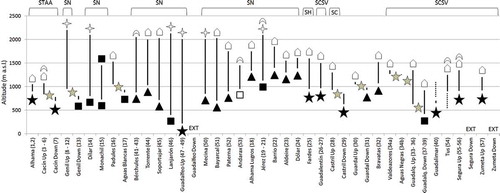
The altitudinal ranges of brown trout populations in Andalusia, the causes of population limits, both upper and lower, and different natural reserves where the species is present are summarized in (more details in the supplemental online data). The highest amplitude of altitude ranges (from 2200 to 200 m above sea level, a.s.l.) have been detected in populations whose upper limits are located in the Sierra Nevada National Park within the Guadalquivir and South basins.
Brown trout is present is Sierra Nevada National Park and it also inhabits some natural parks: Tejeda–Alhama–Almijara, Castril, Cazorla–Segura–Las Villas and Sierra Nevada Natural Park (). The upper limits of all populations are located in these protected areas, except in rivers 16, 17, 30, 31, 32 and 39 (supplemental data), that are completely outside of the natural reserves and account for 6% of the total distribution of the species in the region. The remaining inhabited reaches outside the natural reserves (32% of the Andalusian distribution) are the downstream continuation of populations inhabiting those areas (; supplemental online data) and more than half of them (21% of the Andalusian distribution) are characterized by the species’ reduced population size and fragile demographic status (unpublished data). Thus, currently the species inhabits both protected areas (62%) and unprotected ones (38%) ().
Table II. Kilometers of river inhabited by brown trout in the Andalusian basins, and inhabited partial percentages in each basin. Distribution inside and outside of natural reserves is shown. Gray cells indicate where the trout is managed as a fishing resource (Andalusian law, Orden 6 de mayo de 2014).
In general, populations are delimited by natural causes in their upper limits, and by anthropogenic causes in their lower limits (). In 33 cases, brown trout inhabits from the headwaters of the rivers, in another 13 cases they arrive at the highest possible altitude, above which the low temperatures prevent survival, and in nine cases they are limited by impassable waterfalls. The remaining eight populations are fragmented populations located downstream of reservoirs, whose upper limits are found in the dams. Regarding the 37 lower limits detected, 17 of them are due to the presence of impassable dams to create reservoirs, and 12 to water abstraction for irrigation and human consumption. Finally, the strong alteration of watercourses due to the synergistic effects of agriculture, water diversion, deterioration of water quality (pollution) and habitat fragmentation (reservoirs), causes the disappearance of the species in another seven rivers. In only one lower limit does the cause of the disappearance have a natural origin, i.e. drying in summer due to the natural flow reduction characteristic of the Mediterranean region and the geological characteristics of the substrate (high porosity).
Table III. Causes delimiting the brown trout populations in Andalusia in their upper and lower limits. The types of factors and particular causes are shown.
summarizes all reaches inhabited by rainbow trout (Oncorhynchus mykiss Walbaum, 1792) close to or within the current distribution of the native species. All of these sections are or were inhabited by brown trout in the past. Some naturalized rainbow trout populations (with reproductive capacity) have been detected.
Table IV. Reaches inhabited by rainbow trout coinciding with reaches currently or historically inhabited by the brown trout in Andalusia. Only reaches close to the current distribution of the native salmonid are considered. The rivers designated as “Oncorhynchus mykiss” were inhabited by brown trout in the past (Sáez Gomez Citation2010). The current administrative figure (Andalusian law, Orden 6 de mayo 2014), its length, reaches to which they belong (codes in the supplemental online data) and salmonids detected in each case are indicated. NLFA: non-limited access fishing areas; SFA: stocked fishing areas (some in reaches inhabited by brown trout). Asterisks (*) indicate reaches where rainbow trout reproduction has been confirmed. Shading cells in the “Salmo trutta & Oncorhynchus mykiss” column indicate that both species are present in these reaches; shading cells in the “Oncorhynchus mykiss” column indicate that this species is the only one present in those reaches.
Discussion
Geographical distribution, mountain systems and natural reserves
Brown trout inhabits cold waters (Kottelat & Freyhof Citation2007) and in the Iberian Peninsula is located in mountain streams and upper reaches of the rivers (Nieto et al. Citation2006).
Comparing the current Andalusian distribution of the species () with its distribution in the nineteenth century (Menor & Prenda Citation2006; Sáez Gomez Citation2010), we observed a range contraction and a displacement towards higher altitudes, the western populations having disappeared, closer to the great valley of the Guadalquivir River, where the average water temperature is higher. Its current distribution is associated with rivers whose sources are in the eastern mountain systems, the only ones with areas above 1500 m a.s.l. () and temperature and flow regimens (REDIAM Citation2014) that can sustain optimum ecological conditions for the species. Hence, the eastern Andalusian mountain systems are the last habitat available for the species in its southwestern European distribution edge. In the absence of metapopulation studies, it can be noted that this species in Andalusia is represented by resident populations with a high degree of population isolation between basins and even among rivers ().
On the other hand, the organisms living at the edge of their distribution range are subjected to intense habitat pressures that increase their vulnerability to threats: they often persist in small isolated populations, facing ecological conditions very different from those of the main distribution area and from those considered optimum (Sanz et al. Citation2006).
A lack of protection measures in the middle and lower reaches of rivers (urbanized, fragmented and used in agriculture) has endangered many endemic species of these areas, and has eliminated river connectivity between nearby mountain ranges, removing the natural corridors that kept relatively distant brown trout populations connected. Therefore, brown trout populations in Andalusia are isolated in rivers and streams from protected natural spaces (62% of the distribution) and downstream of those watercourses (32%). Very few populations inhabit areas completely out of them (6% of the distribution). Furthermore, the inhabited river reaches outside of protected natural areas present impacts by damming, water extraction and invasive species, and trout densities are much lower than those detected in the interior of the natural reserves (unpublished data).
It is also known that freshwater biodiversity is being lost at an alarming rate, even more rapidly than terrestrial biodiversity (Moyle & Yoshiyama Citation1994; Cowx & Collares-Pereira Citation2002). Moreover, most current reserves were designed for preserving terrestrial organisms, based on insufficient criteria for adequate management of freshwater biodiversity. Also, there is an urgent need to protect native fish species in light of the ongoing anthropogenic degradation of aquatic environments (Filipe et al. Citation2004).
Causes and typologies of the distribution limits
The fact that the upper limits in the fluvial networks have a natural origin, while the lower limits are anthropogenic (), means that the geographical range of the species under undisturbed conditions would be larger downstream than currently observed. These lower limits are mainly disturbances related to the management of water (reservoirs and water abstraction), a resource that is often scarce in the study area.
Generally, the lower limits at Sierra Nevada are more related to the presence of urban settlements and water management (ditches/untreated discharges), while in other areas the dams and the arrival at lower altitudes more suitable for cyprinid species are the limit factors (). Also, in the Guadalquivir basin, there is a strong impact of olive farming on the riverbed by increased turbidity and runoff, as well as by the current and historical presence of stocked fishing areas (SFA): reaches established by the regional administration where specimens of fishable size of rainbow trout are or were introduced ().
Upper limits
In 33 upper limits, the species was detected to the source of the water course (). Due to the high interannual variability that characterizes the Mediterranean climate, rivers may suffer sharp declines in flow during summer (Tierno de Figueroa et al. Citation2013). During the field work, we detected several times how the natural retractions were so intense that the sources in summer were displaced up to hundreds of meters downstream, forcing fish communities to restrict their range temporarily. The rains of spring and autumn increased again the altitude of the sources and favored the upstream spawning migration of the adults during late fall and early winter.
In 13 cases, the upper limits had an ecological component related to water temperature, and all of them were located in the Sierra Nevada Mountains. This mountain massif has some of the highest peaks of the Iberian Peninsula, and is the southernmost refuge for many species of higher latitudes (Molero Mesa et al. Citation1992).
Temperature has pervasive influences on rates of chemical and physiological reactions of fish, affecting metabolic rate, swimming, feeding, growth and reproduction (Jonsson & Jonsson Citation2011). It is assumed that the lower lethal temperature for brown trout is at, or slightly below, 0°C (Ojanguren & Braña Citation2003; Elliott & Elliott Citation2010), and in these 13 limits, the species reaches the highest level (about 2100 to 2200 m a.s.l.) at which the winter temperature allows it to survive in these latitudes.
In another nine cases, the populations reach up to impassable waterfalls (), although above them the optimal habitat is maintained. It is possible that the trout have never inhabited above these waterfalls.
One only population presents an anthropogenic upper limit, due to the synergistic interaction of water extraction and fragmentation of habitat (water diversion associated with a dam for the production of hydroelectric power).
The eight cases noted as Da in are populations inhabiting downstream of reservoirs (). These barriers are also the lower limits of the isolated populations upstream. The effects of damming will be discussed below, in the lower limits section.
Lower limits
The rivers of Mediterranean regions tend to be more heavily impounded than rivers in humid climates, because the demand for water is greater (Kondolf & Batalla Citation2005; Grantham et al. Citation2010). Water abstraction has been a cause described in numerous studies analyzing anthropogenic impacts on river ecosystems and fish populations in different Mediterranean climate regions (e.g. in Australia: Kingsford Citation2000; in California: Moyle et al. Citation2011; in Chile: Habit et al. Citation2007). Dewatering by diversion reduces the habitat available in streams and sometimes renders it entirely hostile (e.g. through warming) or serves as a barrier to fish passage (e.g. loss of surface flow through riffle crests) (Williams Citation2006). In the study area, the 12 cases of water abstraction are associated with irrigation ditches, mainly for agriculture and human consumption. The extractions are so intense that the water flow decreases strongly all year round, and in summer drying is complete. In addition, in these and other rivers, numerous ditches were detected along their entire length, regardless of the ditches that cause the lower limits. These ditches tend to decrease the flow in summer, drying up some reaches, fragmenting the populations and threatening the survival of individuals that become isolated in pools. We observed how the first 100 m inside some of these ditches are used for spawning by trout, as noted by Williams (Citation2006) in the Columbia River Basin, because the granulometry and the laminar flow inside ditches are very suitable to the egg incubation. However, they can become death traps for adults and fry that die in irrigation ponds or farmland.
There are a large number of reservoirs in the headwaters of the rivers inhabited by brown trout (). Thus, 17 lower limits are due to the presence of dams (). Downstream of them, nine trout populations disappear, while on eight occasions the ancestral populations have been fragmented. Dams are one of the greatest threats to river biodiversity worldwide (Poff et al. Citation2007) and one of the major problems described in the European Water Framework Directive (2000/60/CE). Dams produce drastic changes in river courses: they change the natural flow regimes (e.g. Kingsford Citation2000; Alonso-González et al. Citation2008), alter the dynamics of the whole catchment basin and the periodic patterns of temperature, sediment grain size and composition of the rivers (e.g. Shieh et al. Citation2007; Maddock et al. Citation2008; Kishi & Maekawa Citation2009) and promote the invasion of exotic species (Clavero et al. Citation2004; Johnson et al. Citation2008), modifying their structure and the natural dynamics of the biota (Bunn & Arthington Citation2002). Thus, damming is a cataclysmic event in the life of a riverine system (Ligon et al. Citation1995). Many authors have studied the effects of damming on river ecosystems and fish populations (e.g. Hart & Poff Citation2002), with more than twice as many studies being conducted on salmonids than on any other fish family (Murchie et al. Citation2008). Alteration of natural flow regimes modifies the natural rates of growth, development, habitat use, reproduction or size (Yrjänä et al. Citation2002; Kishi & Maekawa Citation2009) and the construction of hydrological barriers fragments populations into discrete units regardless of historical connections, and causes genetic isolation (Rieman & Dunham Citation2000; Gosset et al. Citation2006; Heggenes & Røed Citation2006). To these general effects we have to join the high natural stochasticity of the Mediterranean systems, due to the highly unpredictable interannual flow variations associated with natural seasonal events (Gortázar et al. Citation2007). Thus, the isolation of Mediterranean fishes has devastating effects: the resilience of patchy populations decreases when facing stochastic events, particularly when carrying capacity is low (Morita & Yokota Citation2002). For this reason, isolated brown trout populations in the study area are subjected to high stress and the processes of extinction could be intensified in these peripheral populations.
Currently, the eight populations located downstream of reservoirs () have much lower densities than upstream populations (unpublished data). Considering both adverse effects of fragmentation and their current low densities, we can assume that these populations are severely endangered (16% of current distribution). In fact, during the realization of a previous study, three populations located downstream of dams have recently disappeared ().
Severe droughts have marked effects on salmonid populations by reducing the volume of water available to the fish, impeding their migration and adversely affecting water quality, especially water temperature and dissolved oxygen (Elliott Citation2000), causing increased mortality and decreased growth of the trout (Elliott et al. Citation1997). Thus, summer drought is the only natural cause detected in one of the lower limits in the study area (53), due both to the natural flow retracting and the river bed permeability. In periods of strong increases in flow, this river can connect with a small reach, 10 km downstream, where water remains throughout the year and a remnant population inhabits, which could behave as sink within a metapopulation structure.
Sometimes many factors contribute to the extinction of a species, and often it is difficult to identify a single cause (Allan & Flecker Citation1993). Furthermore, in freshwater fishes, it has been demonstrated that some factors acts more synergistically than additively (Leuven & Poudevigne Citation2002). This is the case of seven lower limits (named “multiple” in ). In them, agriculture, pollution, water abstraction and invasive species interact with different intensity. They are worldwide proven causes of declines and extinctions of riverine communities in general, and of fish populations in particularly (e.g. Malmqvist & Rundle Citation2002; Smith & Darwall Citation2006).
Mediterranean climate regions have an unpredictable annual precipitation and limited water availability during the dry season (Grantham et al. Citation2010), so intensive agriculture threatens the conservation of natural inland waters in the study area. This threat may be indirect, by water infrastructure development (e.g. reservoirs or water extractions) or direct, due to crop expansion and traditional land management. The lower Guadalquivir population (37 to 39 in the supplemental online data) is the only case detected in Andalusia where a brown trout population is directly affected by agriculture, due to intensive olive monoculture widely distributed throughout the affected subbasin. The land is highly plowed, without vegetation cover, and large amounts of silts and fine sands flow into streams by runoff from farmland, even when the intensity of rainfall is very low, resulting in a reduction of both the redd permeability and the oxygen supply to incubate eggs, inhibiting the emergence of fry (Acornley & Sear Citation1999; Moyle et al. Citation2011). This runoff increases the water turbidity, which reduces in brown trout its ability of capturing food, and modifies its feeding (Stuart-Smith et al. Citation2004). It is also known (Maceda-Veiga Citation2013) that the use of pesticides and herbicides affects fish populations, and especially brown trout (Rodríguez-Cea et al. Citation2003). Furthermore, this population is also affected by the presence of a reservoir, upstream, and one SFA, downstream, so that the probability of local extinction in this case increases.
On the other hand, brown trout stand as symbols of clean, cold water from the northern hemisphere (Jonsson & Jonsson Citation2011). In the study area, the species is found from the upper reaches of rivers until the appearance of the first urban settlements, where water management carried out by its inhabitants often causes a sharp reduction in flow (water abstraction or damming in particular; ). Moreover, sometimes these villages discharge their untreated waste water into the rivers. Pollution has deleterious effects for the survival of this species, but being mountain villages with few inhabitants, the small size of their discharges does not cause the disappearance of the species by itself. However, the deleterious effect of discharges increases under low-flow conditions (Gasith & Resh Citation1999), acting synergistically.
It is known (Clavero et al. Citation2004) that in Mediterranean systems, the number of introduced species is positively related to the presence of reservoirs. In fact, in most of the reservoirs included in the distribution area of trout in Andalusia (), invasive species can be found (e.g. Esox lucius Linnaeus, 1758, Cyprinus carpio Linnaeus, 1758, Micropterus salmoides Lacépède, 1802, etc.). Moreover, since the late nineteenth century, rainbow trout (Oncorhynchus mykiss Walbaum, 1792) has been stocked in Spain for angling purposes (Elvira & Almodóvar Citation2001), in agreement with the “human activity” hypothesis for the species invasions (Leprieur et al. Citation2008). We detected this alien species cohabiting with brown trout along almost 69 km of rivers (), which represents 9.7% of the current distribution of the native species in Andalusia. Furthermore, rainbow trout was the only fish detected in another 76 km of rivers (), in reaches that had harbored indigenous trout populations in the past (Sáez Gomez Citation2010). Of these 145 km invaded, 32 km correspond to SFA in reaches inhabited by brown trout, or very close to the current range of the native species. The remaining alien populations come from deliberate introductions made in the past (uncontrolled genetic stocks for infertility) or from escapes of rainbow trout detected in all Andalusian river reaches located downstream of fish farms. In many of these reaches (including some SFAs), the alien species has become naturalized, and we detected active reproduction and representation of all age classes ().
In this way, acclimatization of exotic freshwater fishes in Iberian rivers is probably one of the most important negative factors affecting the survival of the native species (Elvira & Almodóvar Citation2001), because they may cause predation, competition, disease transmissions, hybridization and behavioral interference (Crawford & Muir Citation2008; Leunda Citation2010). Thus, rainbow trout affects brown trout habitat selection and survival (Blanchet et al. Citation2007; Fausch Citation2007), and its spawn destroy native trout spawning redds (Landergren Citation1999). When the spawning space is limited, the interspecific competitive pressure due to redd superimposition can eliminate native populations of trout (Scott & Irvine Citation2000). It seems likely that these phenomena have taken place in the “Oncorhynchus mykiss” reaches reported in .
Supplemental data
Supplemental data for this article can be accessed here.
Supplementary Data
Download MS Word (57.2 KB)Acknowledgements
This study is framed in the Conservation Program for Brown trout in Andalusia (1589/2007/M/00 and 143/2010/M/00) developed by the Environmental Council of the Andalusian Government. The authors thank Dr. López-Rodríguez for his valuable comments.
References
- Acornley RM, Sear DA. 1999. Sediment transport and siltation of brown trout (Salmo trutta L.) spawning gravels in chalk streams. Hydrological Processes 13:447–458. doi:10.1002/(SICI)1099-1085(19990228)13:3%3C447::AID-HYP749%3E3.0.CO;2-G.
- Allan JD, Flecker AS. 1993. Biodiversity conservation in running waters. Identifying the major factors that threaten destruction of riverine species and ecosystems. Bioscience 43:32–43. doi:10.2307/1312104.
- Almodóvar A, Nicola GG. 2004. Angling impact on conservation of Spanish stream–dwelling brown trout Salmo trutta. Fisheries Management and Ecology 11:173–182. doi:10.1111/j.1365-2400.2004.00402.x.
- Almodóvar A, Nicola GG, Elvira B, Garcia–Marin JL. 2006. Introgression variability among Iberian brown trout evolutionary significant units: The influence of local management and environmental features. Freshwater Biology 51:1175–1187. doi:10.1111/j.1365-2427.2006.01556.x.
- Almodóvar A, Nicola GG, Leal S, Elvira B. 2010. Análisis genético de las poblaciones de Trucha Común Salmo trutta en la Comunidad Autónoma de Andalucía. Memoria Final Proyecto Egmasa–Junta de Andalucía y Universidad Complutense de Madrid, Sevilla.
- Alonso-González C, Gortázar J, Baeza Sanz D, García de Jalón D. 2008. Dam function rules based on brown trout flow requirements: Design of environmental flow regimes in regulated streams. Hydrobiologia 609:253–262. doi:10.1007/s10750-008-9408-y.
- Berryman AA. 1999. Principles of population dynamics and their application. Cheltenham: Stanley Thornes.
- Blanchet S, Loot G, Grenouillet G, Brosse S. 2007. Competitive interactions between native and exotic salmonids: A combined field and laboratory demonstration. Ecology of Freshwater Fish 16:133–143. doi:10.1111/j.1600-0633.2006.00205.x.
- Blondel J, Aronson J, Bodiou JY, Boeuf G. 2010. The Mediterranean region: Biological diversity through time and space. Oxford: Oxford University Press.
- Bunn SE, Arthington AH. 2002. Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environmental Management 30:492–507. doi:10.1007/s00267-002-2737-0.
- Clavero M, Blanco-Garrido F, Prenda J. 2004. Fish fauna in Iberian Mediterranean river basins: Biodiversity, introduced species and damming impacts. Aquatic Conservation: Marine and Freshwater Ecosystems 14:575–585. doi:10.1002/aqc.636.
- Clavero M, Hermoso V, Levin N, Kark S. 2010. Biodiversity research: Geographical linkages between threats and imperilment in freshwater fish in the Mediterranean Basin. Diversity and Distributions 16:744–754. doi:10.1111/j.1472-4642.2010.00680.x.
- Cowx IG, Collares–Pereira MJ. 2002. Freshwater fish conservation: Options for the future. In: Collares–Pereira MJ, Cowx IG, Coelho MM, editors. Conservation of freshwater fishes: Options for the future. Oxford: Fishing News Books/ Blackwell Science. pp. 443–452.
- Crawford SS, Muir AM. 2008. Global introductions of salmon and trout in the genus Oncorhynchus: 1870–2007. Reviews in Fish Biology and Fisheries 18:313–344. doi:10.1007/s11160-007-9079-1.
- De Silva SS. 2012. Aquaculture: A newly emergent food production sector and perspectives of its impacts on biodiversity and conservation. Biodiversity and Conservation 21:3187–3220. doi:10.1007/s10531-012-0360-9.
- Doadrio I. 2001. Atlas y libro rojo de los peces continentales de España. Madrid: Ministerio de Medio Ambiente.
- Elliott JM. 2000. Pools as refugia for brown trout during two summer droughts: Trout responses to thermal and oxygen stress. Journal of Fish Biology 56:938–948. doi:10.1006/jfbi.1999.1220.
- Elliott JM, Elliott JA. 2010. Temperature requirements of Atlantic salmon Salmo salar, brown trout Salmo trutta and Arctic charr Salvelinus alpinus: Predicting the effects of climate change. Journal of Fish Biology 77:1793–1817. doi:10.1111/j.1095-8649.2010.02762.x.
- Elliott JM, Hurley MA, Elliott JA. 1997. Variable effects of droughts on the density of a sea–trout Salmo trutta population over 30 years. The Journal of Applied Ecology 34:1229–1238. doi:10.2307/2405234.
- Elvira B, Almodóvar A. 2001. Freshwater fish introductions in Spain: Facts and figures at the beginning of the 21st century. Journal of Fish Biology 59:323–331. doi:10.1111/j.1095-8649.2001.tb01393.x.
- Fausch KD. 2007. Introduction, establishment and effects of non–native salmonids: Considering the risk of rainbow trout invasion in the United Kingdom. Journal of Fish Biology 71:1–32. doi:10.1111/j.1095-8649.2007.01682.x.
- Filipe AF, Marques TA, Seabra S, Tiago P, Ribeiro F, Moreira L et al. 2004. Selection of priority areas for fish conservation in Guadiana River Basin, Iberian Peninsula. Conservation Biology 18:189–200. doi:10.1111/j.1523-1739.2004.00620.x.
- Franco Ruíz A, Rodríguez de los Santos M. 2001. Libro rojo de los vertebrados amenazados de Andalucía. Sevilla: Consejería de Medio Ambiente Junta de Andalucía.
- Freyhof J. 2011. Salmo trutta. In: The IUCN Red List of Threatened Species. Version 2013.1. Available: http://www.iucnredlist.org/details/19861/0. Accessed July 2013 30.
- García de Jalón D, Schmidt G. 1995. Manual práctico para la gestión sostenible de la pesca fluvial. Girona: A.E.M.S.
- Gasith A, Resh VH. 1999. Streams in Mediterranean climate regions: Abiotic influences and biotic responses to predictable seasonal events. Annual Review of Ecology and Systematics 30:51–81. doi:10.1146/annurev.ecolsys.30.1.51.
- Gortázar J, García De Jalón D, Alonso-González C, Vizcaíno P, Baeza Sanz D, Marchamalo M. 2007. Spawning period of a southern brown trout population in a highly unpredictable stream. Ecology of Freshwater Fish 16:515–527. doi:10.1111/j.1600-0633.2007.00246.x.
- Gosset C, Rives J, Labonne J. 2006. Effect of habitat fragmentation on spawning migration of brown trout (Salmo trutta L.). Ecology of Freshwater Fish 15:247–254. doi:10.1111/j.1600-0633.2006.00144.x.
- Grantham TE, Merenlender AM, Resh VH. 2010. Climatic influences and anthropogenic stressors: An integrated framework for streamflow management in Mediterranean–climate California, U.S.A. Freshwater Biology 55:188–204. doi:10.1111/j.1365-2427.2009.02379.x.
- Habit E, Belk M, Victoriano P, Jaque E. 2007. Spatio–temporal distribution patterns and conservation of fish assemblages in a Chilean coastal river. Biodiversity and Conservation 16:3179–3191. doi:10.1007/s10531-007-9171-9.
- Hart DD, Poff LN. 2002. A special section on dam removal and river restoration. Bioscience (Special Issue) 52:653–747. doi:10.1641/0006-3568(2002)052%5B0653:ASSODR%5D2.0.CO;2.
- Heggenes J, Røed KH. 2006. Do dams increase genetic diversity in brown trout (Salmo trutta)? Microgeographic differentiation in a fragmented river. Ecology of Freshwater Fish 15:366–375. doi:10.1111/j.1600-0633.2006.00146.x.
- Johnson DH, Shrier BM, O´Neal JS, Knutzen JA, Augerot X, O´Neil TA, Pearsons TN. 2007. Salmonid field protocols handbook. Techniques for assessing status and trends in Salmon and trout populations. Bethesda: American Fisheries Society in association with State of the Salmon.
- Johnson PTJ, Olden JD, Vander Zanden MJ. 2008. Dam invaders: Impoundments facilitate biological invasions into freshwaters. Frontiers in Ecology and the Environment 6:357–363. doi:10.1890/070156.
- Johnston FD, Arlinghaus R, Dieckmann U. 2012. Fish life history, angler behaviour and optimal management of recreational fisheries. Fish and Fisheries 14:554–579. doi:10.1111/j.1467-2979.2012.00487.x.
- Jonsson B, Jonsson N. 2011. Ecology of Atlantic Salmon and Brown Trout. Habitat as a template for life histories. Corvallis: Fish & Fisheries Series, Vol. 33.
- Kingsford RT. 2000. Ecological impacts of dams, water diversions and river management on floodplain wetlands in Australia. Austral Ecology 25:109–127. doi:10.1046/j.1442-9993.2000.01036.x.
- Kishi D, Maekawa K. 2009. Stream-dwelling Dolly Varden (Salvelinus malma) density and habitat characteristics in stream sections installed with low-head dams in the Shiretoko Peninsula, Hokkaido, Japan. Ecological Research 24:873–880. doi:10.1007/s11284-008-0562-5.
- Kondolf GM, Batalla RJ. 2005. Hydrological effects of dams and water diversions on rivers of Mediterranean–climate regions: Examples from California. In: Garcia C, Batalla RJ, editors. Catchment dynamics and river processes: Mediterranean and other climate regions. Amsterdam, London: Elsevier Science. pp. 197–211.
- Kottelat M, Freyhof J. 2007. Handbook of European freshwater fishes. Berlin, Germany: Kottelat, Cornol and Freyhof.
- Laikre L. 1999. Conservation genetic management of brown trout (Salmo trutta) in Europe. Report by the Concerted action on identification, management and exploitation. Silkeborg: Danish Institute for Fisheries Research.
- Landergren P. 1999. Spawning of anadromous rainbow trout, Oncorhynchus mykiss (Walbaum): A threat to sea trout, Salmo trutta L., populations? Fisheries Research 40:55–63. doi:10.1016/S0165-7836(98)00215-X.
- Leprieur F, Beauchard O, Blanchet S, Oberdorff T, Brosse S. 2008. Fish invasions in the world’s river systems: When natural processes are blurred by human activities. PLoS Biology 6:e28. doi:10.1371/journal.pbio.0060028.
- Leunda P. 2010. Impacts of non-native fishes on Iberian freshwater ichthyofauna: Current knowledge and gaps. Aquatic Invasions 5:239–262. doi:10.3391/ai.2010.5.3.03.
- Leuven R, Poudevigne I. 2002. Riverine landscape dynamics and ecological risk assessment. Freshwater Biology 47:845–865. doi:10.1046/j.1365-2427.2002.00918.x.
- Ligon FK, Dietrich WE, Trush WJ. 1995. Downstream ecological effects of dams. Bioscience 45:183–192. doi:10.2307/1312557.
- Maceda–Veiga A. 2013. Towards the conservation of freshwater fish: Iberian rivers as an example of threats and management practices. Reviews in Fish Biology and Fisheries 23:1–22. doi:10.1007/s11160-012-9275-5.
- Maceda-Veiga A, De Sostoa A. 2011. Observational evidence of the sensitivity of some fish species to environmental stressors in Mediterranean rivers. Ecological Indicators 11:311–317. doi:10.1016/j.ecolind.2010.05.009.
- Machordom A, Suárez J, Almodóvar A, Bautista JM. 2000. Mitochondrial haplotype variation and phylogeography of Iberian brown trout populations. Molecular Ecology 9:1324–1338. doi:10.1046/j.1365-294x.2000.01015.x.
- Maddock I, Smolar–Žvanut N, Hill G. 2008. The effect of flow regulation on the distribution and dynamics of channel geomorphic units (CGUs) and implications for Marble Trout (Salmo marmoratus) spawning habitat in the Soča River, Slovenia. IOP Conf. Ser.: Earth Environ. Sci. (Vol. 4). Available: http://iopscience.iop.org/1755–1315/4/1/012026. Accessed Jan 2013 15.
- Madeira MJ, Gómez–Moliner BJ, Machordom Barbé A. 2005. Genetic introgression on freshwater fish populations caused by restocking programmes. Biological Invasions 7:117–125. doi:10.1007/s10530-004-9641-x.
- Malmqvist B, Rundle S. 2002. Threats to the running water ecosystems of the world. Environmental Conservation 29:134–153. doi:10.1017/S0376892902000097.
- Menor A, Prenda J. 2006. Análisis histórico de las poblaciones de trucha (Salmo trutta Linnaeus, 1758) en Andalucía y Castilla la Mancha en el siglo XIX. In: XIII Congreso de la Asociación Española de Limnología y V Congreso Ibérico de Limnología. Barcelona: Asociación Española de Limnología.
- Molero Mesa J, Pérez Raya F, Valle F. 1992. Parque Natural de Sierra Nevada: Paisaje, Fauna, Flora e Itinerarios. Madrid: Rueda.
- Morita K, Yokota A. 2002. Population viability of stream-resident salmonids after habitat fragmentation: A case study with white-spotted charr (Salvelinus leucomaenis) by an individual based model. Ecological Modelling 155:85–94. doi:10.1016/S0304-3800(02)00128-X.
- Moyle PB, Katz JVE, Quiñones RM. 2011. Rapid decline of California’s native inland fishes: A status assessment. Biological Conservation 144:2414–2423. doi:10.1016/j.biocon.2011.06.002.
- Moyle PB, Yoshiyama RM. 1994. Protection of aquatic biodiversity in California: A five-tiered approach. Fisheries 19:6–18. doi:10.1577/1548-8446(1994)019%3C0006:POABIC%3E2.0.CO;2.
- Murchie KJ, Hair KPE, Pullen CE, Redpath TD, Stephens HR, Cooke SJ. 2008. Fish response to modified flow regimes in regulated rivers: Research methods, effects and opportunities. River Research and Applications 24:197–217. doi:10.1002/rra.1058.
- Nieto K, Lizana M, Velasco JC. 2006. Distribución de los peces continentales de España asociada a las características físicas, meteorológicas e hidrológicas de las cuencas hidrográficas. Ecosistemas 15:69–76.
- Ojanguren AF, Braña F. 2003. Effects of size and morphology on swimming performance in juvenile brown trout (Salmo trutta L.). Ecology of Freshwater Fish 12:241–246. doi:10.1046/j.1600-0633.2003.00016.x.
- Poff NL, Olden JD, Merritt DM, Pepin DM. 2007. Homogenization of regional river dynamics by dams and global biodiversity implications. Proceedings of the National Academy of Sciences 104:5732–5737. doi:10.1073/pnas.0609812104.
- REDIAM. 2014. Valores climatológicos anuales en Andalucía: 1961-1990. Available: http://www.juntadeandalucia.es/medioambiente/site/rediam. Accessed Apr 2014 21.
- Rieman BE, Dunham JB. 2000. Metapopulations of salmonids: Synthesis of life history patterns and empirical observations. Ecology of Freshwater Fish 9:51–64. doi:10.1034/j.1600-0633.2000.90106.x.
- Rodríguez-Cea A, Ayllon F, García–Vazquez E. 2003. Micronucleus test in freshwater fish species: An evaluation of its sensitivity for application in field surveys. Ecotoxicology and Environmental Safety 56:442–448. doi:10.1016/S0147-6513(03)00073-3.
- Sáez Gomez P. 2010. Análisis de la distribución histórica de la trucha común (Salmo trutta, Linnaeus, 1758) en Andalucía. Datos Preliminares. Ríos Con Vida 85:16–19.
- Sanz N, Cortey M, Pla C, Garcia–Marin JL. 2006. Hatchery introgression blurs ancient hybridization between brown trout (Salmo trutta) lineages as indicated by complementary allozymes and mtDNA markers. Biological Conservation 130:278–289. doi:10.1016/j.biocon.2005.12.023.
- Scott D, Irvine JR. 2000. Competitive exclusion of brown trout Salmo trutta L., by rainbow trout Oncorhynchus mykiss Walbaum, in lake tributaries, New Zealand. Fisheries Management and Ecology 7:225–237. doi:10.1046/j.1365-2400.2000.00177.x.
- Shieh C-L, Guh Y-R, Wang S-Q. 2007. The application of range of variability approach to the assessment of a check dam on riverine habitat alteration. Environmental Geology 52:427–435. doi:10.1007/s00254-006-0470-3.
- Smith KG, Darwall WRT. 2006. The status and distribution of freshwater fish endemic to the Mediterranean basin. Gland, Switzerland and Cambridge: IUCN.
- Stuart–Smith RD, Richardson AMM, White RWG. 2004. Increasing turbidity significantly alters the diet of brown trout: A multi-year longitudinal study. Journal of Fish Biology 65:376–388. doi:10.1111/j.0022-1112.2004.00456.x.
- Tierno de Figueroa JM, López–Rodríguez MJ, Fenoglio S, Sánchez–Castillo P, Fochetti R. 2013. Freshwater biodiversity in the rivers of the Mediterranean Basin. Hydrobiologia 719:137–186. doi:10.1007/s10750-012-1281-z.
- Williams RN. 2006. Return to the river. Restoring salmon to the Columbia River. Burlington: Academic Press.
- Yrjänä T, Meer OVD, Riihimäki J, Sinisalmi T. 2002. Contributions of short–term flow regulation patterns to trout habitats in a boreal river. Boreal Environment Research 7:77–89.
