Abstract
Cancellariidae is a poorly known family of Neogastropods, including about 300 soft-bottom dwelling species, with a cosmopolitan distribution. As cancellariids are seldom found alive, anatomical data on this family are limited. In particular, no anatomical descriptions have been available thus far for the trigonostomatine group, a conchologically well-defined monophyletic lineage of Cancellariidae that was given a subfamilial rank in former classifications. This is the first description of the anatomy of two closely related and conchologically very similar trigonostomatine Cancellariidae: Scalptia androyensis and S. foveolata. Evident differences were detected in these two species, including the shape and arrangement of the salivary glands, the relative size of the mid and posterior oesophagous, and the structure of the pallial oviduct. Such an unexpected degree of anatomical differentiation, if more widespread in cancellariids, may prove useful in species identification of conchologically similar taxa. Instead, different shell colour morphs of S. androyensis did not display anatomical differences, corroborating the hypothesis that they are conspecific.
Introduction
The neogastropod family Cancellariidae (nutmeg shells) includes ca. 300 worldwide distributed living species, mainly soft-bottom dwellers, and with variable shell morphology. They are characterised by a peculiar nematoglossan radula, with a single, elongated and flexible rachidian tooth per row, and apical cusps that allow a reversible interlocking with adjacent teeth (Petit & Harasewych Citation1986). This particular arrangement is plausibly related to the suctorial feeding reported for some members of the family (Talmadge Citation1972; Loch Citation1987; O’Sullivan et al. Citation1987) and is a strong synapomorphy supporting the monophyly of Cancellariidae, that was further confirmed by molecular data (Modica et al. Citation2009).
A general issue when dealing with Cancellariidae is the relative scarcity of anatomical data: living cancellariid specimens are infrequently found and, consequently, anatomical literature is remarkably scant, with a low number of published papers on both internal anatomy (Graham Citation1966; Harasewych & Petit Citation1982, Citation1984, Citation1986; Strong Citation2003; Modica et al. Citation2009, Citation2011) and radular structure (e.g. Troschel Citation1865; Barnard Citation1958; Olsson Citation1970; Oliver Citation1982; Schremp & Richmond Citation1983; Petit & Harasewych Citation1986; Verhecken & Prelle Citation2014). This is especially true for some species of Trigonostoma and allies, for which no anatomical data are available so far.
Previous classifications gave even a subfamily rank to the Trigonostomatinae (e.g. Cossmann Citation1899), while modern classifications (e.g. Bouchet & Rocroi Citation2005) include Trigonostoma and allies in the subfamily Cancellariinae Forbes and Hanley, 1851, and recognised two additional subfamilies: Admetinae Troschel, Citation1865 and Plesiotritoninae Beu & Maxwell, 1987.
The genus Scalptia Jousseaume, Citation1887 includes about 20 recognised species (Hemmen Citation2007), similar to Trigonostoma species in their shell characters, namely a strong shoulder, an open umbilicus and 2–3 columellar plaits. Noteworthy, a close affinity between Scalptia and Trigonostoma is also supported by molecular data, defining a monophyletic “trigonostomatine” clade inside the Cancellariinae (Modica et al. Citation2011).
The internal anatomy of two closely related trigonostomatine species is here investigated: Scalptia androyensis Verhecken & Bozzetti, Citation2006 and S. foveolata (Sowerby, Citation1849).
Scalptia androyensis was originally described from Lavanono, on the southeast coast of Madagascar, based on empty shells, mainly of a purplish-red colour (Verhecken & Bozzetti Citation2006). Pale fawn shells are also found, and it was recently assumed that these are part of the natural colour variation of the species (Verhecken & Prelle Citation2014). Specimens of both shell colours are included in the anatomical analysis here.
Scalptia foveolata from eastern South Africa is conchologically similar to S. androyensis (Verhecken & Bozzetti Citation2006; Verhecken & Prelle Citation2014). Cancellaria foveolata Sowerby was included in the genus Nevia when introduced by Jousseaume (Citation1887) (type species: Cancellaria spirata Lamarck, Citation1822, from Australia). This generic placement has rarely been used (see Hemmen Citation2007); Garrard (Citation1975) used it as a subgenus of the genus Cancellaria, but did not mention Cancellaria foveolata, which has often been incorrectly placed in Cancellaria (e.g. Petit & Harasewych Citation1990).
Material and methods
Specimens of Scalptia androyensis were collected in Lavanono, southeast Madagascar, and preserved in alcohol. Typical purplish-red specimens were found alive in an intertidal rocky area, buried in small areas of very fine sand between the rocks. Pale-shelled specimens were collected along the sandy shore.
Live specimens of Scalptia foveolata were collected intertidally on the sandy beach at Kei Mouth, Cape Province, South Africa, and preserved in alcohol.
Four specimens of S. androyensis (two of each colour variety; all females; collection numbers AV1363 and AV1365 for pale shells, AV1371 and AV1372 for purplish-red shells) and two specimens of S. foveolata (one female and one male; collection numbers respectively AV0738A and AV0738B) were dissected under a stereomicroscope; sketches were made with a camera lucida and then assembled in the final drawings. Voucher shells are deposited in the personal collections of A. Verhecken (S. androyensis) and H. Hemmen (S. foveolata). Shell size ranged between 14 mm and 15.7 mm for S. androyensis, while for S. foveolata the shells, unavailable to the author, were reported as in the size range for the species (18–24.5 mm).
Results
Anatomy of Scalptia androyensis ()
Figure 1. (A–D) Anatomy of Scalptia androyensis. (A) General body appearance, left view; (B) mantle organs; (C) foregut organs (body wall dorsally dissected longitudinally; only right accessory salivary gland shown); (D) pallial oviduct. (E–H) Anatomy of Scalptia foveolata. (E) General body appearance (male specimen, right view; mantle removed); (F) mantle organs, male specimen; (G) foregut organs (only right accessory salivary gland shown); (H) pallial oviduct. Scale bars: 1 mm. Abbreviations: a, anus; ag, albumen gland; aoe, anterior oesophagous; asd, accessory salivary gland duct; asg, accessory salivary gland; bc, bursa copulatrix; bm, buccal mass; cg, capsule gland; cm, columellar muscle; ct, ctenidium; cv, ctenidial vessel; dr, dorsal region of the capsule gland; e, eye; f, foot; fo, female genital opening; hg, hypobranchial gland; m, mantle; mo, male genital opening; mr, medial region of the capsule gland; mt, mouth; moe, mid oesophagous; os, osphradium; p, penis; poe, posterior oesophagous; pr, proboscis; prs, prostate gland; r, rectum; ry, rhynchodaeum; s, siphon; sg, salivary gland; t, tentacle; v, vestibule; vc, ventral channel; vL, valve of Leiblein; vr, ventral region of the capsule gland.
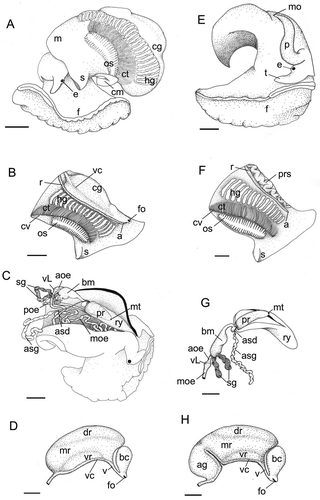
Soft-part morphology was identical in the two shell colour forms and as described below.
Head-foot
Colour homogeneous whitish on the body, light pink on the foot. Head well differentiated. Tentacles well developed, short, dorsoventrally flattened. Eyes dark, on slight swellings at the base of the tentacles. Rhynchostome small, transverse, located between the tentacles. Foot occupying about a half-whorl when the animal is retracted, without evident regionalisation. Columellar muscle as long as about half a spire whorl, white, thin.
Mantle organs
Mantle edge thin, simple, not pigmented. Siphon scarcely developed. Mantle length, measured from the medial point of mantle edge, approximately behind the head, to the beginning of the visceral spire, of about 12 mm. Osphradium elliptical, bipectinate, brown, long – about half the ctenidium length. Osphradium axis thick. Right osphradium filaments about twice the length of the left filaments. Ctenidium whitish, as long as about three quarters of the pallial cavity; anterior end not reaching the mantle border; gill filaments triangular, quite flat. Ctenidial vein uniformly narrow. Hypobranchial gland narrow, as long as about half of the mantle length, occupying the central area of the mantle cavity, whitish, at left of the rectum. Rectum very thin, as long as about three quarters of the mantle length; ending in a small anus surmounted by a short, slender papilla. Female gonoduct running along the right margin of the pallial cavity.
Digestive system
Rhynchodaeum of medium length (about 6 mm in a semi-retracted condition). Buccal mass elongated, basal, outside the posterior end of the proboscis, with a ventral bulge before tapering towards the proboscis tip in an oral tube. Proboscis of medium length, slender. Anterior oesophagous extremely short (about 1 mm). Valve of Leiblein small, placed shortly behind the posterior end of the buccal mass. Mid oesophagous extremely long (about 23 mm), convoluted, translucent white showing internal longitudinal ridges. Posterior oesophagous broader than mid-oesophagous, with no internal structures, straight, running from the nerve ring to the left of the cephalic haemocoel towards the visceral spire. Salivary glands tubular, short, linked by their posterior ends, opening at the base of the buccal cavity with a broad connection. Accessory salivary glands (asd) tubular, elongated, about twice the length of the salivary gland with long ducts (6 mm) entering the proboscis and opening in the oral tube, at the tip of the proboscis.
Female reproductive system
No albumen gland or ingesting gland was detected. Capsule gland well developed, as large as about half of the mantle length, cylindrical, broad. Three different regions identified in the capsule gland: a main medial region (mr), yellowish, thick; a ventral region, orange, divided by the ventral channel in two equal lobes; a whitish, gelatinous dorsal strip, partially covering the main region, slightly asymmetrical (more developed on the right). Poorly muscularised vestibulum anterior to the ventral channel. Sac-like muscular bursa copulatrix, opening by a short duct into the vestibulum. Female opening terminal, slit-like, small.
Anatomy of Scalptia foveolata (–H)
Head-foot
Colour homogeneous whitish on the body, head well differentiated. Tentacles well developed, short, dorsoventrally flattened. Eyes dark, on slight swellings at the base of the tentacles. Rhynchostome small, transverse, located between the tentacles. Foot occupying about a half-whorl when the animal is retracted, not divided in different regions, bowed on the anterior part. Columellar muscle as long as about half a spire whorl, white, thin.
Mantle organs
Mantle border thin, simple, not pigmented. Siphon poorly developed. Mantle length, measured from the medial point of mantle edge, approximately behind the head, to the beginning of the visceral spire, of about 12 mm. Osphradium elliptical, bipectinate, brown, as long as about half the ctenidium length. Osphradium ganglion thick. Right osphradium filaments long – about twice the left filaments. Ctenidium cream coloured, as long as about three quarters of the pallial cavity; anterior end not reaching the mantle border; gill filaments triangular, quite flat. Ctenidial vein uniformly narrow. Hypobranchial gland whitish, quite broad, as long as about half the mantle length, occupying the central area of the mantle cavity, partially covered by the ctenidium in the left and in females by the pallial oviduct on the right. Rectum very thin, as long as about three quarters of the mantle length; ending in a small anus with no papilla. Female gonoduct running along the right margin of the pallial cavity.
Digestive system
Rhynchodaeum of medium length (about 5 mm in a semi-retracted condition). Buccal mass elongated, basal, outside the posterior end of the proboscis, with a ventral bulge before tapering towards the proboscis tip in an oral tube. Proboscis of medium length, slender. Anterior oesophagous extremely short (about 1 mm). Valve of Leiblein small, placed shortly behind the posterior end of the buccal mass. Mid oesophagous long about half of the posterior esophagous, thin, convoluted, translucent white. Posterior oesophagous broader than mid-oesophagous and extremely long (about 25 mm) and convoluted, running from the nerve ring to the left of the cephalopodial cavity towards the visceral spire. Salivary glands tubular, short but rather broad, opening at the base of the buccal cavity with no ducts but a broad connection. Accessory salivary glands tubular, elongated, about 4 times longer than the salivary glands, with a long duct entering the proboscis and opening in the oral tube, at the tip of the proboscis.
Female reproductive system
Pallial oviduct large, about two thirds of the mantle length and one third of the mantle width, cylindrical, broad. Globular albumen gland posterior to the capsule gland, connected to it by the ventral channel, and easily recognisable due to its lighter colouration. Three different regions identified in the capsule gland: a main median region, yellowish, thick; a ventral region, orange, divided by the ventral channel in two equal lobes; a whitish, gelatinous dorsal strip, partially covering the main region, slightly asymmetrical (more developed on the right). Scarcely muscularized vestibulum anterior to the ventral channel. Sac-like muscular bursa copulatrix, opening by a short duct into the vestibulum. Female opening terminal, slit-like, small.
Male reproductive system
Pallial spermiduct as long as about two thirds of the mantle length, entirely occupied by a convoluted, swollen prostate gland. Penis with a rounded section, gradually tapering toward the tip. Male genital duct opening on a small papilla at the tip of the penis.
Discussion
Recently published conchological data showed that, apart from the shell colour, there is no difference between the reddish and the pale shell varieties of Scalptia androyensis (Verhecken & Prelle Citation2014). Dissection did not reveal any divergent anatomical character between the two colour morphs, supporting the conchological results. Colour variations may be attributed to dietary differentiation in different microhabitats (Verhecken & Prelle Citation2014), but may also represent alternative colour phenotypes in a polymorphic species.
Anatomically, S. foveolata and S. androyensis can be easily distinguished on the basis of several features. The salivary glands in S. foveolata are much shorter and more globular than in S. androyensis, and not connected at their posterior ends. A posterior connection of the salivary glands was reported, with different degrees, in two plesiotritonine cancellariids: in Tritonoharpa antiquata (Modica et al. Citation2009) showing a situation similar to S. androyensis, and in Loxotaphrus deshayesii (Modica et al. Citation2011), in which salivary glands are fused for the posterior third. This characteristic seems to be rather common and scattered in different cancellariid groups, and thus is probably not informative at high taxonomic levels. Fused salivary glands retaining two salivary ducts were previously reported in some species of Conoidea (Taylor et al. Citation1993; Ball et al. Citation1997), in some buccinoids such as Chlanidota densesculpta (Harasewych & Kantor Citation1999), in the Nassariidae Ilyanassa obsoleta (Brown Citation1969; Strong Citation2003) and in at least some members of the Columbellidae, Mitridae, Volutomitridae and Ptychatractidae (Marcus & Marcus Citation1962; Ponder Citation1972; Kantor & Harasewych Citation1992; Bouchet & Kantor Citation2000). Fusion of the salivary glands seems to be a widespread feature throughout Neogastropoda, although its functional significance and taxonomic value remains to be investigated. The posterior oesophagous is convoluted and remarkably longer than the mid-oesophagous in S. foveolata, while in S. androyensis it is short and straight. Again, a functional explanation of these foregut characteristics is lacking, especially as no information is available on the feeding habits of these species, which might be related to anatomical variations. A major difference is evident in the pallial oviduct, which in S. foveolata includes a globular albumen gland, connected to the capsule gland by the ventral channel. The albumen gland is missing in S. androyensis. The possibility that such absence could be due to an incomplete sexual maturation of the examined S. androyensis specimens can be ruled out, as the size of all dissected specimens was close to or beyond the maximum value of the range reported for this species (Verhecken & Bozzetti Citation2006; Verhecken & Prelle Citation2014). The few species of Cancellariidae anatomically described to date are reported to possess an albumen gland; in any case, such variations in reproductive anatomy are quite common within neogastropods and have proven to be phylogenetically informative in several groups (e.g. Columbellidae, deMaintenon Citation1999; Rapaninae, Kool Citation1993; Coralliophilinae, Richter & Luque Citation2003). Additionally, there may be a correlation between the presence/absence of the albumen gland and the larval ecology of the species. In the present case, the two species differ significantly in their larval shell size (1.25 whorls in S. androyensis and 1.75 whorls in S. foveolata), but based only on published shell morphology (Verhecken & Prelle Citation2014) it is not clear if a dichotomy in lecitotrophy vs planktotrophy is involved. However, this is a good working hypothesis that might be worth investigating in detail.
Unfortunately, well-preserved samples included only female specimens of S. androyensis; thus, male reproductive tracts of S. androyensis and S. foveolata could not be compared.
The few anatomical works on cancellariid anatomy (Graham Citation1966; Ponder Citation1973; Harasewych & Petit Citation1982, Citation1984, Citation1986; Strong Citation2003; Modica et al. Citation2009, Citation2011) did not focus on the comparison of closely related species. The present results seem to indicate that in Cancellariidae, conchologically similar species (as Scalptia foveolata and S. androyensis appear to be) may display a remarkable degree of anatomical differentiation, allowing a better definition of species boundaries and highlighting the high levels of homoplasy of shell characters traditionally used in cancellariid systematics, as reported elsewhere (Modica et al. Citation2011). More anatomical data on other species of Cancellariidae could help to evaluate the levels of anatomical differentiation in this insufficiently known gastropod group and may provide informative characters useful for phylogenetic and evolutionary studies. In particular, given the basal placement of the Cancellariidae in the neogastropod phylogeny (Oliverio & Modica Citation2010), a better understanding of their anatomical variability can give us crucial insights into the evolution of the whole Neogastropoda, the most successful clade of marine gastropods, for which putative homologies and evolutionary patterns of anatomical characters are still to be completely clarified.
Acknowledgements
André Verhecken (Royal Belgian Institute of Natural Sciences, Brussels, Belgium) is gratefully acknowledged for sharing ideas on Cancellariidae anatomy and evolution. Christa and the late Jens Hemmen (Wiesbaden, Germany) and Giovanni Prelle (Superga, Italy) are acknowledged for donating animals for comparative anatomical study. Thanks are due to Prof. Marco Oliverio (Sapienza University of Rome, Italy) and the anonymous reviewers for helpful comments on the manuscript.
References
- Ball AD, Taylor JD, Andrews EB. 1997. Development of the acinous and accessory salivary glands in Nucella lapillus (Neogastropoda: Muricoidea). Journal of Molluscan Studies 63:245–260. doi:10.1093/mollus/63.2.245.
- Barnard KH. 1958. The radula of Cancellaria. Journal of Conchology 24:243–244.
- Bouchet P, Kantor YI. 2000. The anatomy and systematics of Latiromitra, a genus of tropical deep-water Ptychatractinae (Gastropoda: Turbinellidae). The Veliger 43:1–23.
- Bouchet P, Rocroi J-P. 2005. Classification and nomenclator of gastropod families. Malacologia 47:1–397.
- Brown SC. 1969. The structure and function of the digestive system of the mud snail Nassarius obsoletus (Say). Malacologia 9:447–500.
- Cossmann M. 1899. Essais de paléoconchologie comparée, 3. Paris: the author and Comptoir Géologique. 201 pp. 8 pls.
- deMaintenon M.J. 1999. Phylogenetic analysis of the Columbellidae (Mollusca: Neogastropoda) and the evolution of herbivory from carnivory. Invertebrate Biology 118:258–288. doi:10.2307/3226997.
- Garrard TA. 1975. A revision of Australian Cancellariidae (Gastropoda: Mollusca). Records of the Australian Museum 30:1–62. doi:10.3853/j.0067-1975.30.1975.212.
- Graham A. 1966. The fore-gut of some marginellid and cancellariid prosobranchs. Studies in Tropical Oceanography 4:134–151.
- Harasewych MG, Kantor YI. 1999. A revision of the Antarctic genus Chlanidota (Gastropoda: Neogastropoda: Buccinulidae). Proceedings of the Biological Society of Washington 112:253–302.
- Harasewych MG, Petit RE. 1982. Notes on the morphology of Cancellaria reticulata (Gastropoda: Cancellariidae). The Nautilus 96:104–113.
- Harasewych MG, Petit RE. 1984. Notes on the morphology of Olssonella smithii (Gastropoda: Cancellariidae). The Nautilus 98:37–44.
- Harasewych MG, Petit RE. 1986. Notes on the morphology of Admete viridula (Gastropoda: Cancellariidae). The Nautilus 100:85–91.
- Hemmen J. 2007. Annotated and illustrated catalogue of recent Cancellariidae. Wiesbaden: Hemmen.
- Jousseaume FP. 1887. La famille des Cancellariidae (Mollusques Gastéropodes). Le Naturaliste Année 9, 2e série:155–157, 192–194, 213–214, 221–223.
- Kantor YI, Harasewych MG. 1992. Morphology of the digestive system of Volutomitra alaskana Dall, 1902 (Gastropoda: Pectinibranchia: Volutomitridae), with notes on the possible mechanism of feeding. Ruthenica 2:45–53.
- Kool SP. 1993. Phylogenetic analysis of the Rapaninae (Neogastropoda: Muricidae). Malacologia 35:155–259.
- Lamarck JB. 1822. Histoire naturelle des animaux sans vertèbres. Paris 7:1–711.
- Loch I. 1987. Man bites dog. Australian Shell News 9:59–60.
- Marcus E, Marcus E. 1962. Studies on Columbellidae. Boletim da Faculdade de Filosofia e Ciências e Letras, Universidade de São Paulo. Zoologia 261, 24:335–402.
- Modica MV, Kosyan A, Oliverio M. 2009. The relationships of the enigmatic gastropod Tritonoharpa: New data on early neogastropod evolution? The Nautilus 123:177–188.
- Modica MV, Verhecken A, Oliverio M. 2011. The relationships of the enigmatic neogastropod Loxotaphrus (Cancellariidae). New Zealand Journal of Geology and Geophysics 54:115–124. doi:10.1080/00288306.2011.537610.
- Oliver GP. 1982. A new species of cancellariid gastropod from Antarctica with description of the radula. British Antarctic Survey Bulletin 57:15–20.
- Oliverio M, Modica MV. 2010. Relationships of the haematophagous marine snail Colubraria (Rachiglossa: Colubrariidae), within the neogastropod phylogenetic framework. Zoological Journal of the Linnean Society 158:779–800. doi:10.1111/j.1096-3642.2009.00568.x.
- Olsson AA. 1970. The cancellariid radula and its interpretation. Paleontographic American 7:19–27.
- O’Sullivan JB, McConnaughey RR, Huber ME. 1987. A blood-sucking snail: The Cooper’s nutmeg, Cancellaria cooperi Gabb, parasitizes the California electric ray, Torpedo californica Ayres. Biological Bulletin 172:362–366. doi:10.2307/1541716.
- Petit RE, Harasewych MG. 1986. New Philippine Cancellariidae (Gastropoda: Cancellariacea), with notes on the fine structure and function of the nematoglossan radula. The Veliger 28:436–443.
- Petit RE, Harasewych MG. 1990. Catalog of the Superfamily Cancellarioidea Forbes & Hanley, 1851 (Gastropoda: Prosobranchia). The Nautilus Suppl. 1:1–69.
- Ponder WF. 1972. The morphology of some mitriform gastropods with special reference to their alimentary and reproductive system (Neogastropoda). Malacologia 11:295–342.
- Ponder WF. 1973. The origin and evolution of the Neogastropoda. Malacologia 12:295–338.
- Richter A, Luque Á A.. 2003. Reproductive anatomy of three Mediterranean species of Coralliophilidae (Mollusca: Gastropoda: Neogastropoda). Journal of the Marine Biological Association of the UK 83:1029–1045. doi:10.1017/S0025315403008245h.
- Schremp L, Richmond R. 1983. The cancellariid radula. Western Society of Malacology 15:16–17.
- Sowerby GB. 1849. Descriptions of some new species of Cancellaria in the collection of Mr. H. Cuming. Proceedings of the Zoological Society of London 1848:136–138.
- Strong EE. 2003. Refining molluscan characters: Morphology, character coding and a phylogeny of the Caenogastropoda. Zoological Journal of the Linnean Society 137:447–554. doi:10.1046/j.1096-3642.2003.00058.x.
- Talmadge R. 1972. Pinky. Of Sea and Shore 3: 189.
- Taylor JD, Kantor Y, Sysoev AV. 1993. Foregut anatomy, feeding mechanisms, relationships and classification of Conoidea (Toxoglossa) (Gastropoda). Bulletin of the Natural History Museum (Zoology) 59:125–169.
- Troschel FH. 1865 [in 1856–1891]. Das Gebiss der Schnecken, zur Begründung einer natürlichen Classification 2. Berlin: Nicolai. 1–48.
- Verhecken A, Bozzetti L. 2006. New data on East-African Mericella species, and description of a new species of Scalptia (Neogastropoda: Cancellarioidea: Cancellariidae). Gloria Maris 45:14–25.
- Verhecken A, Prelle G. 2014. Additional data on Scalptia androyensis Verhecken & Bozzetti, 2006, (Neogastropda, Cancellarioidea) from Madagascar. Gloria Maris 53:92–100.
